The 3D Modeling System for Bioaerosol Distribution Based on Planar Laser-Induced Fluorescence
Abstract
1. Introduction
2. System and Experiment
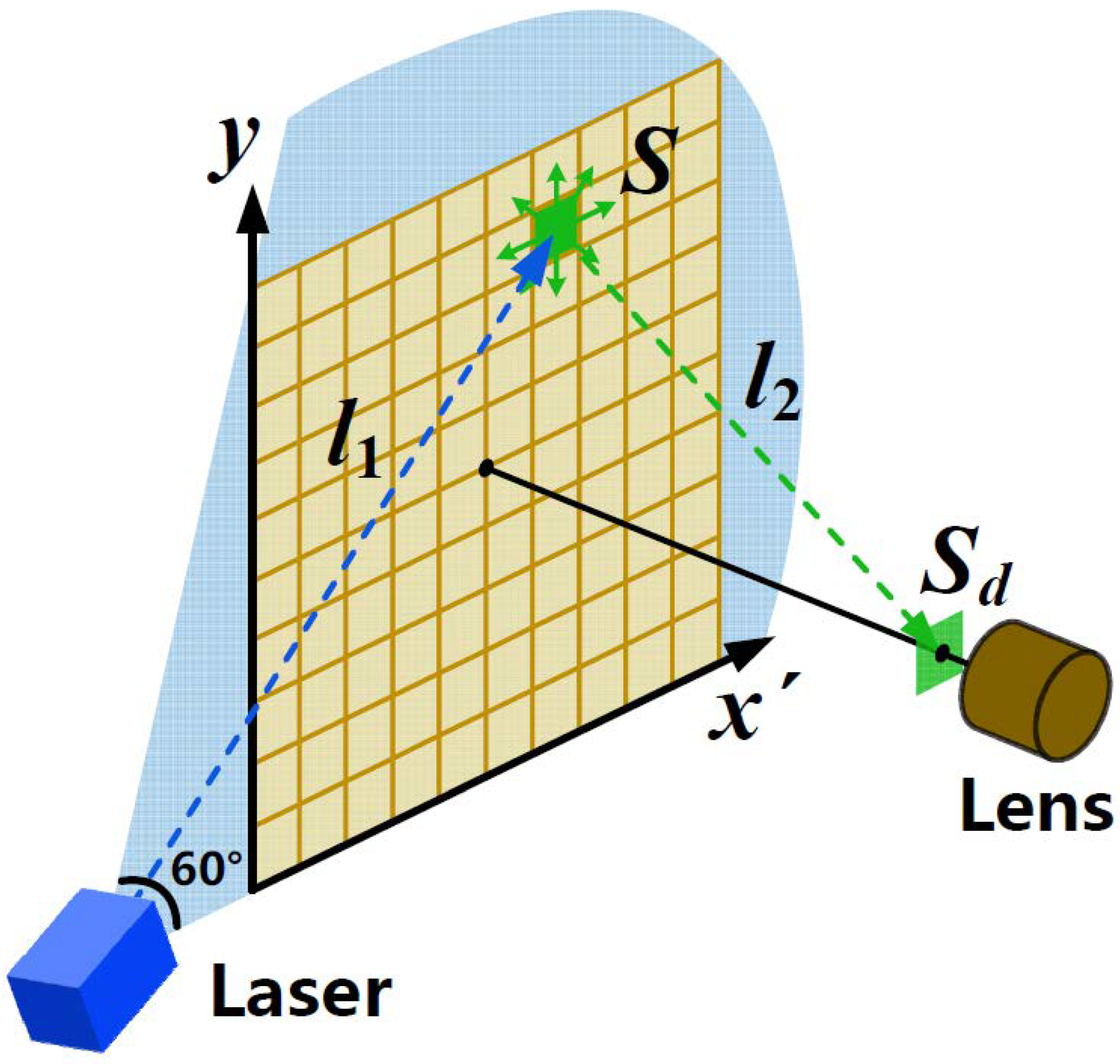
2.1. Structure and Equipment
2.2. Experimental Design
3. Data Processing
3.1. Image Denoising
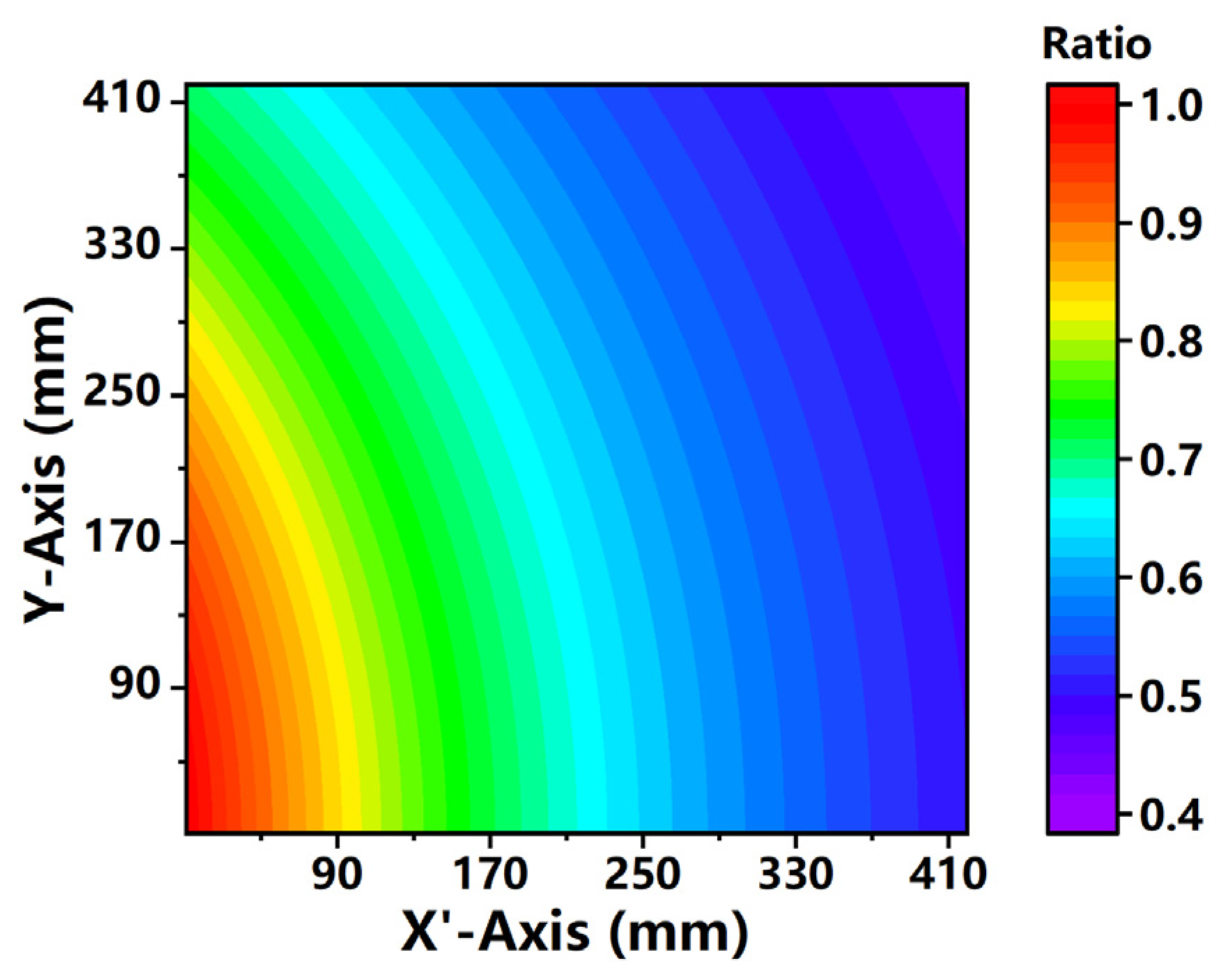
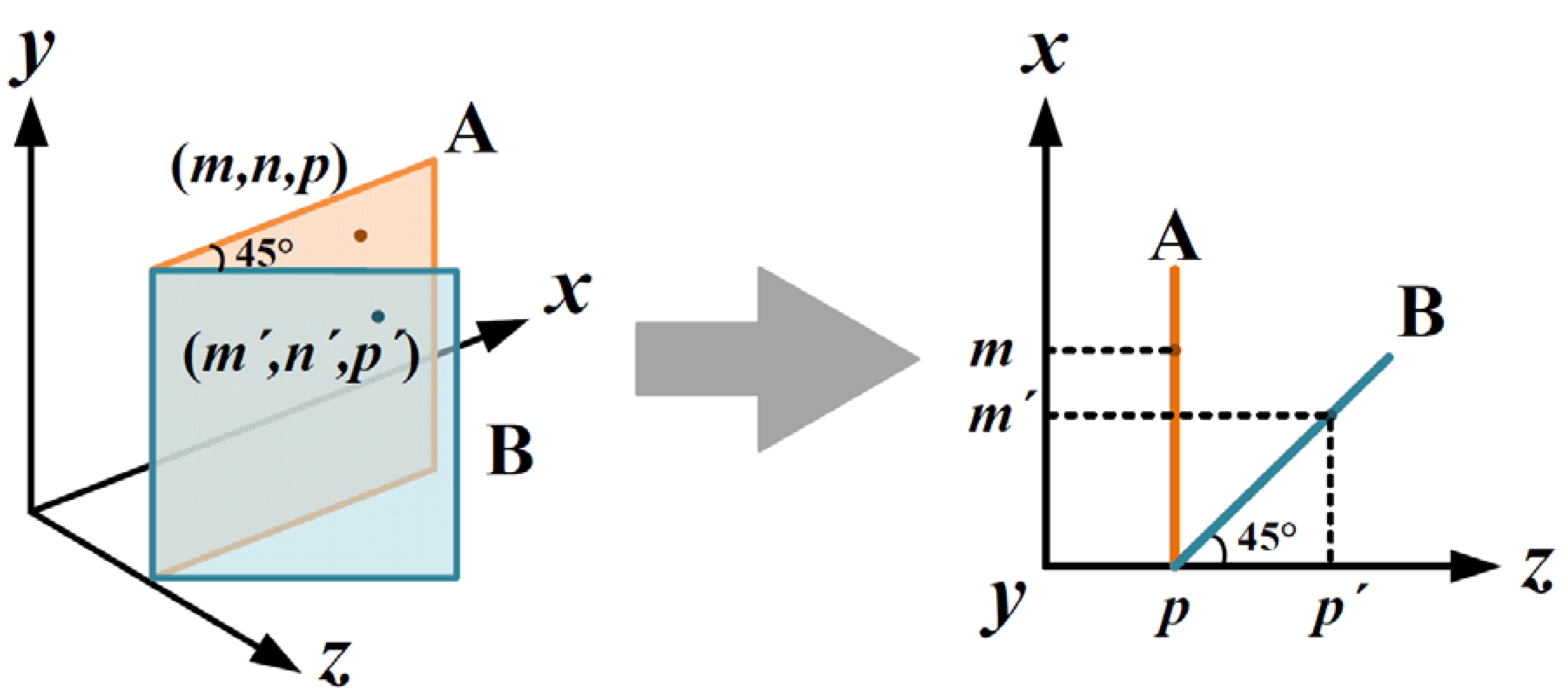
3.2. Geometric Correction of Laser Intensity
3.3. 3D Reconstruction
4. Result and Discussion
4.1. Analysis of Fluorescence Attenuation
4.2. Analysis of Image Processing
4.3. Comparison of 2D Intensity Distributions
4.4. Diffusion Process of 3D Distribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Pöschl, U. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Fiegel, J.; Clarke, R.; Edwards, D.A. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov. Today. 2006, 11, 51–57. [Google Scholar] [CrossRef]
- Fung, F.; Hughson, W.G. Health effects of indoor fungal bioaerosol exposure. Appl. Occup. Environ. Hyg. 2003, 18, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.J.; Lim, C.E.; Kim, H.B.; Lee, B.U. Effects of human activities on concentrations of culturable bioaerosols in indoor air environments. J. Aerosol Sci. 2017, 104, 58–65. [Google Scholar] [CrossRef]
- Holler, S.; Chang, R.K.; Hill, S.C.; Pinnick, R.G.; Niles, S.; Bottiger, J.R. Single-shot fluorescence spectra of individual micrometer-sized bioaerosols illuminated by a 351- or a 266-nm ultraviolet laser. Opt. Lett. 1999, 24, 116–118. [Google Scholar]
