Immobilized Antibodies on Mercaptophenylboronic Acid Monolayers for Dual-Strategy Detection of 20S Proteasome
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
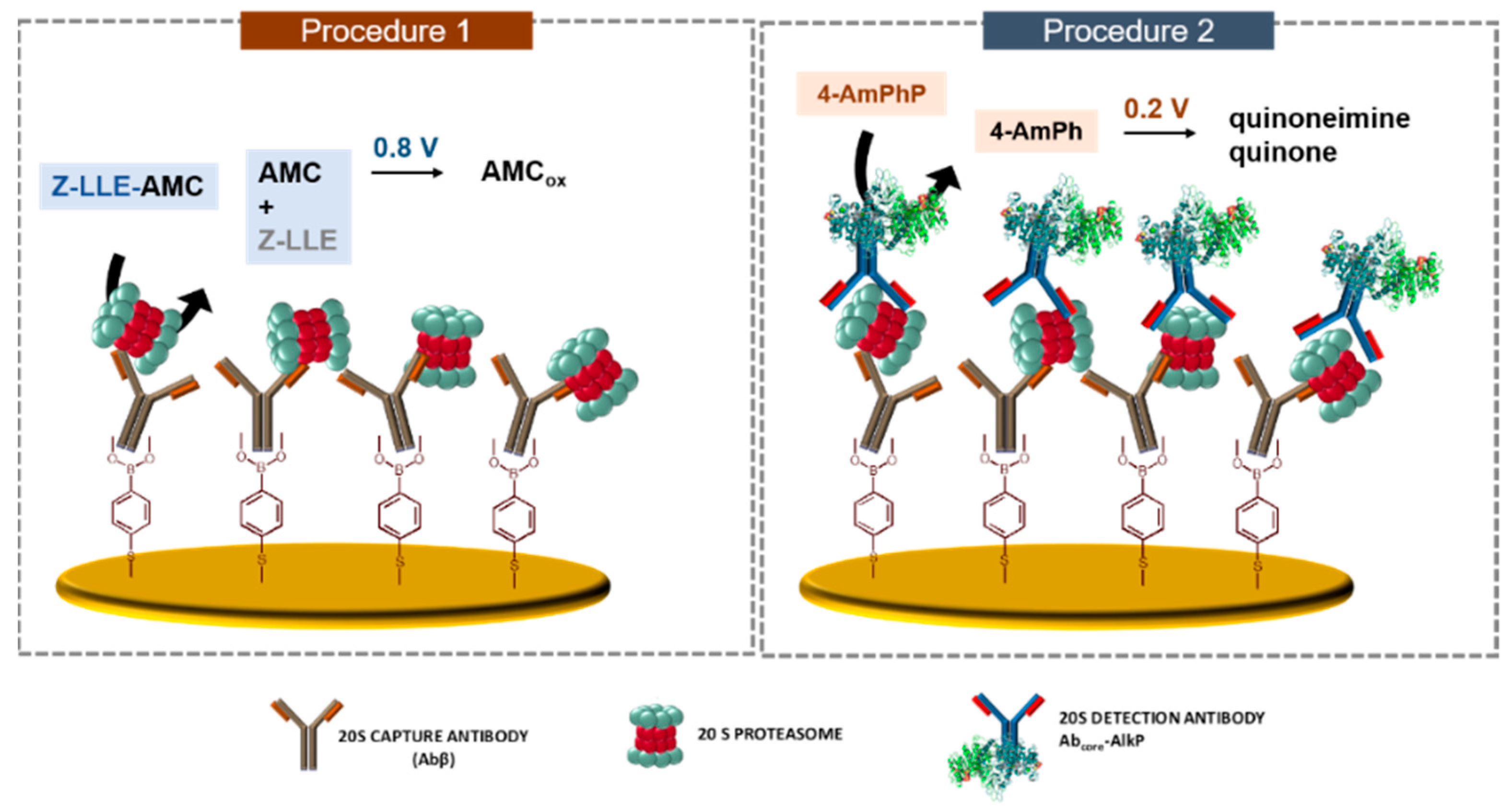
2.3. Preparation of the Immunosensor
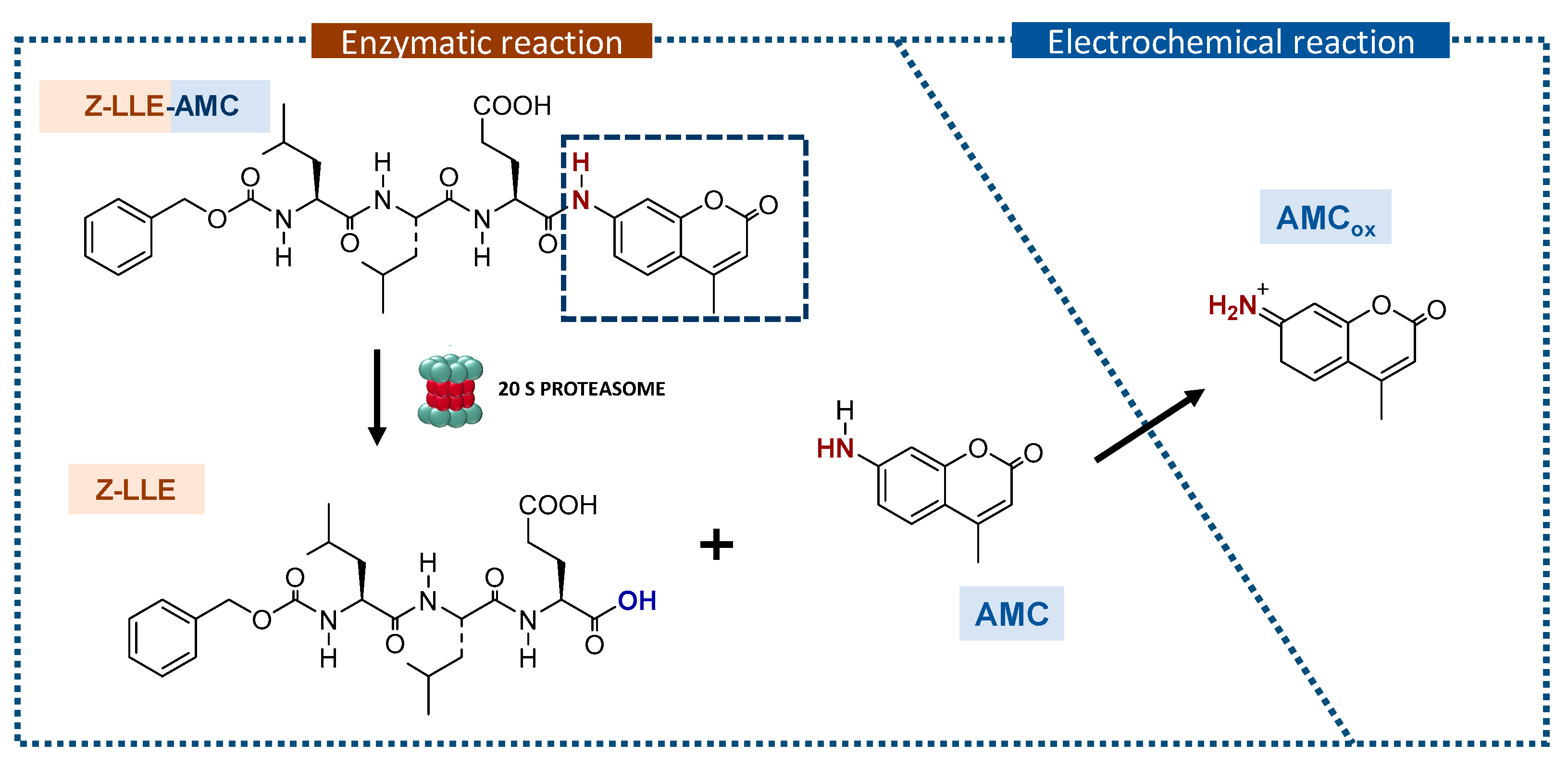
2.4. Procedure for 20S Detection
2.5. Real Sample Analysis
3. Results and Discussion
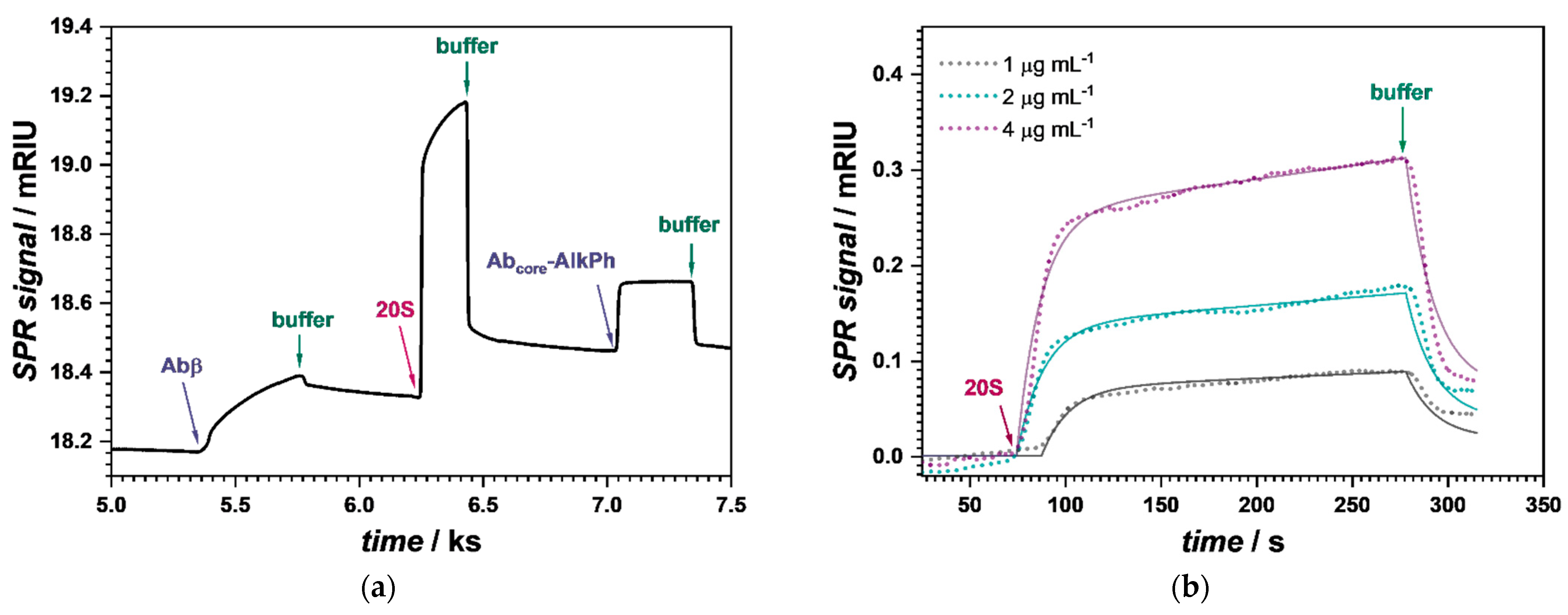
3.1. Monitoring the Step-by-Step Construction
3.1.1. Surface Plasmon Resonance
3.1.2. Gravimetric Analysis
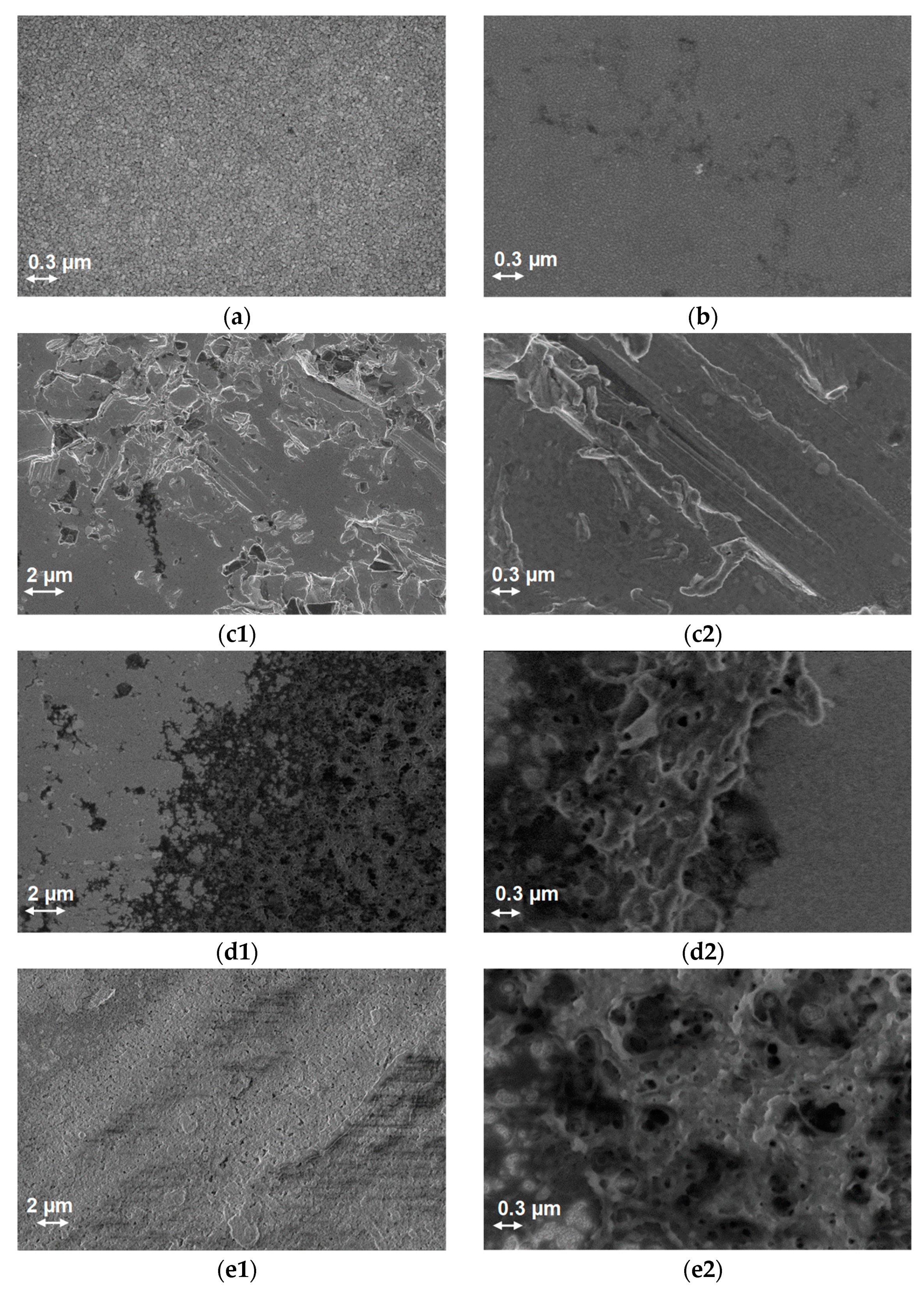
3.2. Characterization
3.2.1. Morphological—Scanning Electron Microscopy
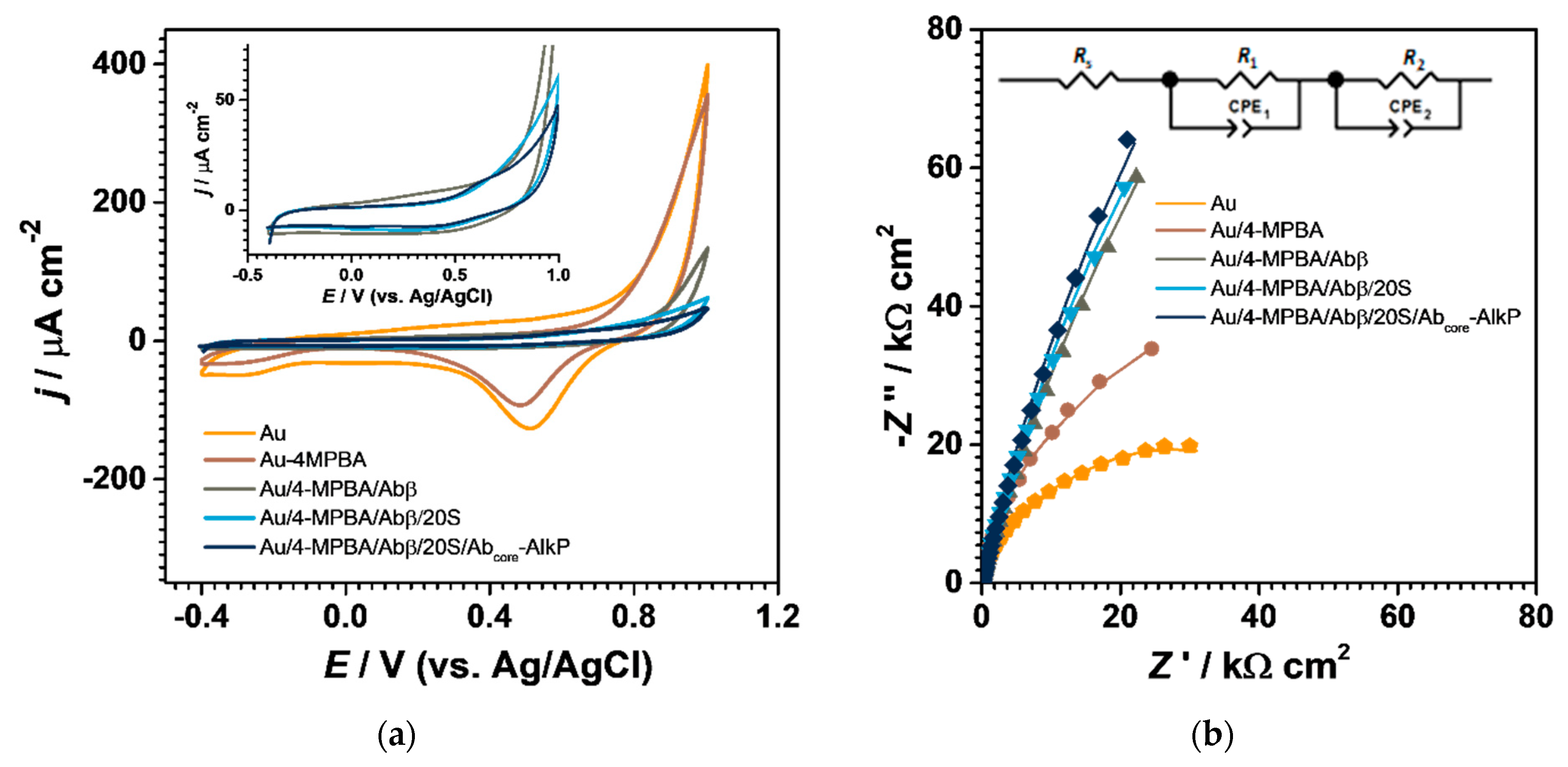
3.2.2. Electrochemical
- Cyclic voltammetry
- Electrochemical impedance spectroscopy (EIS)
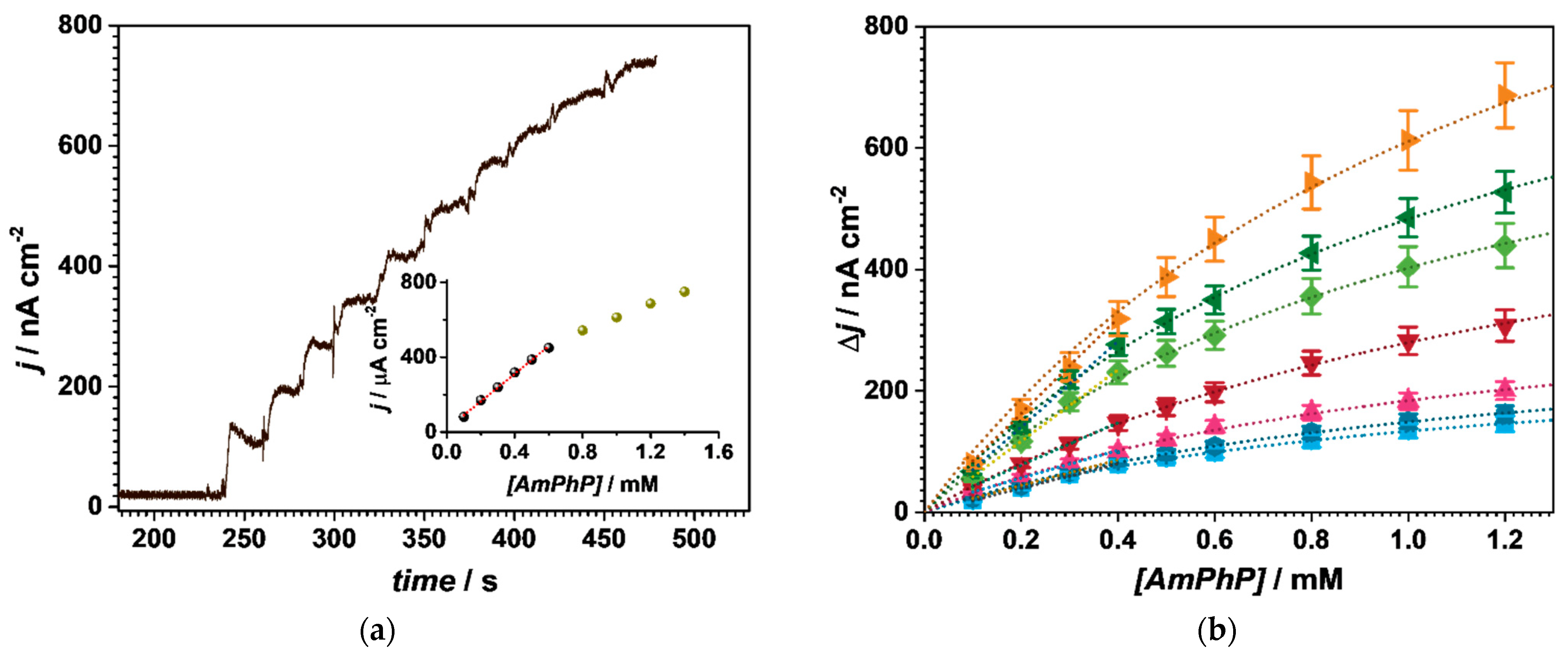
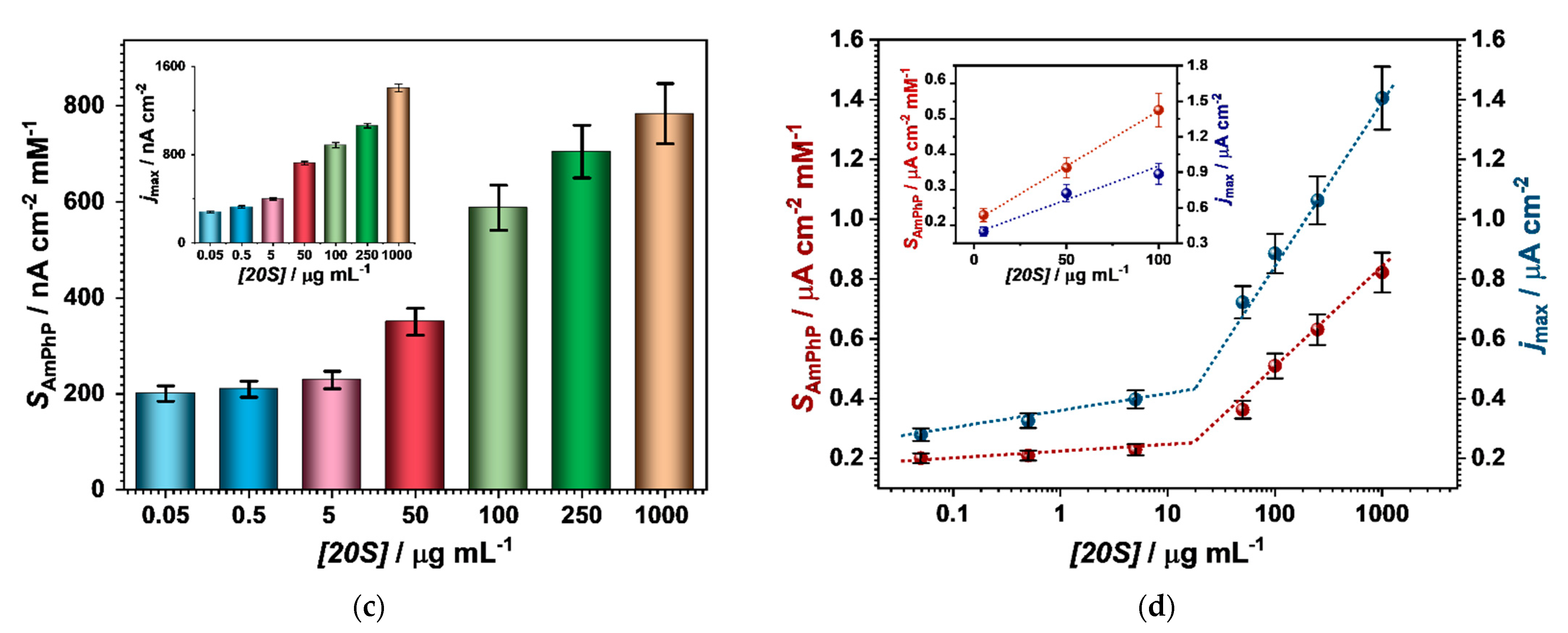
3.3. Applications—Quantification of 20S Proteasome
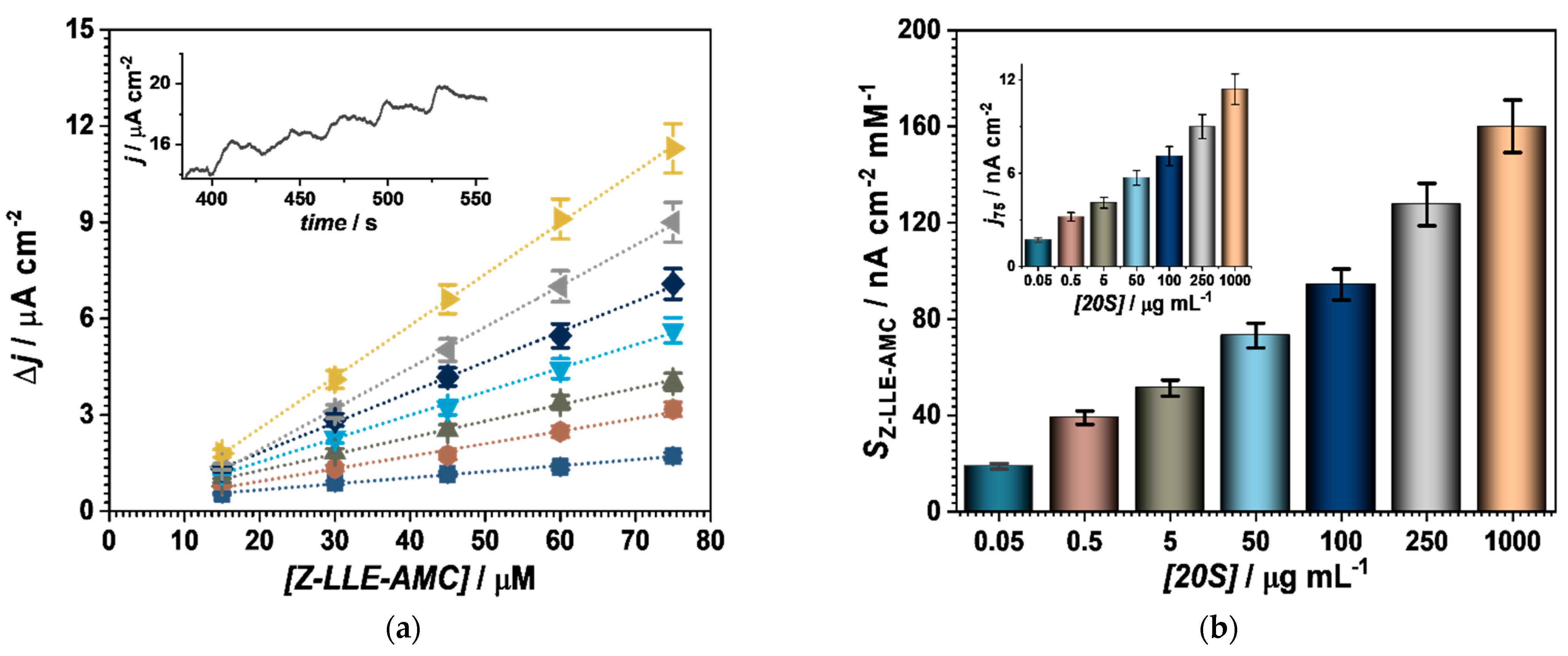
3.3.1. Au/4-MPBA/Abβ/20S
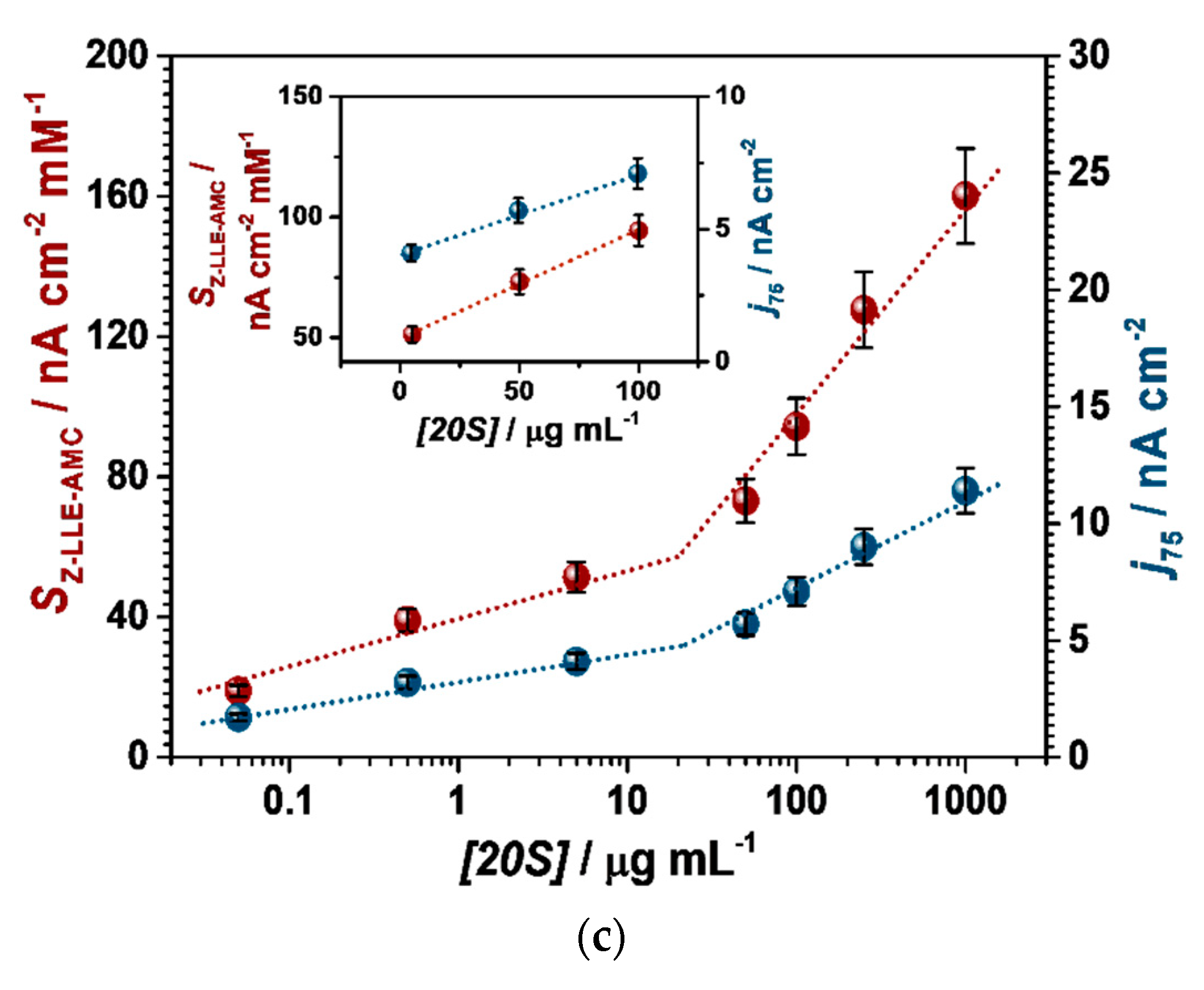
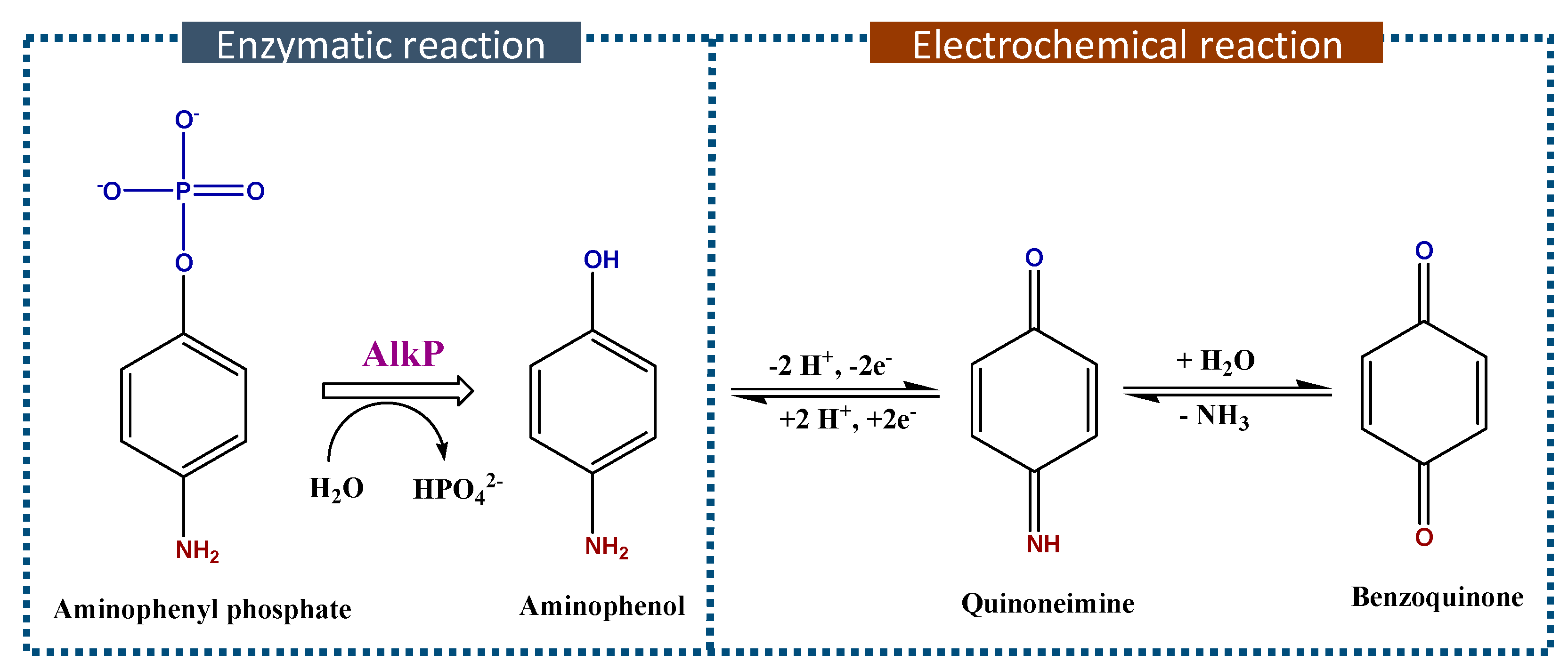
3.3.2. Au/4-MPBA/Abβ/20S/Abcore-AlkP
3.3.3. Recovery
3.3.4. Comparison with Other AlkP Based Electrochemical Immunosensors
3.3.5. Comparison with Other 20S Detection Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, A.; Young, M.A.; Donato, N.J. Emerging Potential of Therapeutic Targeting of Ubiquitin-Specific Proteases in the Treatment of Cancer. Cancer Res. 2014, 74, 4955–4966. