Enzymatic Activity and Its Relationship with Organic Matter Characterization and Ecotoxicity to Aliivibrio fischeri of Soil Samples Exposed to Tetrabutylphosphonium Bromide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Experimental Design
2.3. Determination of Soil Enzyme Activity
2.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.5. Microtox® Assay
2.6. Data Analysis
3. Results and Discussion
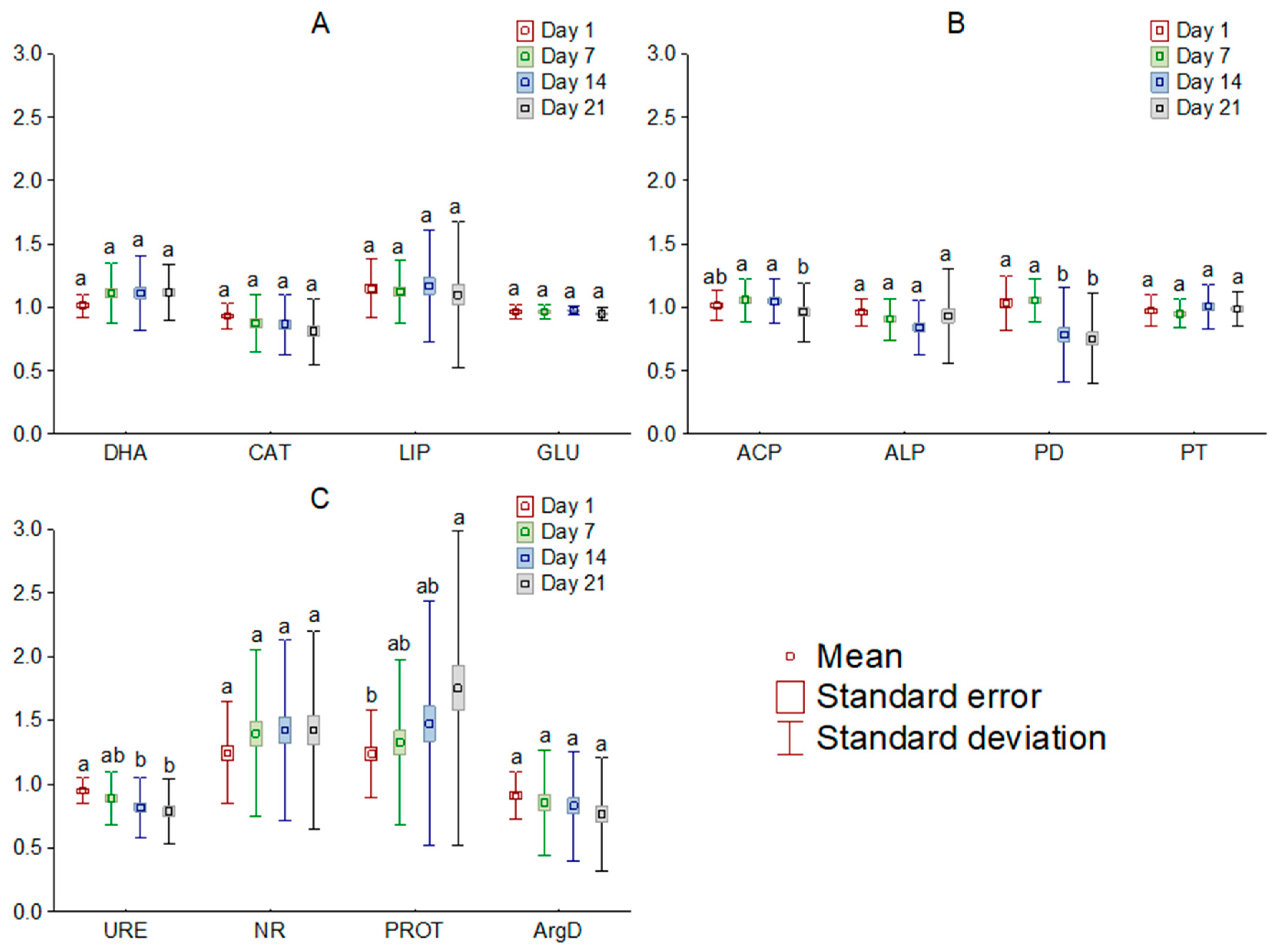
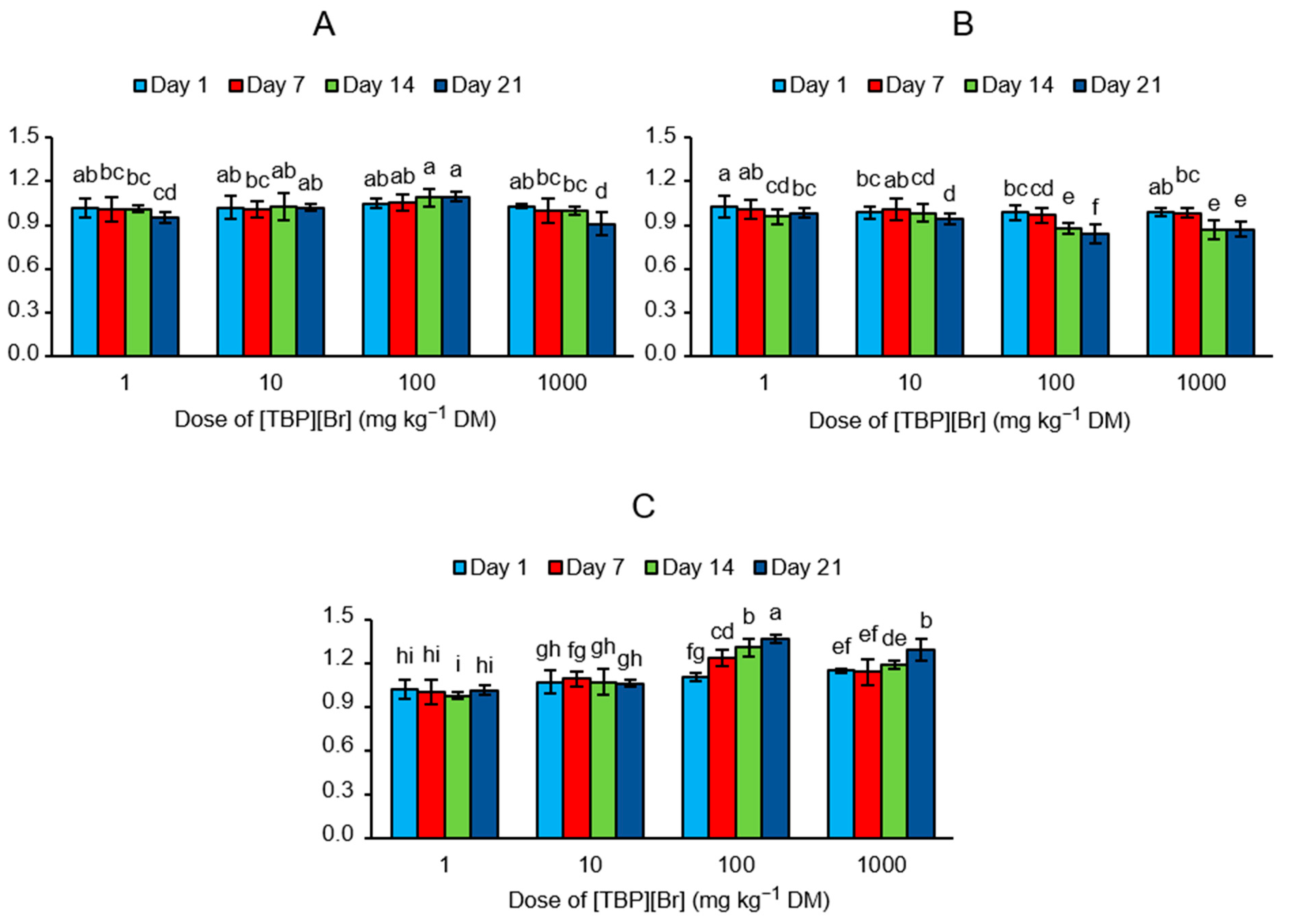
3.1. Enzyme Activity in the Soil Exposed to Tetrabutylphosphonium Bromide [TBP][Br]
3.2. Enzyme Activity Index (EAI) of the Soil Exposed to Tetrabutylphosphonium Bromide [TBP][Br]
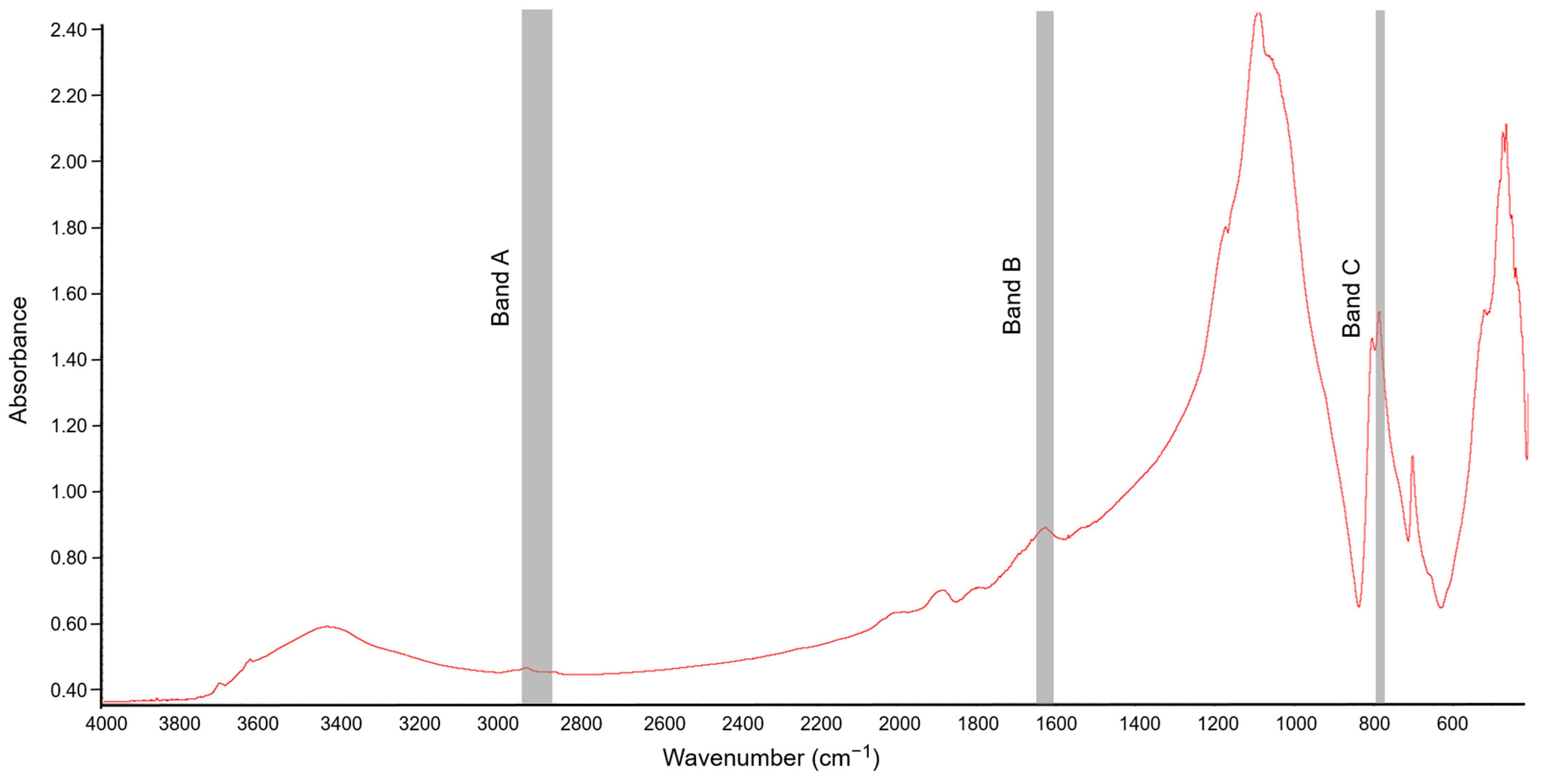