- Pan, Y.L.; Hartings, J.; Pinnick, R.G.; Hill, S.C.; Halverson, J.; Chang, R.K. Single-particle fluorescence spectrometer for ambient aerosols. Aerosol Sci. Tech. 2003, 37, 628–639. [Google Scholar] [CrossRef][Green Version]
- Kiselev, D.; Bonacina, L.; Wolf, J.P. Individual bioaerosol particle discrimination by multi-photon excited fluorescence. Opt. Express 2011, 19, 24516–24521. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Kumar, D.; Maini, A.K.; Sharma, R.C. Detection of biological warfare agents using ultra violet-laser induced fluorescence LIDAR. Spectrochim. Acta A. 2013, 112, 446–456. [Google Scholar] [CrossRef]
- Gottfried, J.L.; De Lucia, F.C.; Munson, C.A.; Miziolek, A.W. Standoff detection of chemical and biological threats using laser-induced breakdown spectroscopy. Appl. Spectrosc. 2008, 62, 353–363. [Google Scholar] [CrossRef]
- Sharma, R.C.; Kumar, D.; Kumar, S.; Joshi, D.; Srivastva, H.B. Standoff detection of biomolecules by ultraviolet laser-induced fluorescence LIDAR. IEEE Sens. J. 2015, 15, 3349–3352. [Google Scholar] [CrossRef]
- Kim, M.S.; Lefcourt, A.M.; Chen, Y.R. Multispectral laser-induced fluorescence imaging system for large biological samples. Appl. Optics 2003, 42, 3927–3934. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Satija, A.; Pourpoint, T.L.; Son, S.F.; Lucht, R.P. High-repetition-rate three-dimensional OH imaging using scanned planar laser-induced fluorescence system for multiphase combustion. Appl. Optics 2014, 53, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.A.; Troutman, V.A.; Hanson, R.K. Near-kHz 3D tracer-based LIF imaging of a co-flow jet using toluene. Mea. Sci. Technol. 2014, 25, 075403. [Google Scholar] [CrossRef]