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Kaplan, G.S.; Torcun, C.C.; Grune, T.; Ozer, N.K.; Karademir, B. Proteasome inhibitors in cancer therapy: Treatment regimen and peripheral neuropathy as a side effect. Free Radic. Biol. Med. 2017, 103, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gozzetti, A.; Papini, G.; Candi, V.; Brambilla, C.Z.; Sirianni, S.; Bocchia, M. Second Generation Proteasome Inhibitors in Multiple Myeloma. Anti-Cancer Agents Med. Chem. 2017, 17, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Dantuma, N.P.; Bott, L.C. The ubiquitin-proteasome system in neurodegenerative diseases: Precipitating factor, yet part of the solution. Front. Mol. Neurosci. 2014, 7, 70. [Google Scholar] [CrossRef]
- Sixt, S.U.; Dahlmann, B. Extracellular, circulating proteasomes and ubiquitin—Incidence and relevance. Biochim. Biophys. Acta Mol. Basis Dis. 2008, 1782, 817–823. [Google Scholar] [CrossRef]
- Wada, M.; Kosaka, M.; Saito, S.; Sano, T.; Tanaka, K.; Ichihara, A. Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J. Lab. Clin. Med. 1993, 121, 215–223. [Google Scholar]
- Qu, K.Z.; Zhang, K.; Ma, W.; Li, H.; Wang, X.; Zhang, X.; Giles, F.; Lai, M.; Afdhal, N.H.; Albitar, M. Ubiquitin-proteasome profiling for enhanced detection of hepatocellular carcinoma in patients with chronic liver disease. J. Gastroenterol. Hepatol. 2010, 26, 751–758. [Google Scholar] [CrossRef]
