Phacoemulsification and IOL-Implantation without Using Viscoelastics: Combined Modeling of Thermo Fluid Dynamics, Clinical Outcomes, and Endothelial Cell Density
Abstract
1. Introduction
2. Materials and Methods
2.1. Medical Study Part
2.1.1. Non-Contact Corneal Endothelium Microscopy
2.1.2. Surgery Method with BSS
2.1.3. Surgery Method with Healon
2.1.4. Statistical Analysis
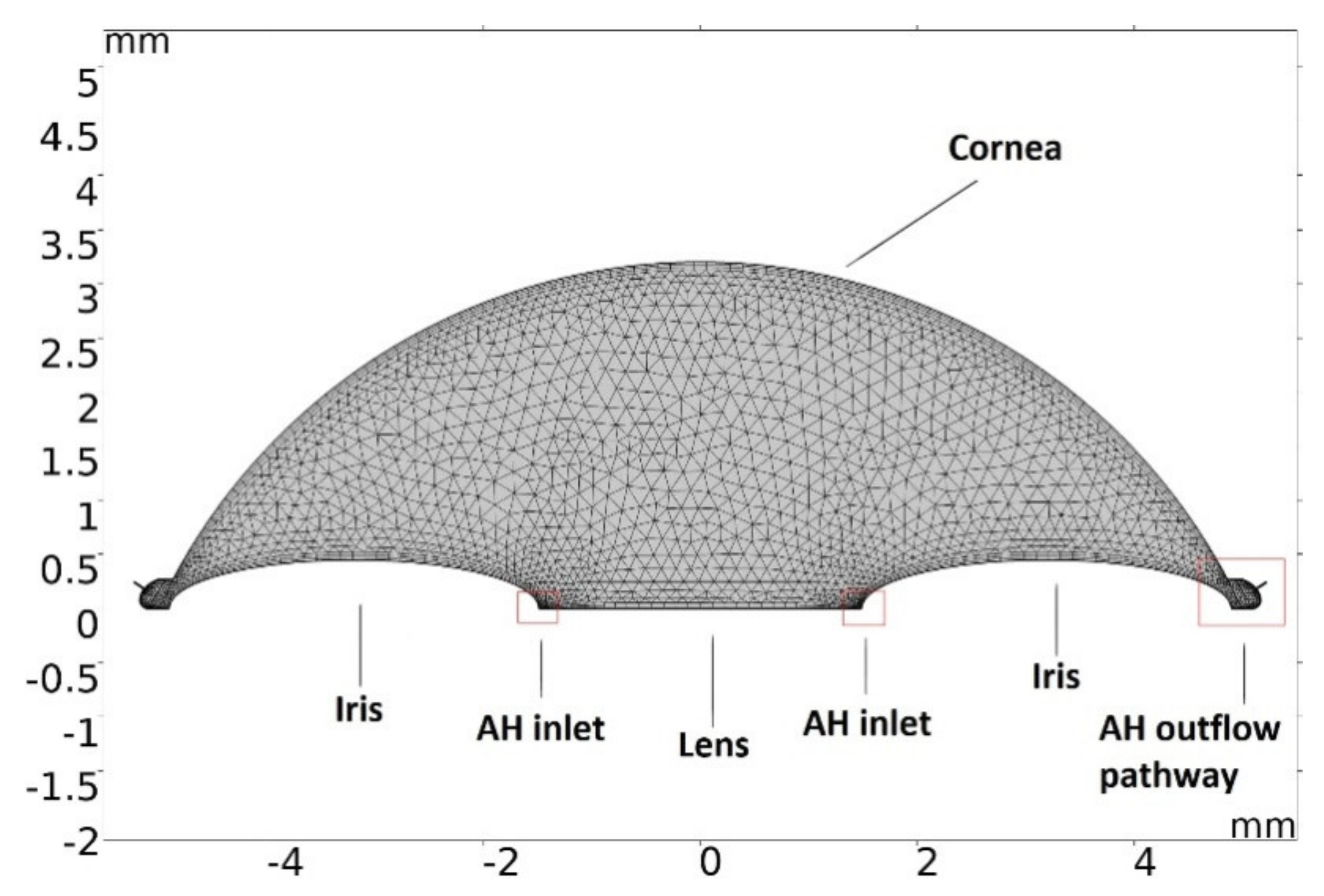
2.2. Modeling of Temperature Dissipation in the Anterior Chamber of the Eye during Phacoemulsification
3. Results
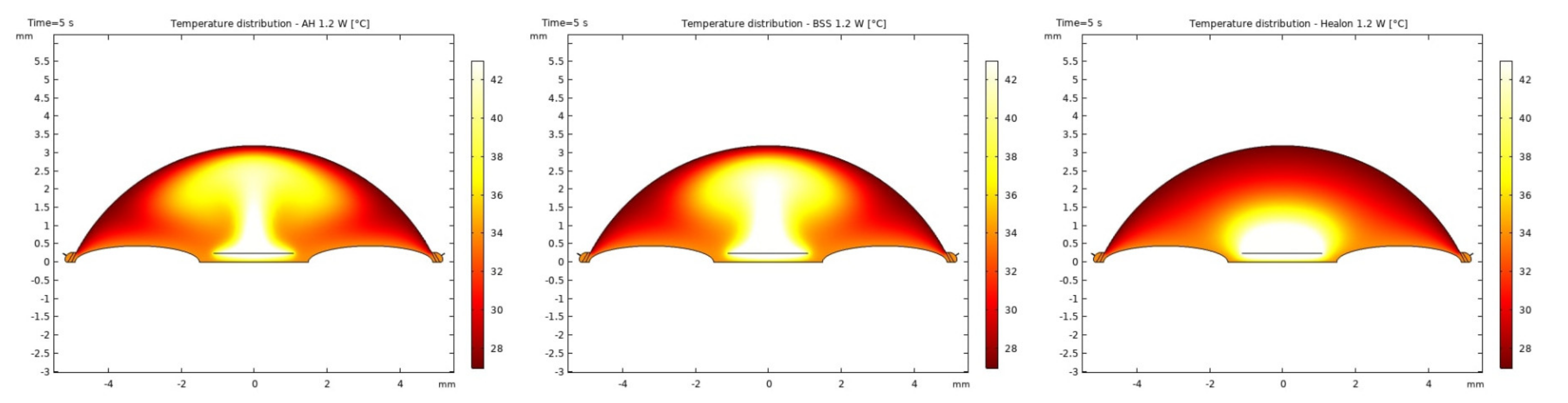
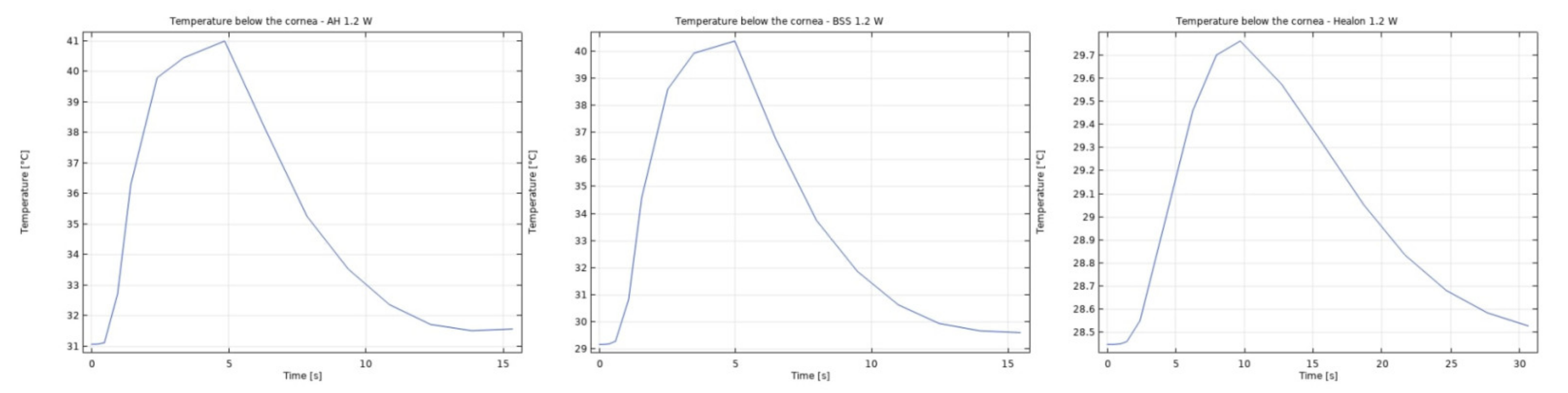
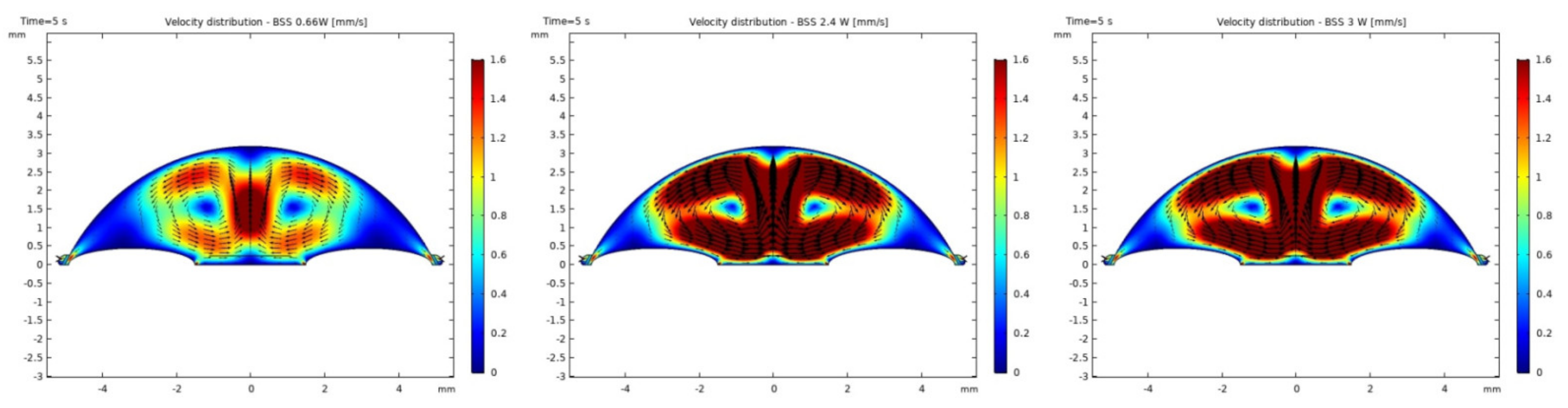
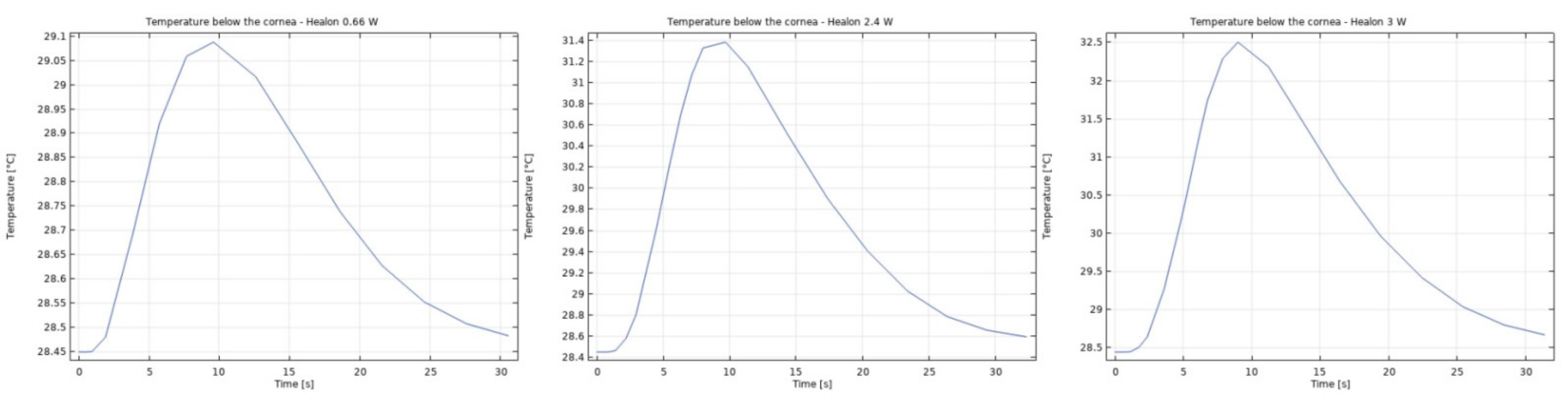
3.1. Modeling of Temperature and Velocity Distribution in the Anterior Chamber of the Eye
3.2. Results in Medical Part
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AC—anterior chamber | EPT—effective phaco time |
| AVE—average cell area | IOL—intraocular lens |
| AVG—average cell size | IOP—intraocular pressure |
| BCVA—best corrected visual acuity | LOCS—lens opacities classification system |
| BSCVA—best sphere corrected visual acuity | MAX—cell maximum area |
| BSS—balanced salt solution | MIN—cell minimum area |
| CD—cell density | SC—Schlemm’s canal |
| CV—coefficient of variation | SD—standard deviation |
| ECD—endothelial cell density | TM—Trabecular meshwork |
| ECL—endothelial cell loss | VA—visual acuity |
| ECM—endothelial cell morphology | 6A—percent of hexagonal cell |
| ECS—endothelial cell size |
References
- Murphy, C.; Alvarado, J.; Juster, R.; Maglio, M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Investig. Ophthalmol. Vis. Sci. 1984, 25, 312–322. [Google Scholar]