- Cai, W.; Li, X.; Ma, L. Practical aspects of implementing three-dimensional tomography inversion for volumetric flame imaging. Appl. Optics 2013, 52, 8106–8116. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lei, Q.; Capil, T.; Hammack, S.D.; Carter, C.D. Direct comparison of two-dimensional and three-dimensional laser-induced fluorescence measurements on highly turbulent flames. Opt. Lett. 2017, 42, 267–270. [Google Scholar] [CrossRef]
- Halls, B.R.; Thul, D.J.; Michaelis, D.; Roy, S.; Meyer, T.R.; Gord, J.R. Single-shot, volumetrically illuminated, three-dimensional, tomographic laser-induced-fluorescence imaging in a gaseous free jet. Opt. Express 2016, 24, 10040–10049. [Google Scholar] [CrossRef]
- Li, T.; Pareja, J.; Fuest, F.; Schütte, M.; Zhou, Y.; Dreizler, A.; Böhm, B. Tomographic imaging of OH laser-induced fluorescence in laminar and turbulent jet flames. Mea. Sci. Technol. 2017, 29, 015206. [Google Scholar] [CrossRef]
- Farsund, Ø.; Rustad, G.; Kasen, I.; Haavardsholm, T.V. Required spectral resolution for bioaerosol detection algorithms using standoff laser-induced fluorescence measurements. IEEE Sens. J. 2010, 10, 655–661. [Google Scholar] [CrossRef]