- Majetschak, M.; Patel, M.B.; Sorell, L.T.; Liotta, C.; Li, S.; Pham, S.M. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem. Biophys. Res. Commun. 2008, 365, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M.; Sorell, L.T.; Patricelli, T.; Seitz, D.H.; Knöferl, M.W. Detection and possible role of proteasomes in the bronchoalveolar space of the injured lung. Physiol. Res. 2009, 58, 363–372. [Google Scholar] [CrossRef]
- Gorodkiewicz, E.; Ostrowska, H.; Sankiewicz, A. SPR imaging biosensor for the 20S proteasome: Sensor development and application to measurement of proteasomes in human blood plasma. Microchim. Acta 2011, 175, 177–184. [Google Scholar] [CrossRef]
- Anna, S.; Agnieszka, M.; Zenon, L.; Beata, P.; Ewa, G. Methods for 20S Immunoproteasome and 20S Constitutive Proteasome Determination Based on SPRI Biosensors. Cell. Mol. Bioeng. 2017, 10, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors–A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-S.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Yndart, A.; Kumar, S.; Jayant, R.D.; Vashist, A.; Brown, A.N.; Li, C.-Z.; Nair, M. A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-R.; Park, T.J.; Kim, M.S.; Kim, I.-H.; Kim, K.-S.; Chung, K.H.; Ko, S. Functional fusion proteins and prevention of electrode fouling for a sensitive electrochemical immunosensor. Anal. Chim. Acta 2017, 967, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Yean, C.Y.; Shueb, R.H. Screen Printed Carbon Electrode Based Electrochemical Immunosensor for the Detection of Dengue NS1 Antigen. Diagnostics 2014, 4, 165–180. [Google Scholar] [CrossRef]