- Nucci, P.; Brancato, R.; Mets, M.B.; Shevell, S.K. Normal endothelial cell density range in childhood. Arch. Ophthalmol. 1990, 108, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.K.; Park, W.K.; Lee, J.H.; Chi, J.G. A histomorphometric study of corneal endothelial cells in normal human fetuses. Exp. Eye Res. 2001, 72, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Central corneal endothelial cell changes over a ten-year period. Investig. Ophthalmol. Vis. Sci. 1997, 38, 779–782. [Google Scholar]
- Hollingsworth, J.; Perez-Gomez, I.; Mutalib, H.A.; Efron, N. A population study of the normal cornea using an in vivo slit-scanning confocal microscope. Optom. Vis. Sci. 2001, 78, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, G.D.; Sherrard, E.S.; Rice, N.S.C. Specular microscopy of the corneal endothelium. Br. J. Ophtahlmol. 1978, 62, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Roper-Hall, M.J. Effect of age on the endothelial cell count in the normal eye. Br. J. Ophthalmol. 1982, 66, 513–515. [Google Scholar] [CrossRef]
- Waring, G.O., III; Bourn, W.M.; Edelhauser, H.F.; Kenyon, K.R. The corneal endothelium: Normal and pathologic structure and function. Ophthalmology 1982, 89, 531. [Google Scholar] [CrossRef]
- Tuft, S.J.; Coster, D.J. The corneal endothelium. Eye 1990, 4, 389–424. [Google Scholar] [CrossRef]
- Lass, J.H.; Sugar, A.; Benetz, B.A.; Beck, R.W.; Dontchev, M.; Gal, R.L.; Kollman, C.; Gross, R.; Heck, E.; Holland, E.J.; et al. Cornea Donor Study Investigator Group. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch. Ophthalmol. 2010, 128, 63–69. [Google Scholar] [CrossRef]