- Skiba, A.W.; Wabel, T.M.; Carter, C.D.; Hammack, S.D.; Temme, J.E.; Lee, T.; Driscoll, J.F. Reaction layer visualization: A comparison of two PLIF techniques and advantages of kHz-imaging. Proc. Combust. Inst. 2017, 36, 4593–4601. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Lei, Q.; Ma, L. Single-shot volumetric laser induced fluorescence (VLIF) measurements in turbulent flows seeded with iodine. Opt. Express 2015, 23, 33408–33418. [Google Scholar] [CrossRef]
- Blomberg, S.; Zhou, J.; Gustafson, J.; Zetterberg, J.; Lundgren, E. 2D and 3D imaging of the gas phase close to an operating model catalyst by planar laser induced fluorescence. J. Phys. Condens. Matter 2016, 28, 453002. [Google Scholar] [CrossRef] [PubMed]
- Wellander, R.; Richter, M.; Aldén, M. Time-resolved (kHz) 3D imaging of OH PLIF in a flame. Exp. Fluids 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Pareja, J.; Johchi, A.; Li, T.; Dreizler, A.; Böhm, B. A study of the spatial and temporal evolution of auto-ignition kernels using time-resolved tomographic OH-LIF. Proc. Combust. Inst. 2019, 37, 1321–1328. [Google Scholar] [CrossRef]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta A. 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Han, M.; Liu, Y.; Xi, J.; Guo, W. Noise smoothing for nonlinear time series using wavelet soft threshold. IEEE Signal Process. Lett. 2006, 14, 62–65. [Google Scholar] [CrossRef]
- Valencia, D.; Orejuela, D.; Salazar, J.; Valencia, J. Comparison analysis between rigrsure, sqtwolog, heursure and minimaxi techniques using hard and soft thresholding methods. In Proceedings of the Symposium on Signal Processing, Images and Artificial Vision (STSIVA), Bucaramanga, Colombia, 31 August–16 September 2016. [Google Scholar]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta. 