- Martínez-Rojas, F.; Diculescu, V.C.; Armijo, F. Electrochemical Immunosensing Platform for the Determination of the 20S Proteasome Using an Aminophenylboronic/Poly-indole-6-carboxylic Acid-Modified Electrode. ACS Appl. Bio Mater. 2020, 3, 4941–4948. [Google Scholar] [CrossRef]
- Barsan, M.M.; Diculescu, V.C. Antibody-based amperometric biosensor for 20S proteasome activity and for inhibitors screening. Analyst 2021. [Google Scholar] [CrossRef]
- Lin, P.-H.; Huang, S.-C.; Chen, K.-P.; Li, B.-R.; Li, Y.-K. Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization. Sensors 2018, 19, 28. [Google Scholar] [CrossRef]
- Duval, F.; Van Beek, T.A.; Zuilhof, H. Key steps towards the oriented immobilization of antibodies using boronic acids. Analyst 2015, 140, 6467–6472. [Google Scholar] [CrossRef]
- De Jesus, C.S.H.; Paquim, A.-M.C.; Diculescu, V.C. Voltammetric and atomic force microscopy characterization of chymotrypsin, trypsin and caspase activities of proteasome. Catal. Today 2018, 306, 287–293. [Google Scholar] [CrossRef]
- de Jesus, C.S.H.; Chiorcea-Paquim, A.M.; Barsan, M.M.; Diculescu, V.C. Electrochemical assay for 20S proteasome activity and inhibition with anti-cancer drugs. Talanta 2019, 199, 32–39. [Google Scholar] [CrossRef]
