Endoluminal Motion Recognition of a Magnetically-Guided Capsule Endoscope Based on Capsule-Tissue Interaction Force
Abstract
1. Introduction
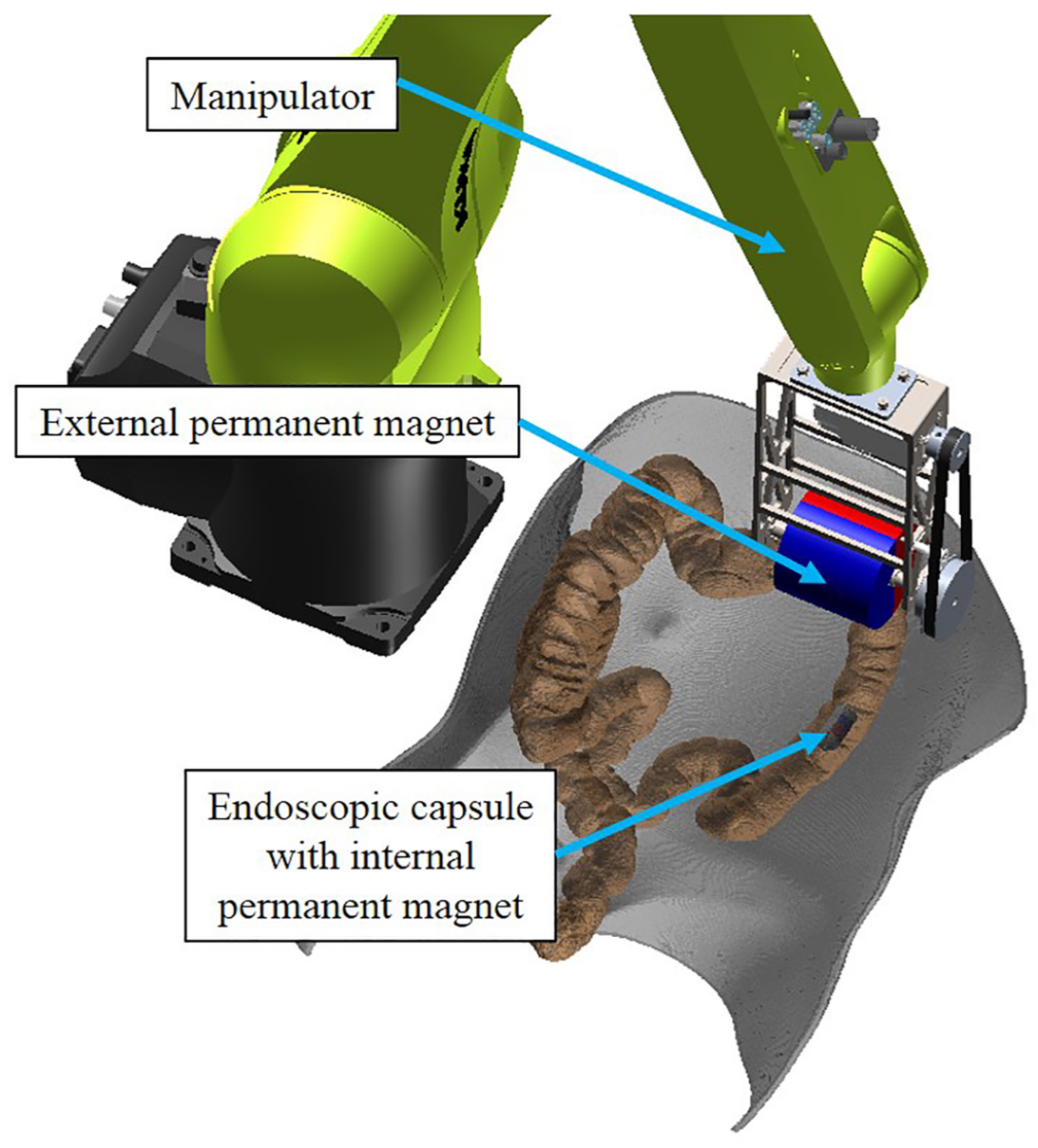
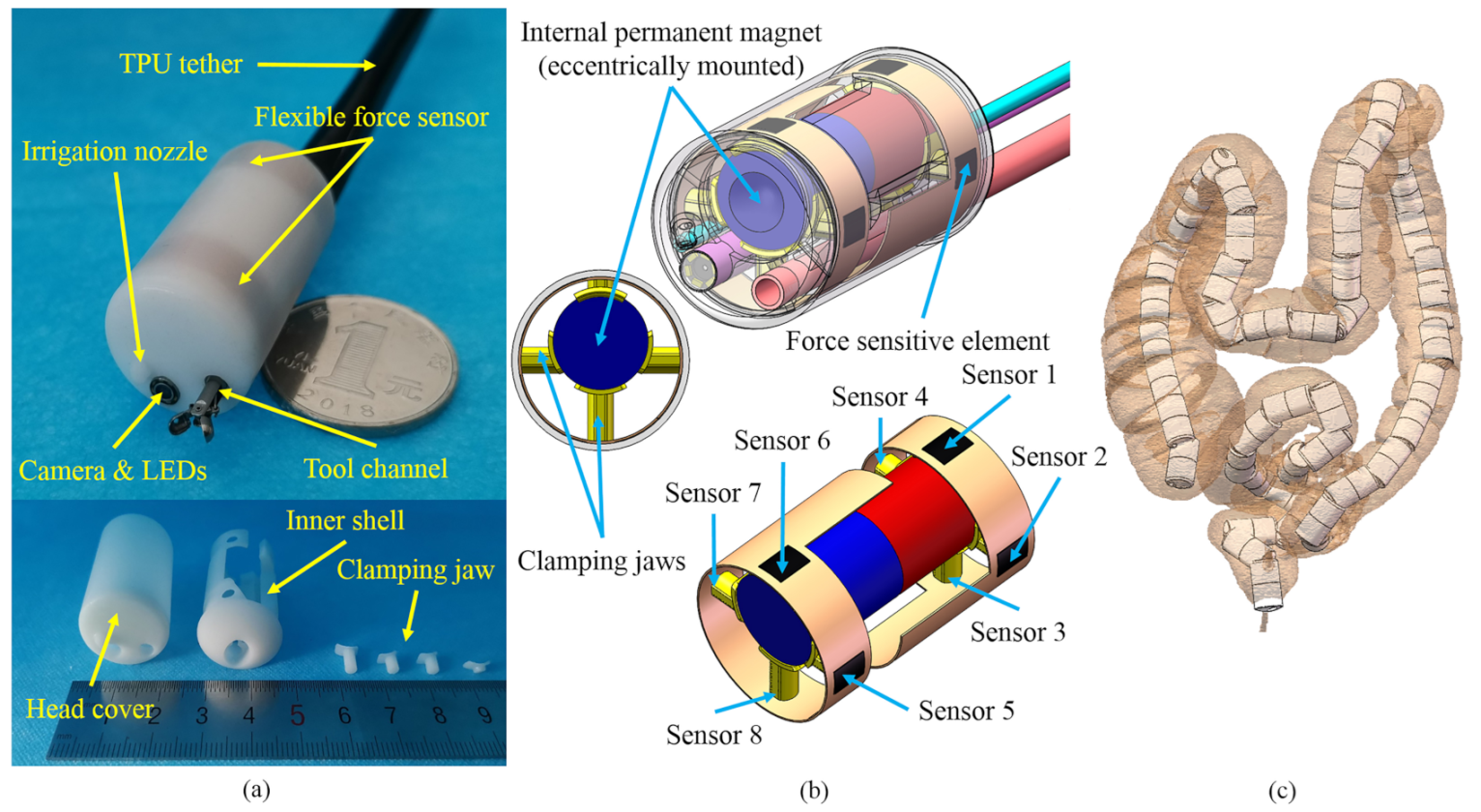
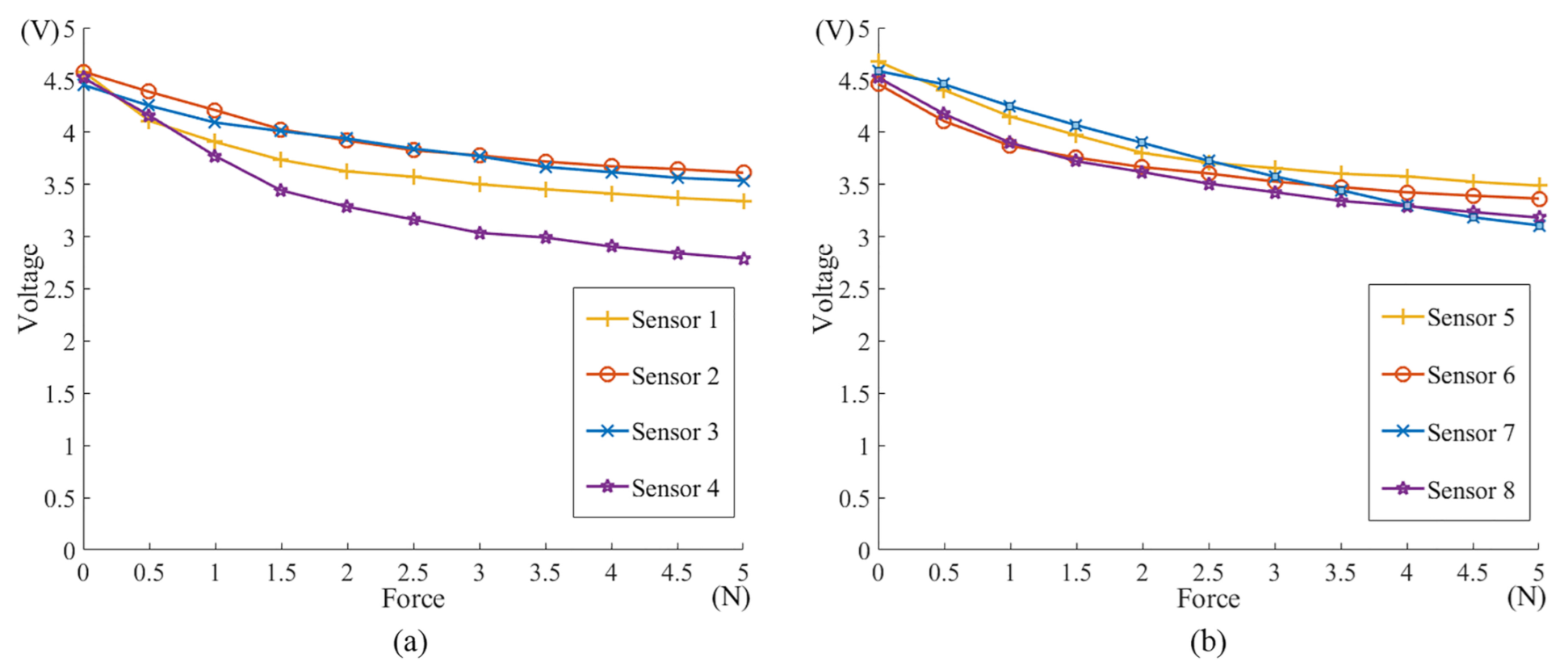
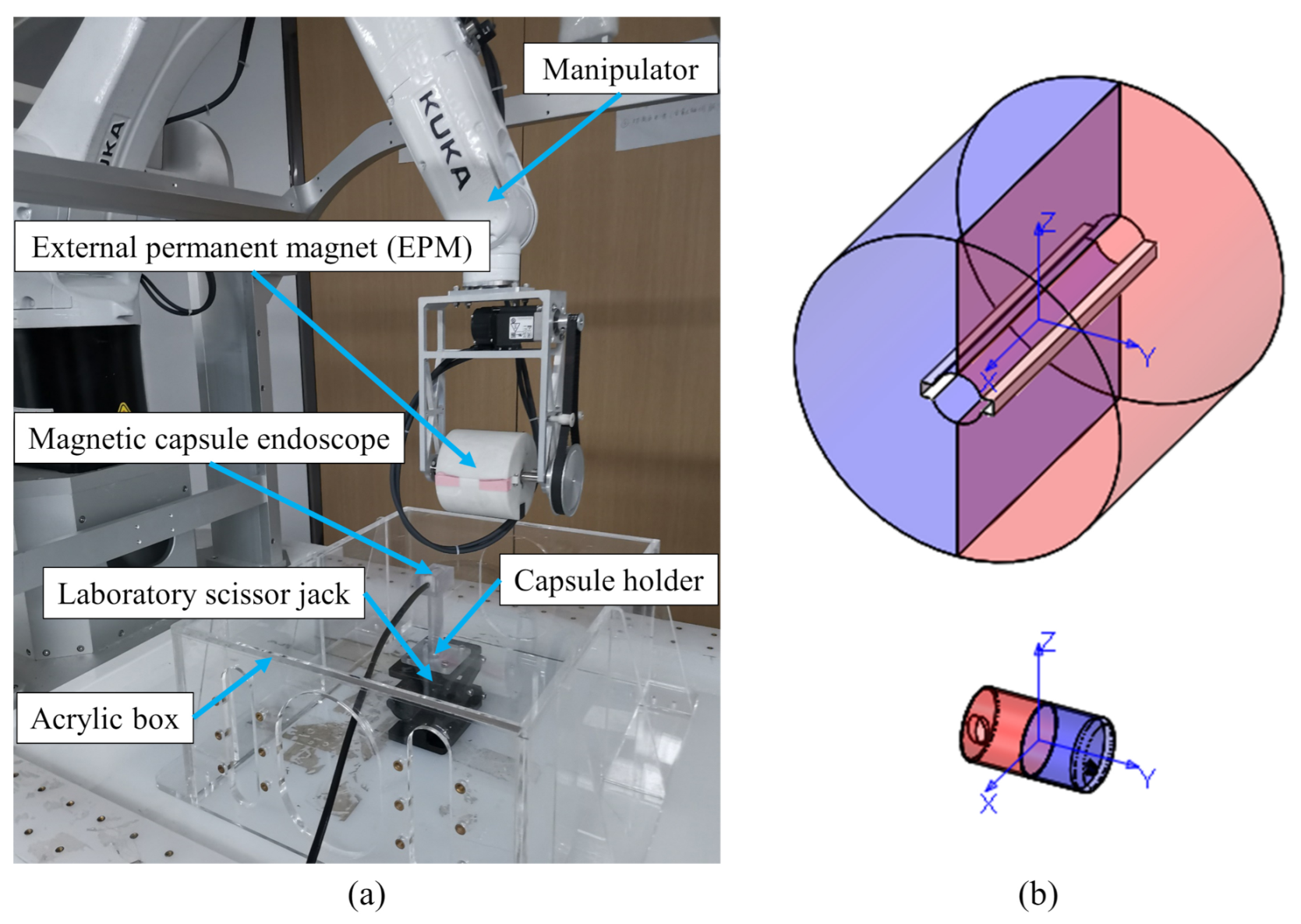
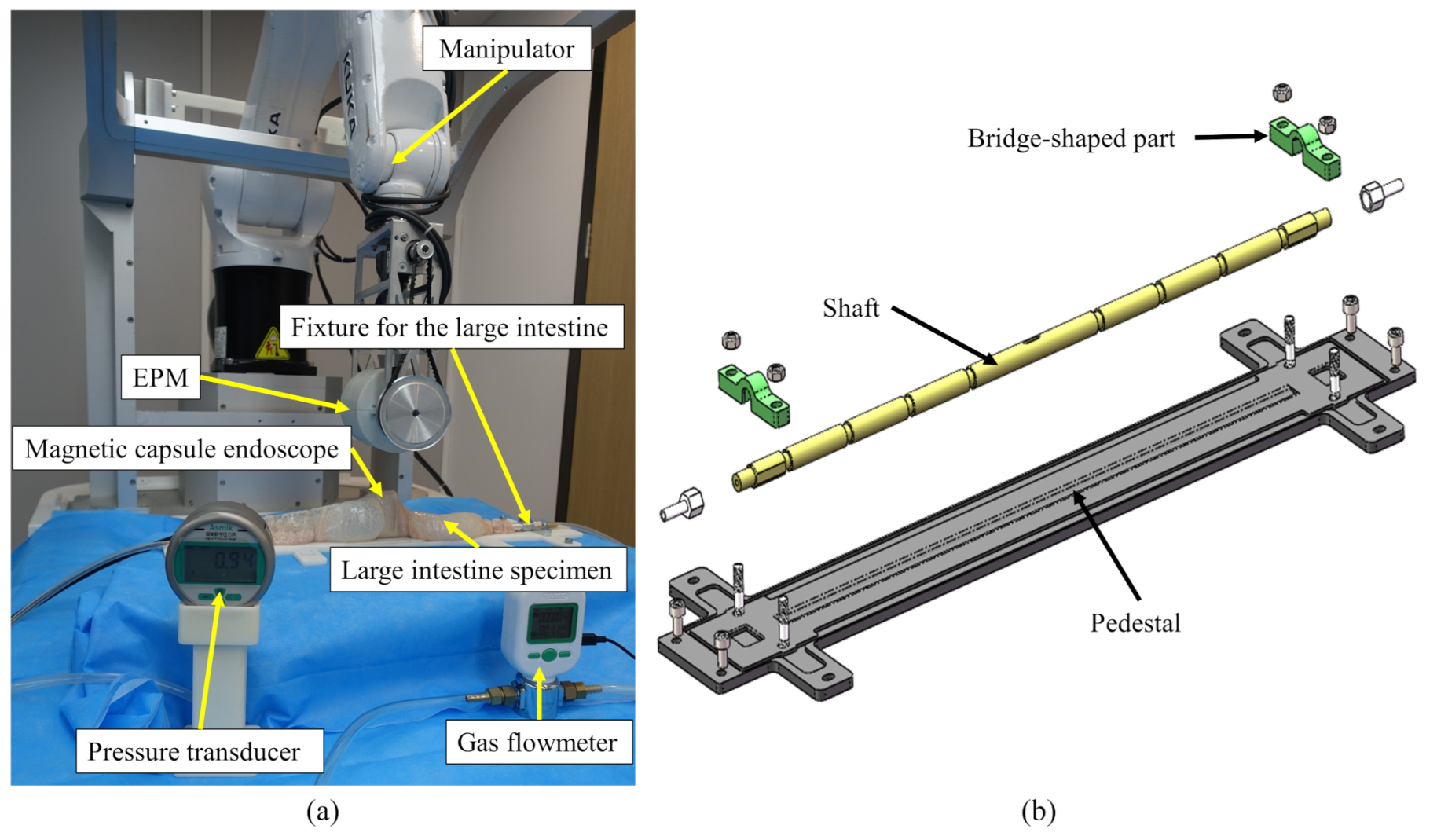
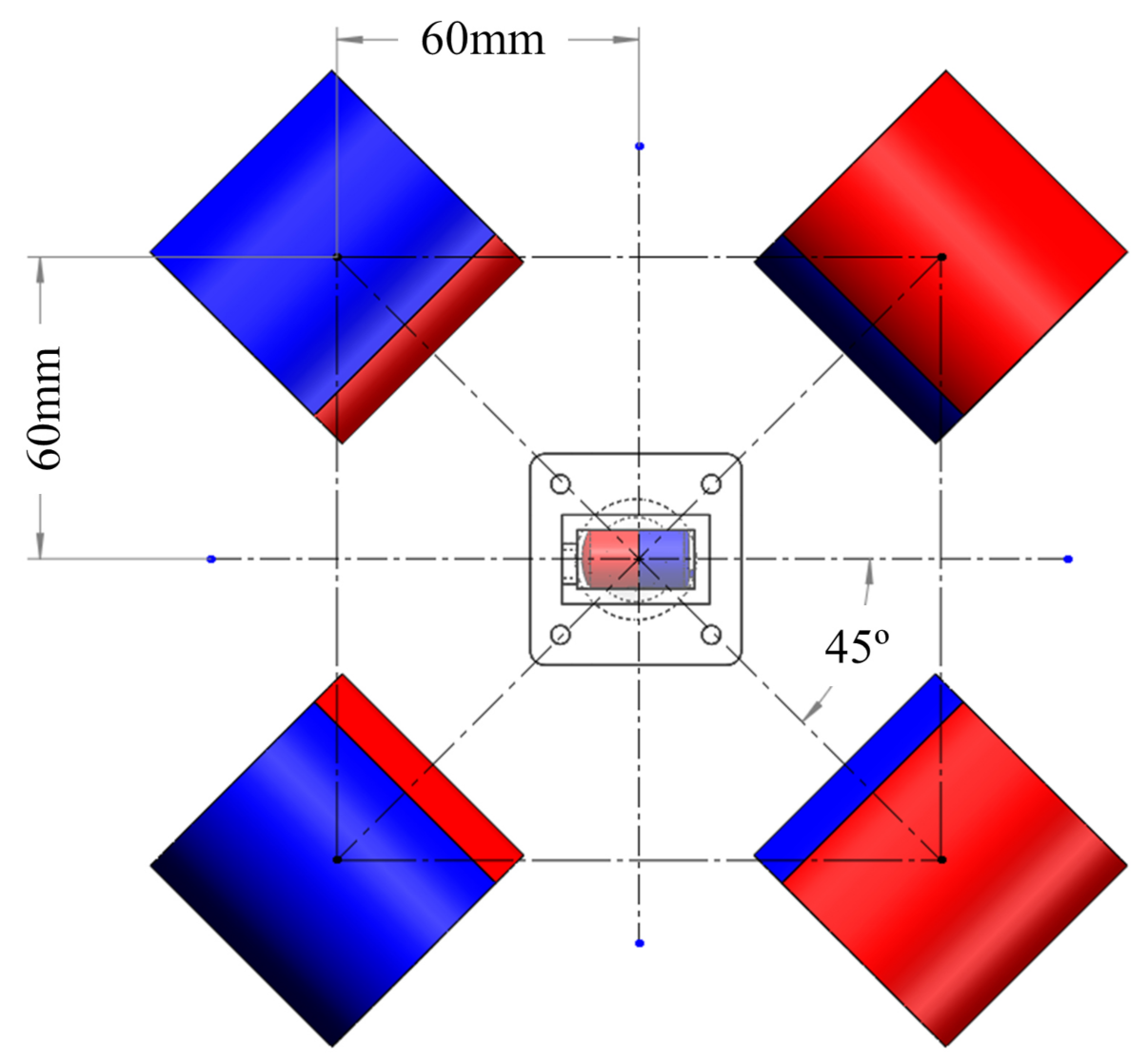
2. Mechanical Design
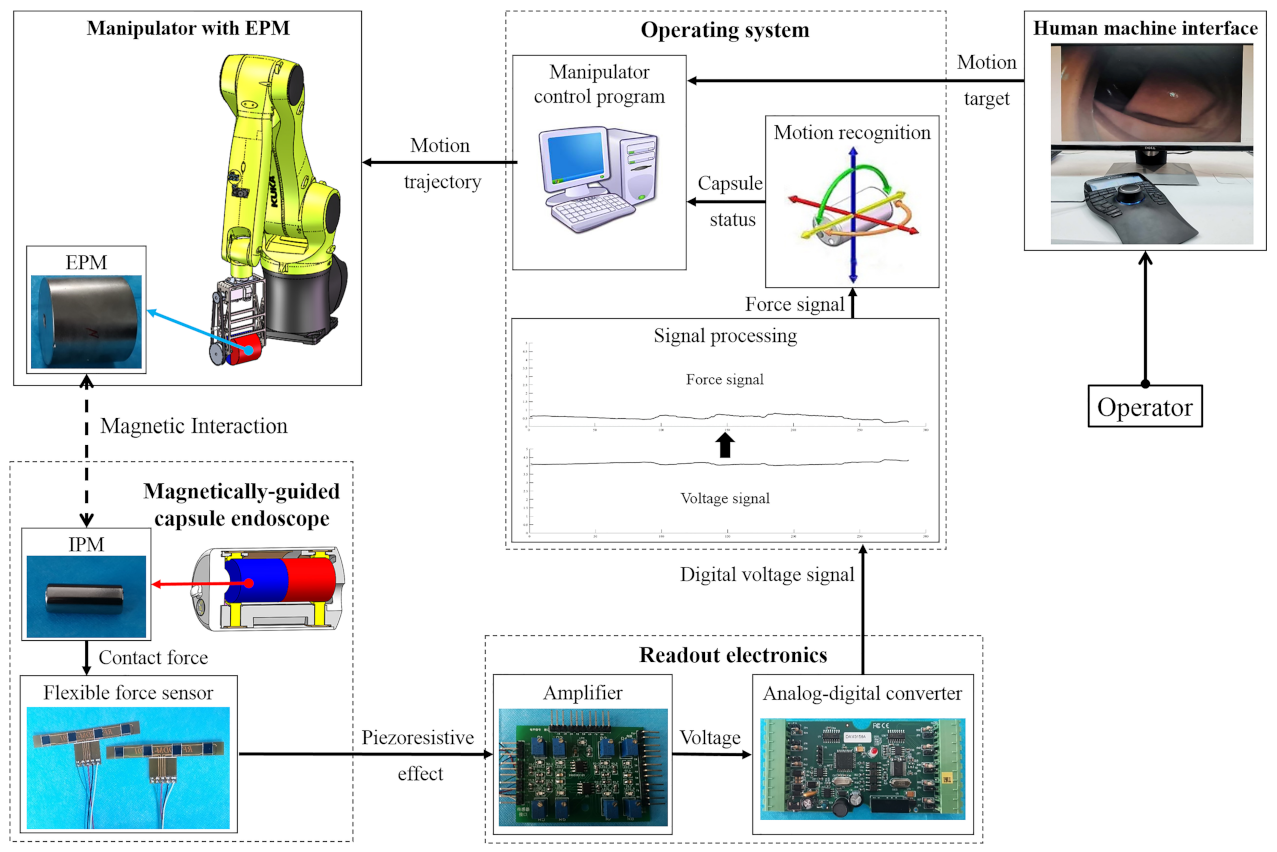