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Wind, L.; Szymanski, W.W. Quantification of scattering corrections to the Beer-Lambert law for transmittance measurements in turbid media. Mea. Sci. Technol. 2002, 13, 270. [Google Scholar] [CrossRef]


| Component | Parameter | Value |
|---|---|---|
| Laser | Laser Power | 100 mW |
| Laser Wavelength | 450 nm | |
| Lens | Focal Length | 16 mm |
| Imaging Distance | 30 cm | |
| Camera | Quantum Efficiency | 0.82 (500–600 nm) |
| Cell Size | 6.5 μm × 6.5 μm | |
| Effective Area | 13.3 mm × 13.3 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Chen, Y.; Zhang, Y.; Guo, P.; Chen, H.; Wu, H. The 3D Modeling System for Bioaerosol Distribution Based on Planar Laser-Induced Fluorescence. Sensors 2021, 21, 2607. https://doi.org/10.3390/s21082607
Chen S, Chen Y, Zhang Y, Guo P, Chen H, Wu H. The 3D Modeling System for Bioaerosol Distribution Based on Planar Laser-Induced Fluorescence. Sensors. 2021; 21(8):2607. https://doi.org/10.3390/s21082607
Chicago/Turabian StyleChen, Siying, Yuanyuan Chen, Yinchao Zhang, Pan Guo, He Chen, and Huiyun Wu. 2021. "The 3D Modeling System for Bioaerosol Distribution Based on Planar Laser-Induced Fluorescence" Sensors 21, no. 8: 2607. https://doi.org/10.3390/s21082607
APA StyleChen, S., Chen, Y., Zhang, Y., Guo, P., Chen, H., & Wu, H. (2021). The 3D Modeling System for Bioaerosol Distribution Based on Planar Laser-Induced Fluorescence. Sensors, 21(8), 2607. https://doi.org/10.3390/s21082607