- Preechaworapun, A.; Dai, Z.; Xiang, Y.; Chailapakul, O.; Wang, J. Investigation of the enzyme hydrolysis products of the substrates of alkaline phosphatase in electrochemical immunosensing. Talanta 2008, 76, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Campàs, M.; De La Iglesia, P.; Le Berre, M.; Kane, M.; Diogène, J.; Marty, J.-L. Enzymatic recycling-based amperometric immunosensor for the ultrasensitive detection of okadaic acid in shellfish. Biosens. Bioelectron. 2008, 24, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Preechaworapun, A.; Ivandini, T.A.; Suzuki, A.; Fujishima, A.; Chailapakul, O.; Einaga, Y. Development of Amperometric Immunosensor Using Boron-Doped Diamond with Poly(o-aminobenzoic acid). Anal. Chem. 2008, 80, 2077–2083. [Google Scholar] [CrossRef]
- Trilling, A.K.; Harmsen, M.M.; Ruigrok, V.J.; Zuilhof, H.; Beekwilder, J. The effect of uniform capture molecule orientation on biosensor sensitivity: Dependence on analyte properties. Biosens. Bioelectron. 2013, 40, 219–226. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, P.C.A.; Morris, E.P. Structure of the Human 26S Proteasome. J. Biol. Chem. 2008, 283, 23305–23314. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, T.-I.; Kim, K.-H.; Kim, J.-H.; Choi, M.-S.; Choi, H.-J.; Koh, K. Formation of a self-assembled phenylboronic acid monolayer and its application toward developing a surface plasmon resonance-based monosaccharide sensor. Anal. Biochem. 2002, 310, 163–170. [Google Scholar] [CrossRef]