3. Motion Recognition of the Magnetically-Guided Capsule
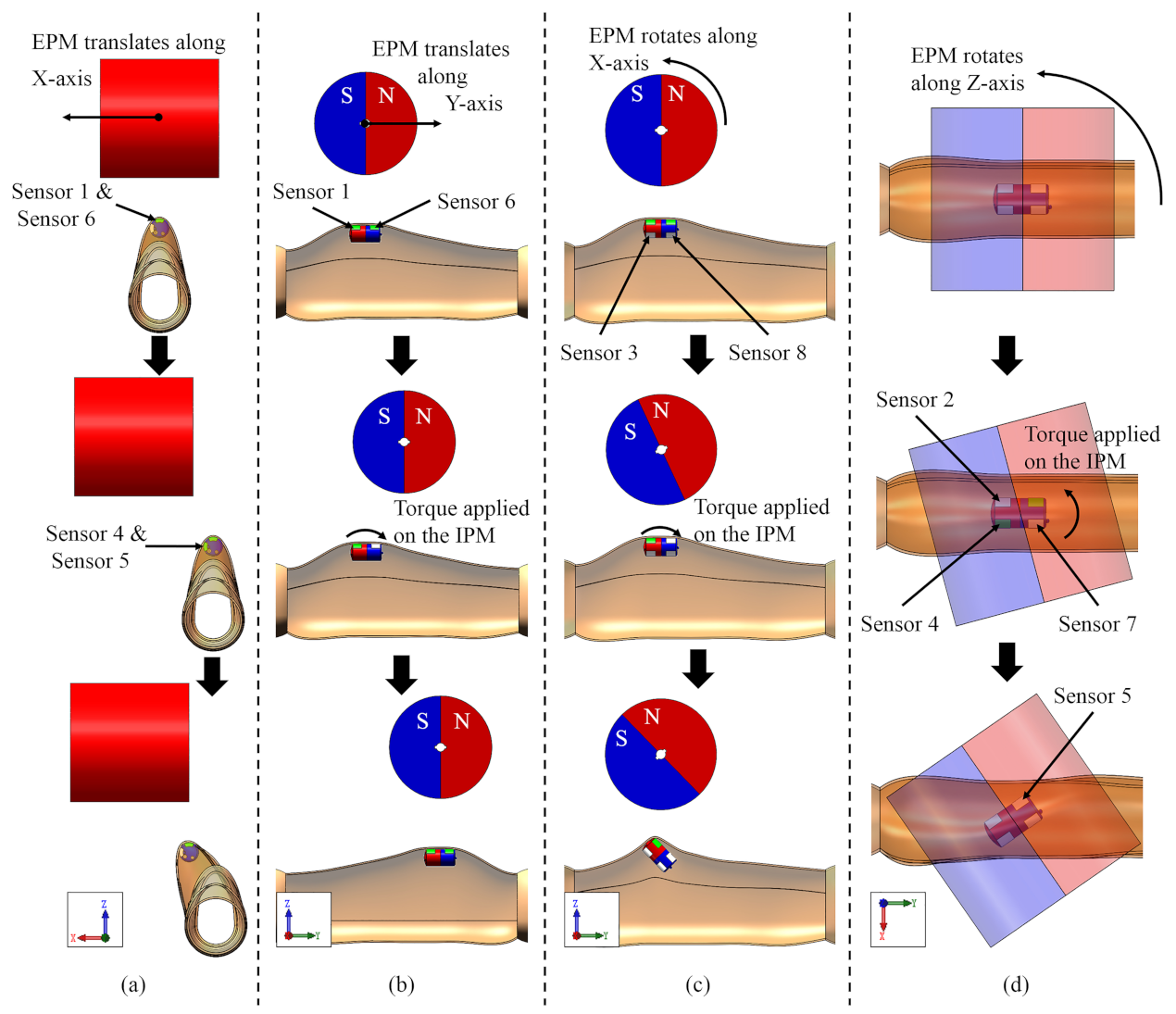
3.1. EPM Translating along Its Z-Axis
3.2. EPM Translating along Its X-Axis
3.3. EPM Translating along Its Y-Axis
3.4. EPM Rotating along Its X-Axis
3.5. EPM Rotating along Its Z-Axis
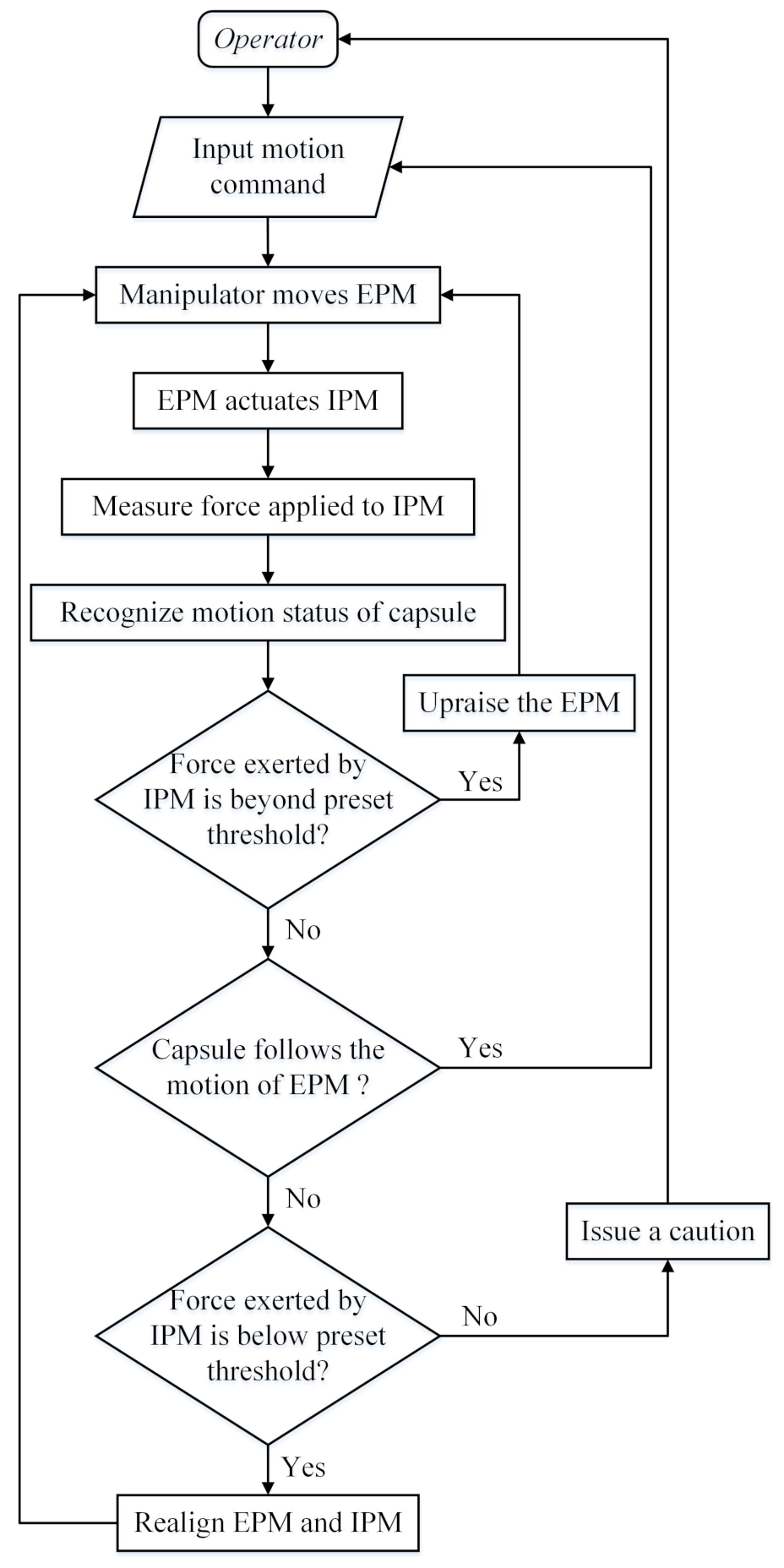
3.6. Realigning the EPM and the IPM
- Step 1.
- rotate the EPM along its X-axis until the Z-axis of the EPM is vertical to the ground.