- Claerhout, I.; Beele, H.; Kestelyn, P. Graft failure I: Endothelial cell loss. Int. Ophthalmol. 2008, 28, 165–173. [Google Scholar] [CrossRef]
- Waltman, S.R.; Cozean, C.H. The effect of phacoemulsification on the corneal endothelium. Ophthalmic. Surg. 1979, 10, 31–33. [Google Scholar] [PubMed]
- Sugar, J.; Mitchelson, J.; Kraff, M. The effect of phacoemulsification on corneal endothelial cell density. Arch. Ophthalmol. 1978, 96, 446–448. [Google Scholar] [CrossRef]
- Bourne, W.M.; Kaufman, H.E. Endothelial damage associated with intraocular lenses. Am. J. Ophthalmol. 1976, 81, 482–485. [Google Scholar] [CrossRef]
- Kaufman, E.; Katz, J.I. Endothelial damage from intraocular lens insertion. Investig. Ophthalmol. Vis. Sci. 1976, 15, 996–1000. [Google Scholar]
- Faulkner, G.D. Endothelial cell loss after phacoemulsification and insertion of silicone lens implants. J. Cataract. Refract. Surg. 1987, 13, 649–652. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakao, F.; Hayashi, F. Corneal endothelial cell loss after phacoemulsification using nuclear cracking procedures. J. Cataract. Refract. Surg. 1994, 20, 44. [Google Scholar] [CrossRef]
- Rosado-Adames, N.; Afshari, N.A. The changing fate of the corneal endothelium in cataract surgery. Curr. Opin. Ophthalmol. 2012, 23, 3–6. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar]
- Jooybar, E.; Abdekhodaie, M.J.; Farhadi, F.; Cheng, Y. Computational modeling of drug distribution in the posterior segment of the eye: Effects of device variables and positions. Math. Biosci. 2014, 255, 11–20. [Google Scholar] [CrossRef]
- Mauro, A.; Massarotti, N.; Mohamed, S.; Uña, I.R.; Romano, M.R.; Romano, V. A novel patient-oriented numerical procedure for glaucoma drainage devices. Int. J. Numer. Meth. Biomed. Enging. 2018, 34, e3141. [Google Scholar] [CrossRef] [PubMed]