- Stevens, T.E.; Pearce, C.J.; Whitten, C.N.; Grant, R.P.; Monson, T.C. Self-Assembled Array of Tethered Manganese Oxide Nanoparticles for the Next Generation of Energy Storage. Sci. Rep. 2017, 7, 44191. [Google Scholar] [CrossRef]
- Góes, M.S.; Rahman, H.; Ryall, J.; Davis, J.J.; Bueno, P.R. A Dielectric Model of Self-Assembled Monolayer Interfaces by Capacitive Spectroscopy. Langmuir 2012, 28, 9689–9699. [Google Scholar] [CrossRef]
- Oldziej, A.; Bolkun, L.; Galar, M.; Kalita, J.; Ostrowska, H.; Romaniuk, W.; Kloczko, J. Assessment of proteasome concentration and chymotrypsin-like activity in plasma of patients with newly diagnosed multiple myeloma. Leuk. Res. 2014, 38, 925–930. [Google Scholar] [CrossRef]
- Scandurra, G.; Antonella, A.; Ciofi, C.; Saitta, G.; Lanza, M. Electrochemical Detection of p-Aminophenol by Flexible Devices Based on Multi-Wall Carbon Nanotubes Dispersed in Electrochemically Modified Nafion. Sensors 2014, 14, 8926–8939. [Google Scholar] [CrossRef]
- Yeh, M.F.; Trela, J.M. Purification and characterization of a repressible alkaline phosphatase from Thermus aquaticus. J. Biol. Chem. 1976, 251, 3134–3139. [Google Scholar] [CrossRef]
- Song, S.; Kim, Y.J.; Shin, I.-S.; Kim, W.-H.; Lee, K.-N.; Seong, W.K. Electrochemical Immunoassay Based on Indium Tin Oxide Activity Toward a Alkaline Phosphatase. BioChip J. 2019, 13, 387–393. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.; Keating, E.; Delerue-Matos, C. High-performance electrochemical immunomagnetic assay for breast cancer analysis. Sens. Actuators B Chem. 2020, 308, 127667. [Google Scholar] [CrossRef]