- Step 2.
- rotate the EPM along its Z-axis direction which decreases the value of until:
- Step 3.
- translate the EPM along its Y-axis direction which decreases the value of until:
- Step 4.
- translate the EPM along its X-axis direction which decreases the value of until:
- Step 5.
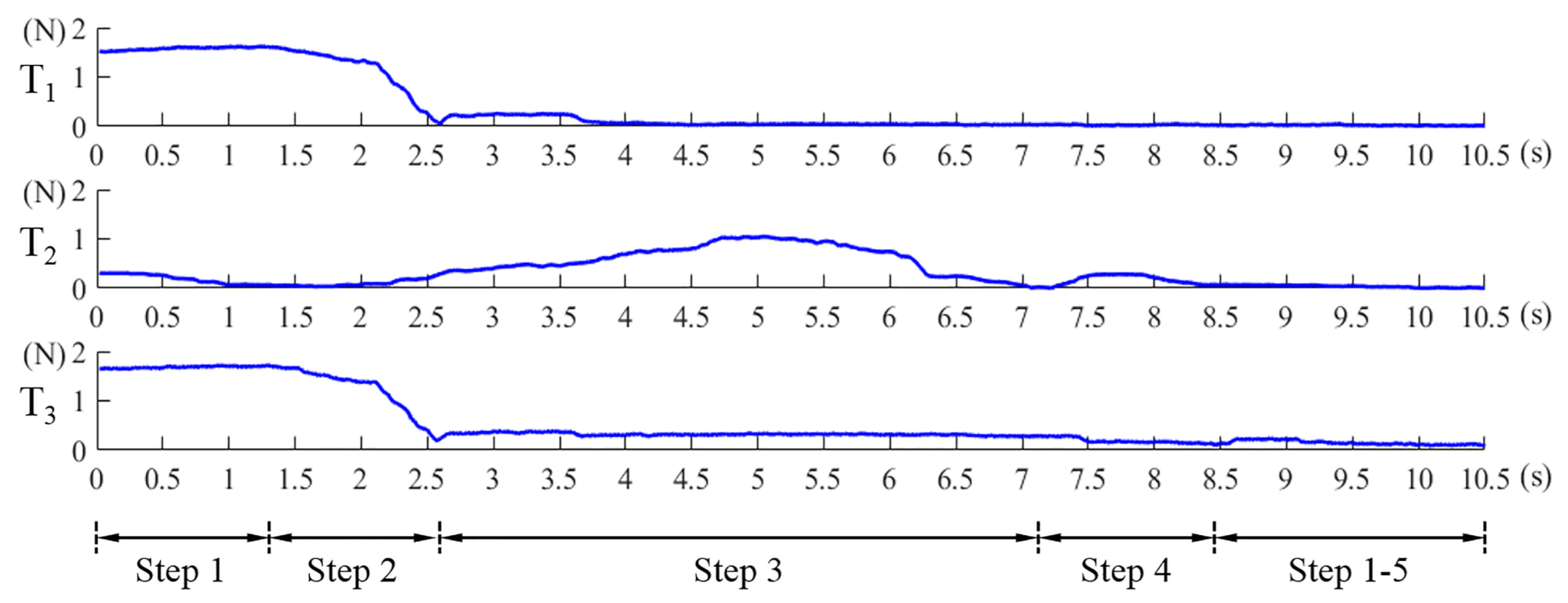
- repeat the Step 1 to Step 4 until the Equations (8) and (9) are simultaneously satisfied. , , are the preset thresholds. Values of Equation (7), Equations (8) and (9) in this alignment process are shown in Figure 10. The abscissa axis of each curve indicates the value of time and the ordinate axis indicates the value of force. If the magnetic link between the EPM and the IPM has already been broken or is not established, such as at the beginning of the magnetic capsule colonoscopy, the EPM can be moved close to the possible position of the magnetic capsule to establish magnetic link. Once the force value of the Sensor 1 plus Sensor 6 is within the preset threshold, we can implement the alignment method to align the EPM and the IPM. The alignment method based on flexible force sensors is suitable for most situation of the magnetic capsule colonoscopy and less likely to be affected by the interference compared with other localization strategies.