- Villamarin, A.; Roy, S.; Hasballa, R.; Vardoulis, O.; Reymond, P.; Stergiopulos, N. 3D simulation of the aqueous flow in the human eye. Med. Eng. Phys. 2012, 34, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Massarotti, N.; Salahudeen, M.; Romano, M.R.; Romano, V.; Nithiarasu, P. A generalised porous medium approach to study thermo-fluid dynamics in human eyes. Med. Biol. Eng. Comput. 2018, 56, 1823–1839. [Google Scholar] [CrossRef]
- Mohamed, S.; Coccarelli, A.; Mauro, A.; Massarotti, N.; Romano, M.R.; Romano, V.; Nithiarasu, P. A novel numerical modelling approach for keratoplasty eye procedure. Biomech. Model. Mechanobiol. 2019, 18, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Mohamed, S. Three dimensional heat and mass transfer in human eye based on porous medium approach. Int. J. Heat Mass Transf. 2020, 158, 119994. [Google Scholar] [CrossRef]
- Corvi, A.; Innocenti, B.; Mencucci, R. Thermography used for analysis and comparison of different cataract surgery procedures based on phacoemulsification. Physiol. Meas. 2006, 27, 371. [Google Scholar] [CrossRef] [PubMed]
- Buschschlüter, S.; Koch, C.; Von Eicken, J.; Höh, H. Computation of the temperature rise at the corneal endothelium during surgery by modeling of heat generation inside anterior chamber. Ultrasound Med. Biol. 2014, 40, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, T.D.; McGlothan, J.S.; Rotkis, W.M. Indications for penetrating keratoplasty. Cornea 1991, 10, 210. [Google Scholar] [CrossRef]
- Robin, J.B.; Gindi, J.J.; Koh, K. An update of the indications for penetrating keratoplasty. Arch. Ophthalmol. 1986, 104, 87. [Google Scholar] [CrossRef]