- Neagu, D.; Micheli, L.; Palleschi, G. Study of a toxin–alkaline phosphatase conjugate for the development of an immunosensor for tetrodotoxin determination. Anal. Bioanal. Chem. 2006, 385, 1068–1074. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Jiang, L.-P.; Zhu, J.-J. Electrochemical immunosensor of tumor necrosis factor α based on alkaline phosphatase functionalized nanospheres. Biosens. Bioelectron. 2011, 26, 1890–1894. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Guerrero, S.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J. Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva. Bioelectrochemistry 2020, 133, 107484. [Google Scholar] [CrossRef] [PubMed]
| Electrode Configuration | R1/ kΩ cm2 | CPE1/ µF cm−2 sα−1 | α1 | R2/kΩ cm2 | CPE2/ µF cm−2 sα−1 | α2 |
|---|---|---|---|---|---|---|
| Au | 12 | 26 | 0.90 | 38 | 45 | 0.93 |
| Au/4-MPBA | 5 | 115 | 0.80 | 44 | 32 | 1.00 |
| Au/4-MPBA/Abβ | 13 | 55 | 0.76 | 320 | 22 | 0.95 |
| Au/4-MPBA/Abβ/20S | 10 | 55 | 0.80 | 360 | 22 | 0.97 |
| Au/4-MPBA/Abβ/20S/Abcore-AlkP | 11 | 65 | 0.76 | 620 | 20 | 0.92 |
| Target Analyte | AlkP Substrate | Technique, E/V | Dynamic Range/ ng mL−1 | LoD/µg mL−1 | Ref. |
|---|---|---|---|---|---|
| α-Tumor Necrosis Factor | 1-NP 1 | DPV, +0.30 | 0.02–200 | 1.0 × 10−5 | [38] |
| IgG | PAA 2 | CA, +0.40 | 3–200 | 1.0 × 10−3 | [24] |