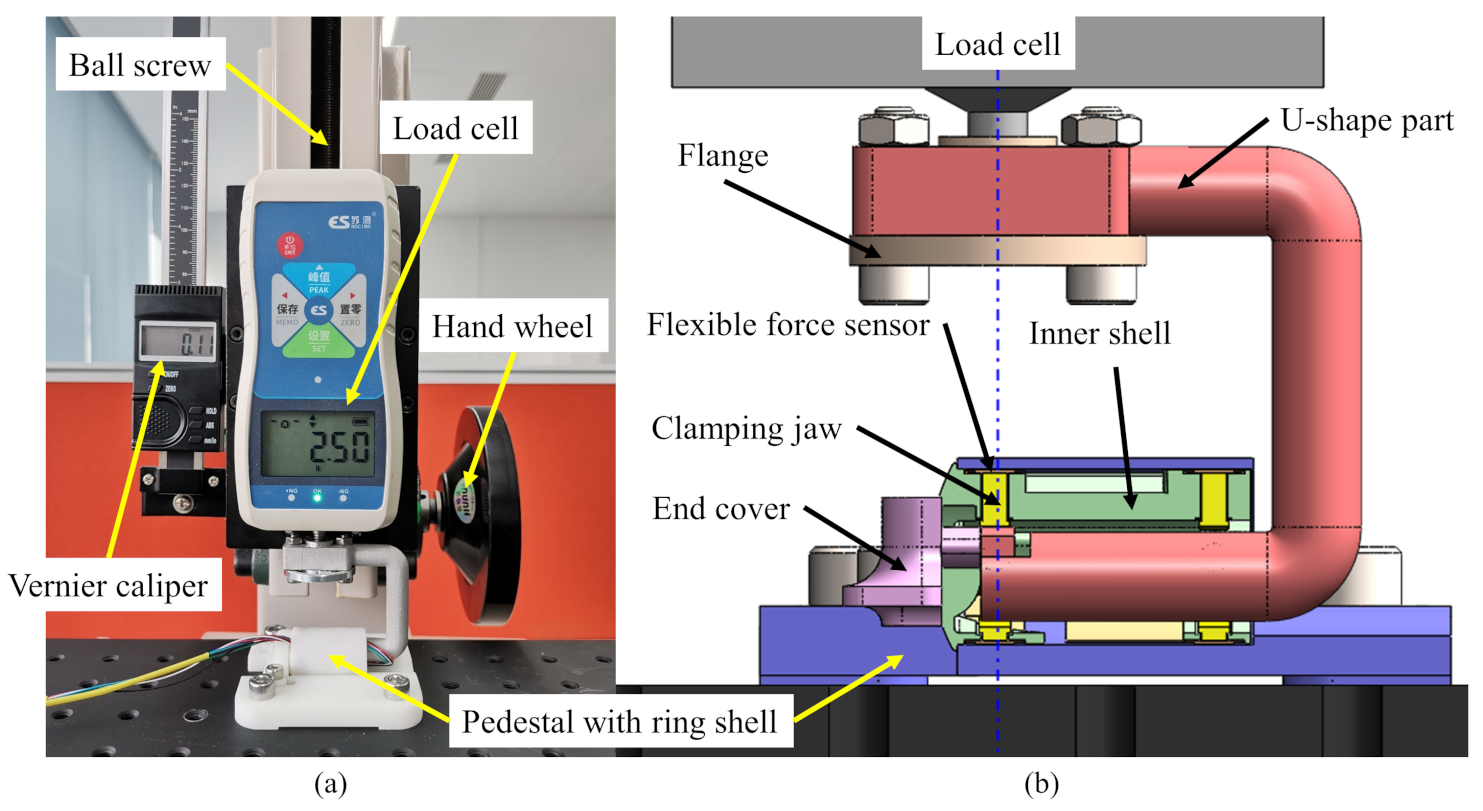
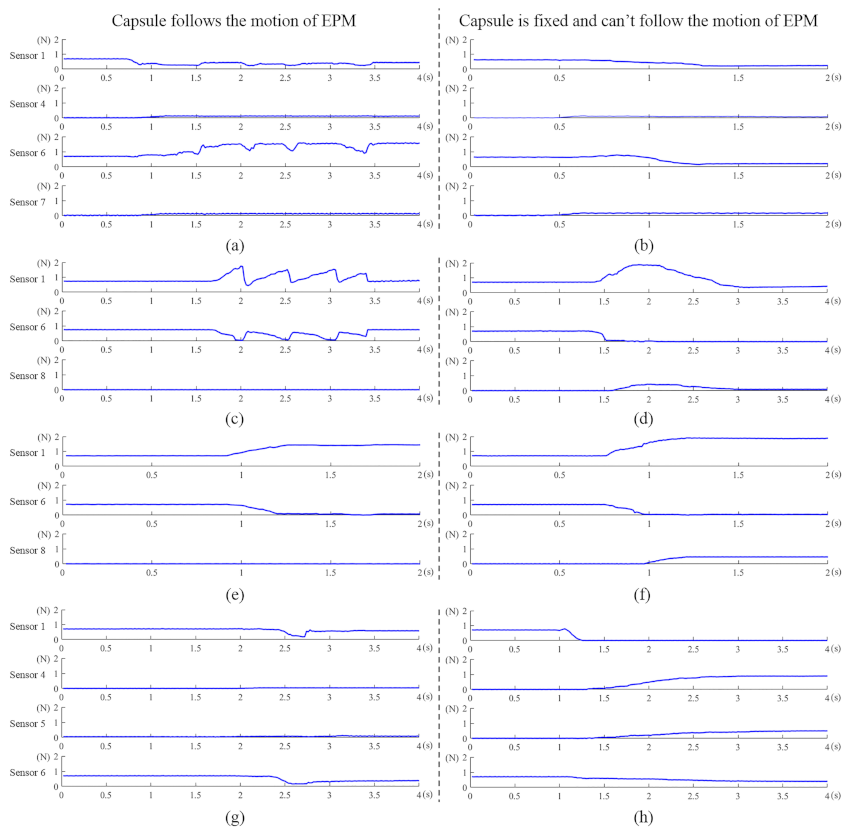
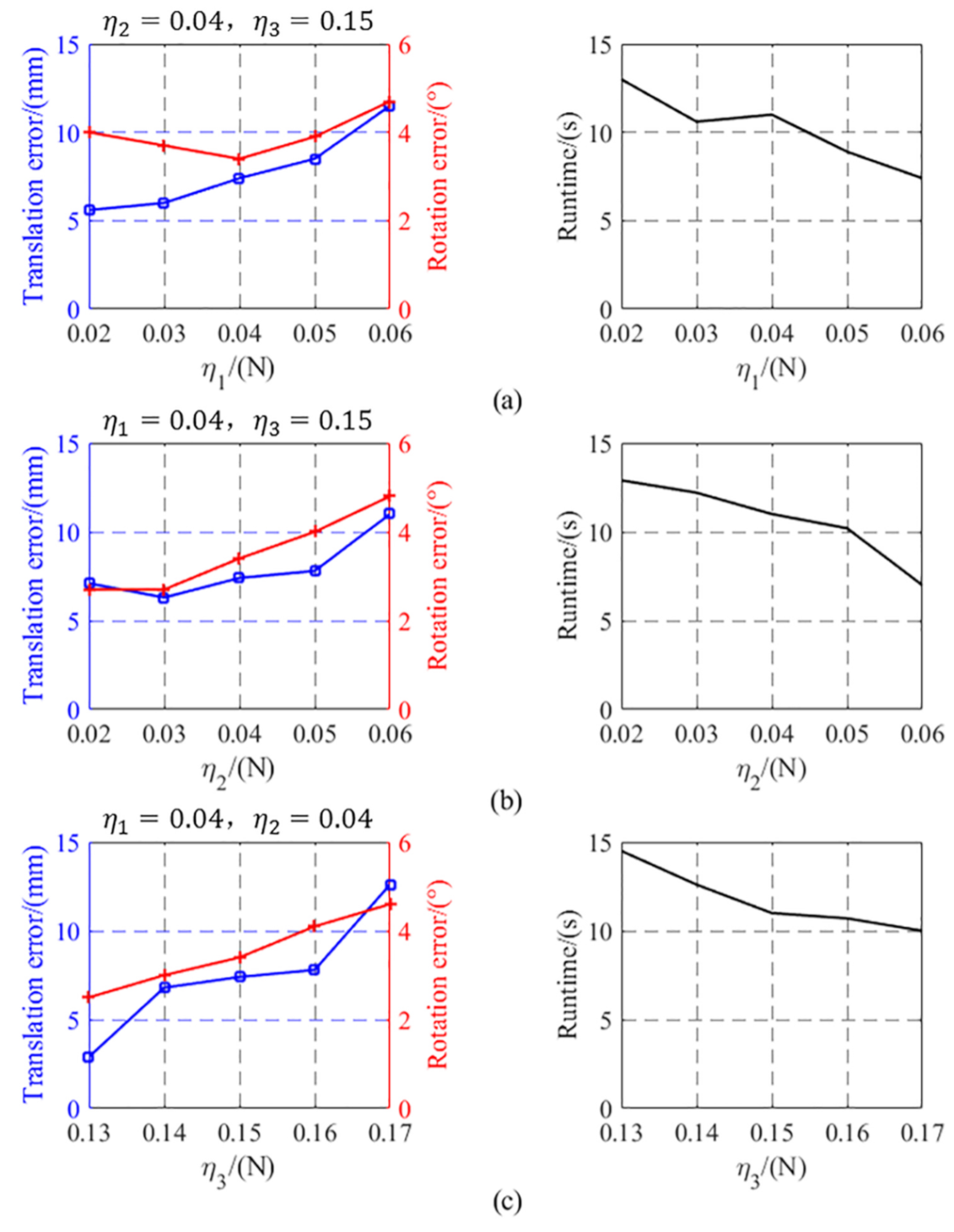
4. Experimental Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPM | Internal permanent magnet |
| EPM | External permanent magnet |
| CRC | Colorectal cancer |
| IMU | Inertial measurement unit |
| EUR | European |
| LEDs | Light-emitting diodes |
| TPU | Thermoplastic polyurethane |
| MDCT | Multiple-detector computed tomography |
| DOF | Degree of freedom |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Robertson, D.J.; Senore, C.; Rex, D.K. Optimizing the Quality of Colorectal Cancer Screening Worldwide. Gastroenterology 2020, 158, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ratushnyak, S.; Hoogendoorn, M.; van Baal, P.H.M. Cost-Effectiveness of Cancer Screening: Health and Costs in Life Years Gained. Am. J. Prev. Med. 2019, 57, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Martin, J.W.; Scaglioni, B.; Norton, J.C.; Subramanian, V.; Arezzo, A.; Obstein, K.L.; Valdastri, P. Enabling the future of colonoscopy with intelligent and autonomous magnetic manipulation. Nat. Mach. Intell. 2020, 2, 595–606. [Google Scholar] [CrossRef]
- Ciuti, G.; Skonieczna-Zydecka, K.; Marlicz, W.; Iacovacci, V.; Liu, H.; Stoyanov, D.; Arezzo, A.; Chiurazzi, M.; Toth, E.; Thorlacius, H.; et al. Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies. J. Clin. Med. 2020, 9, 1648. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Song, S.; Yang, W.A.; Zhang, R.; Meng, M.Q.H. A Cubic 3-Axis Magnetic Sensor Array for Wirelessly Tracking Magnet Position and Orientation. IEEE Sens. J. 2010, 10, 903–913. [Google Scholar] [CrossRef]
- Hu, C.; Ren, Y.P.; You, X.H.; Yang, W.N.; Song, S.; Xiang, S.; He, X.Q.; Zhang, Z.H.; Meng, M.Q.H. Locating Intra-Body Capsule Object by Three-Magnet Sensing System. IEEE Sensors J. 2016, 16, 5167–5176. [Google Scholar] [CrossRef]
- Carpi, F.; Kastelein, N.; Talcott, M.; Pappone, C. Magnetically controllable gastrointestinal steering of video capsules. IEEE Trans. Biomed. Eng. 2011, 58, 231–234. [Google Scholar] [CrossRef]
- Li, J.; Barjuei, E.S.; Ciuti, G.; Hao, Y.; Dario, P. Analytical magnetic model applied to endoscopic robots design: A ready-to-use implementation and a case of study. In Proceedings of the 2016 IEEE International Conference on Information and Automation (ICIA), Ningbo, China, 1–3 August 2016; pp. 1618–1623. [Google Scholar]
- Singh, A.V.; Dad Ansari, M.H.; Dayan, C.B.; Giltinan, J.; Wang, S.; Yu, Y.; Kishore, V.; Laux, P.; Luch, A.; Sitti, M. Multifunctional magnetic hairbot for untethered osteogenesis, ultrasound contrast imaging and drug delivery. Biomaterials 2019, 219, 119394. [Google Scholar] [CrossRef]
- Yim, S.; Sitti, M. Design and Rolling Locomotion of a Magnetically Actuated Soft Capsule Endoscope. IEEE Trans. Robot. 2012, 28, 183–194. [Google Scholar] [CrossRef]
- Ciuti, G.; Salerno, M.; Lucarini, G.; Valdastri, P.; Arezzo, A.; Menciassi, A.; Morino, M.; Dario, P. A comparative evaluation of control interfaces for a robotic-aided endoscopic capsule platform. IEEE Trans. Robot. 2011, 28, 534–538. [Google Scholar] [CrossRef]
- Khare, M.; Singh, A.; Zamboni, P. Prospect of brain-machine interface in motor disabilities: The future support for multiple sclerosis patient to improve quality of life. Ann. Med. Health Sci. Res. 2014, 4, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Fleming, A.J. A novel and compatible sensing coil for a capsule in Wireless Capsule Endoscopy for real time localization. In Proceedings of the SENSORS, Valencia, Spain, 2–5 November 2014; pp. 1607–1610. [Google Scholar]
- Verra, M.; Firrincieli, A.; Chiurazzi, M.; Mariani, A.; Lo Secco, G.; Forcignano, E.; Koulaouzidis, A.; Menciassi, A.; Dario, P.; Ciuti, G.; et al. Robotic-Assisted Colonoscopy Platform with a Magnetically-Actuated Soft-Tethered Capsule. Cancers 2020, 12, 2485. [Google Scholar] [CrossRef]