- Linebarger, E.J.; Hardten, D.R.; Shah, G.K.; Lindstrom, R.L. Phacoemulsification and modern cataract surgery. Surv. Ophthalmol. 1999, 44, 123–147. [Google Scholar] [CrossRef]
- Koch, D.D.; Liu, J.F.; Glasser, D.B.; Merin, L.M.; Haft, E. A comparison of corneal endothelial changes after use of Healon or Viscoat during phacoemulsification. Am. J. Ophthalmol. 1993, 115, 188–201. [Google Scholar] [CrossRef]
- Hejny, C.; Edelhauser, H.F. Surgical pharmacology: Intraocular solutions and drugs used for cataract surgery. In Phacoemulsification; Principles and Techniques, 2nd ed.; Buratto, L., Werner, L., Zanini, M., Apple, D., Eds.; Slack: Thorofare, NJ, USA, 2003; pp. 219–246. [Google Scholar]
- MacRae, S.M.; Edelhauser, H.F.; Hyndiuk, R.A.; Burd, E.M.; Schultz, R.O. The effects of sodium hyaluronate, chondroitin sulfate, and methylcellulose on the corneal endothelium and intraocular pressure. Am. J. Ophthalmol. 1983, 95, 332–341. [Google Scholar]
- Rainer, G.; Schmid, K.E.; Findl, O.; Sacu, S.; Kiss, B.; Heinzl, H.; Menapace, R. Natural course of intraocular pressure after cataract surgery with sodium hyaluronate 1% versus hydroxypropylmethylcellulose 2%. Ophthalmology 2007, 114, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Kinoshita, S.; Ohashi, Y.; Shimomura, Y.; Ohguro, N.; Okamoto, H.; Omoto, T.; Hosotani, H.; Yoshida, H. Comparison of the effects of intraocular irrigating solutions on the corneal endothelium in intraocular lens implantation. Br. J. Ophthalmol. 1991, 75, 476–479. [Google Scholar] [CrossRef] [PubMed]








| AVG±SD | MAX | MIN | CV | 6A | |
|---|---|---|---|---|---|