| tetrodoxin | 1-NP | DPV, +0.30 | 2–50 | 1.0 × 10−3 | [37] |
| S and N proteins, of SARS-CoV2 | 1-NP | DPV, +0.20 | - | 1.9 × 10−2/ 0.8 × 10−2 | [39] |
| Cancer biomarker | PAA | CV, +0.30 | 0.4–100 | 2.0 × 10−4 | [35] |
| IL-1 beta cytokine | 1-NP | DPV, +0.20 | 0.2–1.2 | 5.2 × 10−6 | [40] |
| Cancer biomarker | 3-IP 3 | LSV, +0.15 | 5–50 | 2.8 × 10−3 | [36] |
| 20 S proteasome | AmPhP | CA, +0.20 | 5–100 × 103 | 2.0 × 10−1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsan, M.M.; Sanz, C.G.; Onea, M.; Diculescu, V.C. Immobilized Antibodies on Mercaptophenylboronic Acid Monolayers for Dual-Strategy Detection of 20S Proteasome. Sensors 2021, 21, 2702. https://doi.org/10.3390/s21082702
Barsan MM, Sanz CG, Onea M, Diculescu VC. Immobilized Antibodies on Mercaptophenylboronic Acid Monolayers for Dual-Strategy Detection of 20S Proteasome. Sensors. 2021; 21(8):2702. https://doi.org/10.3390/s21082702
Chicago/Turabian StyleBarsan, Madalina M., Caroline G. Sanz, Melania Onea, and Victor C. Diculescu. 2021. "Immobilized Antibodies on Mercaptophenylboronic Acid Monolayers for Dual-Strategy Detection of 20S Proteasome" Sensors 21, no. 8: 2702. https://doi.org/10.3390/s21082702
APA StyleBarsan, M. M., Sanz, C. G., Onea, M., & Diculescu, V. C. (2021). Immobilized Antibodies on Mercaptophenylboronic Acid Monolayers for Dual-Strategy Detection of 20S Proteasome. Sensors, 21(8), 2702. https://doi.org/10.3390/s21082702