- Ciuti, G.; Valdastri, P.; Menciassi, A.; Dario, P. Robotic magnetic steering and locomotion of capsule endoscope for diagnostic and surgical endoluminal procedures. Robotica 2010, 28, 199–207. [Google Scholar] [CrossRef]
- Taddese, A.Z.; Slawinski, P.R.; Pirotta, M.; De Momi, E.; Obstein, K.L.; Valdastri, P. Enhanced Real-Time Pose Estimation for Closed-Loop Robotic Manipulation of Magnetically Actuated Capsule Endoscopes. Int. J. Robot. Res. 2018, 37, 890–911. [Google Scholar] [CrossRef]
- Norton, J.C.; Slawinski, P.R.; Lay, H.S.; Martin, J.W.; Cox, B.F.; Cummins, G.; Desmulliez, M.P.Y.; Clutton, R.E.; Obstein, K.L.; Cochran, S.; et al. Intelligent magnetic manipulation for gastrointestinal ultrasound. Sci. Robot. 2019, 4, eaav7725. [Google Scholar] [CrossRef]
- Lucarini, G.; Mura, M.; Ciuti, G.; Rizzo, R.; Menciassi, A. Electromagnetic Control System for Capsule Navigation: Novel Concept for Magnetic Capsule Maneuvering and Preliminary Study. J. Med. Biol. Eng. 2015, 35, 428–436. [Google Scholar] [CrossRef]
- Bianchi, F.; Ciuti, G.; Koulaouzidis, A.; Arezzo, A.; Stoyanov, D.; Schostek, S.; Oddo, C.M.; Menciassi, A.; Dario, P. An innovative robotic platform for magnetically-driven painless colonoscopy. Ann. Transl. Med. 2017, 5, 421. [Google Scholar] [CrossRef]
- Nouda, S.; Ota, K.; Higuchi, K. Retrograde colon capsule endoscopy with the self-propelling capsule endoscope: The first human trial. Dig. Endosc. 2018, 30, 117–118. [Google Scholar] [CrossRef]
- Pittiglio, G.; Barducci, L.; Martin, J.W.; Norton, J.C.; Avizzano, C.A.; Obstein, K.L.; Valdastri, P. Magnetic Levitation for Soft-Tethered Capsule Colonoscopy Actuated with a Single Permanent Magnet: A Dynamic Control Approach. IEEE Robot. Autom. Lett. 2019, 4, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, S.; Watson, J.H.P. Magnetic dipole model of a permanent magnet based device. J. Phys. D Appl. Phys. 2001, 34, 2622–2628. [Google Scholar] [CrossRef]
- Hsu, S.W. Analysis of errors in obtaining magnetic dipoles based on magnetic field distribution. J. Magn. Magn. Mater. 2006, 304, 228–230. [Google Scholar] [CrossRef]
- Furlani, E.P.; Reznik, S.; Kroll, A. A 3-Dimensional Field Solution for Radially Polarized Cylinders. IEEE Trans. Magn. 1995, 31, 844–851. [Google Scholar] [CrossRef]
- Guo, X.F.; Yang, Y.; Zheng, X.J. Analytic expression of magnetic field distribution of rectangular permanent magnets. Appl. Math. -Mech. Engl. Ed. 2004, 25, 297–306. [Google Scholar] [CrossRef]
- Li, J.; Barjuei, E.S.; Ciuti, G.; Hao, Y.; Zhang, P.; Menciassi, A.; Huang, Q.; Dario, P. Magnetically-driven medical robots: An analytical magnetic model for endoscopic capsules design. J. Magn. Magn. Mater. 2018, 452, 278–287. [Google Scholar] [CrossRef]
- Sundaram, S.; Kellnhofer, P.; Li, Y.Z.; Zhu, J.Y.; Torralba, A.; Matusik, W. Learning the signatures of the human grasp using a scalable tactile glove. Nature 2019, 569, 698–702. [Google Scholar] [CrossRef]
- Takei, K.; Takahashi, T.; Ho, J.C.; Ko, H.; Gillies, A.G.; Leu, P.W.; Fearing, R.S.; Javey, A. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 2010, 9, 821–826. [Google Scholar] [CrossRef]
- Mu, C.H.; Song, Y.Q.; Huang, W.T.; Ran, A.; Sun, R.J.; Xie, W.H.; Zhang, H.W. Flexible Normal-Tangential Force Sensor with Opposite Resistance Responding for Highly Sensitive Artificial Skin. Adv. Funct. Mater. 2018, 28, 1707503. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z.N. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.; Lin, L.; Zhu, G.; Wu, W.Z.; Zhang, R.; Wang, Z.L. Transparent Triboelectric Nanogenerators and Self-Powered Pressure Sensors Based on Micropatterned Plastic Films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef]