| BSS-group | 457.7 ± 252 µm2 | 942.6 µm2 | 170.1 µm2 | 34.7 ± 5.9 | 60.4% |
| Healon-group | 452.3 ± 126 µm2 | 933.9 µm2 | 158.2 µm2 | 34.9 ± 5.1 | 58.8% |
| AVG ± SD | MAX | MIN | CV | 6A | |
|---|---|---|---|---|---|
| BSS-group | 481.8 ± 211 µm2 | 1014.0 µm2 | 170.0 µm2 | 36.7 ± 6.0 | 57.9% |
| Healon-group | 467.0 ± 116 µm2 | 962.3 µm2 | 150.0 µm2 | 35.5 ± 5.2 | 57.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goles, N.; Nerancic, M.; Konjik, S.; Pajic-Eggspuehler, B.; Pajic, B.; Cvejic, Z. Phacoemulsification and IOL-Implantation without Using Viscoelastics: Combined Modeling of Thermo Fluid Dynamics, Clinical Outcomes, and Endothelial Cell Density. Sensors 2021, 21, 2399. https://doi.org/10.3390/s21072399
Goles N, Nerancic M, Konjik S, Pajic-Eggspuehler B, Pajic B, Cvejic Z. Phacoemulsification and IOL-Implantation without Using Viscoelastics: Combined Modeling of Thermo Fluid Dynamics, Clinical Outcomes, and Endothelial Cell Density. Sensors. 2021; 21(7):2399. https://doi.org/10.3390/s21072399
Chicago/Turabian StyleGoles, Nikola, Marko Nerancic, Sanja Konjik, Brigitte Pajic-Eggspuehler, Bojan Pajic, and Zeljka Cvejic. 2021. "Phacoemulsification and IOL-Implantation without Using Viscoelastics: Combined Modeling of Thermo Fluid Dynamics, Clinical Outcomes, and Endothelial Cell Density" Sensors 21, no. 7: 2399. https://doi.org/10.3390/s21072399
APA StyleGoles, N., Nerancic, M., Konjik, S., Pajic-Eggspuehler, B., Pajic, B., & Cvejic, Z. (2021). Phacoemulsification and IOL-Implantation without Using Viscoelastics: Combined Modeling of Thermo Fluid Dynamics, Clinical Outcomes, and Endothelial Cell Density. Sensors, 21(7), 2399. https://doi.org/10.3390/s21072399