- Rouhani, H.; Favre, J.; Crevoisier, X.; Aminian, K. Ambulatory assessment of 3D ground reaction force using plantar pressure distribution. Gait Posture 2010, 32, 311–316. [Google Scholar] [CrossRef]
- Lai, W.J.; Cao, L.; Tan, R.X.; Tan, Y.C.; Li, X.G.; Phan, P.T.; Tiong, A.M.H.; Tjin, S.C.; Phee, S.J. An Integrated Sensor-Model Approach for Haptic Feedback of Flexible Endoscopic Robots. Ann. Biomed. Eng. 2020, 48, 342–356. [Google Scholar] [CrossRef]
- Bartolozzi, C.; Natale, L.; Nori, F.; Metta, G. Robots with a sense of touch. Nat. Mater. 2016, 15, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.S.; Li, J.; Hao, Y.; Bianchi, F.; Ciuti, G.; Arai, T.; Huang, Q.; Dario, P. The role of computed tomography data in the design of a robotic magnetically-guided endoscopic platform. Adv. Robot. 2018, 32, 443–456. [Google Scholar] [CrossRef]
- Pace, J.L.; Williams, I. Organization of the muscular wall of the human colon. Gut 1969, 10, 352–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saunders, B.P.; Fukumoto, M.; Halligan, S.; Jobling, C.; Moussa, M.E.; Bartram, C.I.; Williams, C.B. Why is colonoscopy more difficult in women? Gastrointest. Endosc. 1996, 43, 124–126. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Hao, Y.; Ciuti, G.; Arai, T.; Huang, Q.; Dario, P. Experimental assessment of intact colon deformation under local forces applied by magnetic capsule endoscopes. J. Mech. Med. Biol. 2020, 20, 2050041. [Google Scholar] [CrossRef]
- Johnson, S.; Schultz, M.; Scholze, M.; Smith, T.; Woodfield, J.; Hammer, N. How much force is required to perforate a colon during colonoscopy? An experimental study. J. Mech. Behav. Biomed. Mater. 2019, 91, 139–148. [Google Scholar] [CrossRef]

| Researcher | Actuation Strategy | IPM Size (mm) | Capsule Size (mm) | Control Method | Sensor Type | Ref |
|---|---|---|---|---|---|---|
| Ciuti et al. 2010 VECTOR EUR project | Permanent magnet | 3.2 × 19.1 (3 magnets) | 13.5 × 29.5 | Position control | Hall effect sensor & IMU | [14,18] |
| Lucarini et al. 2015 SUPCAM EUR project | Electromagnet | 11.4 × 5.5 | 37 × 37 | Force control | Current of electromagnet | [21] |
| Bianchi et al. 2017 ENDOO EUR project | Permanent magnet | - | - | Position control | Hall effect sensor & IMU | [17,22] |
| Nouda et al. 2018 | Electromagnet | - | 11 × 45 | Silicone fin with magnet | - | [23] |
| Taddese et al. 2019 | Permanent magnet | 11.1 × 11.1 | 20 × 22 | Force control | Hall effect sensor & IMU | [19,24] |
| Norton et al. 2019 | Permanent magnet | 11.1 × 11.1 | 21 × 39 | Position control | Micro ultrasound transducer | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Li, J.; Zhang, W.; Hao, Y.; Ciuti, G.; Arai, T.; Dario, P.; Huang, Q. Endoluminal Motion Recognition of a Magnetically-Guided Capsule Endoscope Based on Capsule-Tissue Interaction Force. Sensors 2021, 21, 2395. https://doi.org/10.3390/s21072395
Zhang P, Li J, Zhang W, Hao Y, Ciuti G, Arai T, Dario P, Huang Q. Endoluminal Motion Recognition of a Magnetically-Guided Capsule Endoscope Based on Capsule-Tissue Interaction Force. Sensors. 2021; 21(7):2395. https://doi.org/10.3390/s21072395
Chicago/Turabian StyleZhang, Peisen, Jing Li, Weimin Zhang, Yang Hao, Gastone Ciuti, Tatsuo Arai, Paolo Dario, and Qiang Huang. 2021. "Endoluminal Motion Recognition of a Magnetically-Guided Capsule Endoscope Based on Capsule-Tissue Interaction Force" Sensors 21, no. 7: 2395. https://doi.org/10.3390/s21072395
APA StyleZhang, P., Li, J., Zhang, W., Hao, Y., Ciuti, G., Arai, T., Dario, P., & Huang, Q. (2021). Endoluminal Motion Recognition of a Magnetically-Guided Capsule Endoscope Based on Capsule-Tissue Interaction Force. Sensors, 21(7), 2395. https://doi.org/10.3390/s21072395