3.3. Characterization of Soil Organic Matter by FT-IR Spectroscopy in the Soil Exposed to Tetrabutylphosphonium Bromide [TBP][Br]
3.4. Ecotoxicity of the Tetrabutylphosphonium Bromide [TBP][Br]-Exposed Soil to A. fischeri
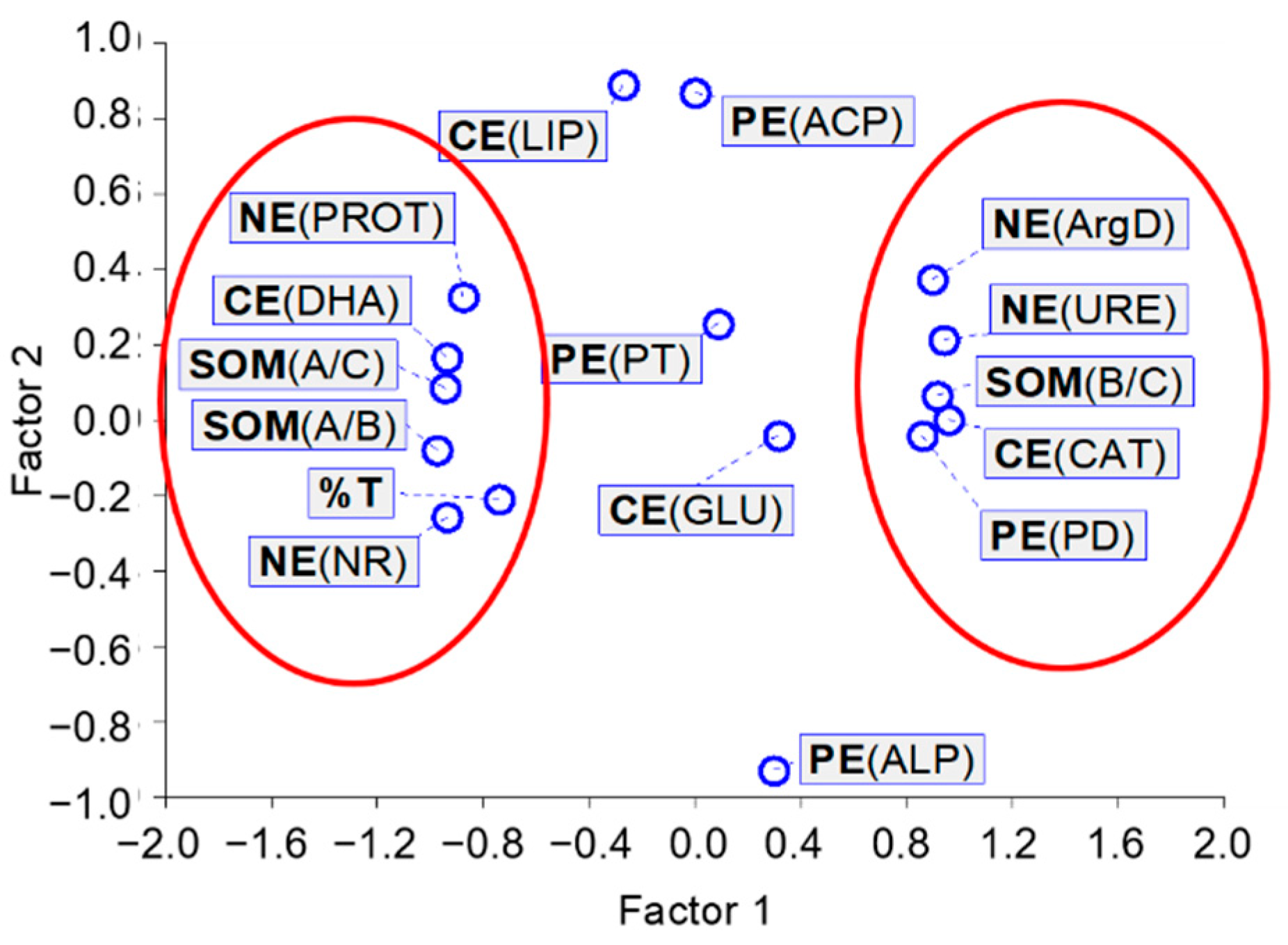
3.5. Relationships Between Enzyme Activity and Organic Matter Characterization and Ecotoxicity to A. fischeri of the Soil Samples Exposed to Tetrabutylphosphonium Bromide [TBP][Br]
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dindar, E.; Şağban, F.O.T.; Başkaya, H.S. Evaluation–of soil enzyme activities as soil quality indicators in sludge-amended soils. J. Environ. Biol. 2015, 36, 919–926. [Google Scholar]
- Acosta-Martinez, V.; Cano, A.; Johnson, J. Simultaneous determination of multiple soil enzyme activities for soil health-biogeochemical indices. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 17, 13630. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afric. J. Biotechnol. 2011, 10, 19840–19849. [Google Scholar]
- Guangming, L.; Xuechen, Z.; Xiuping, W.; Hongbob, S.; Jingsong, Y.; Xiangping, W. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. The role of dactylis glomerata and diesel oil in the formation of microbiome and soil enzyme activity. Sensors 2020, 20, 3362. [Google Scholar] [CrossRef]
- Telesiński, A.; Krzyśko-Łupicka, T.; Cybulska, K.; Pawłowska, B.; Biczak, R.; Śnieg, M.; Wróbel, J. Comparison of oxidoreductive enzyme activities in three coal tar creosote-contaminated soils. Soil Res. 2019, 57, 814–824. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Mocek-Płóciniak, A. Biochemical and chemical indices of soil transformations on goose farms in years 1996–2011. Arch. Environ. Protect. 2015, 41, 81–85. [Google Scholar]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Monitoring soil enzymes activity before and after animal manure application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids–solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R.; Pawłowska, B.; Podsiadło, C.; Śnioszek, M.; Telesiński, A. The reaction of cucumber to the introduction of ionic liquids into the soil. Environ. Sci. Pollut. Res. 2020, 27, 34182–34198. [Google Scholar] [CrossRef]
- Koel, M. Ionic liquids in chemical analysis. Crit. Rev. Anal. Chem. 2005, 35, 177–192. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Radošević, K.; Radojčić Redovniković, I.; Halambek, J.; Srčeka, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef]
- Kim, T.; Yu, C.; Park, C.; Kang, H. Polymer having dicationic structure in dumbbell shape for forward osmosis process. Polymers 2019, 11, 571. [Google Scholar] [CrossRef]
- Saita, S.; Kohno, Y.; Ohno, H. Detection of small differences in the hydrophilicity of ions using the LCST-type phase transition of an ionic liquid-water mixture. Chem. Commun. 2013, 49, 93–95. [Google Scholar] [CrossRef]
- You, S.-K.; Kwon, H.-H.; Lee, J.-M.; Shin, S.-C.; Cho, C.-W. Studies on the formation of hydrophobic ion-pairing complex of alendronate. Arch. Pharm. Res. 2009, 32, 1055–1060. [Google Scholar] [CrossRef]
- Stepnowski, P.; Mrozik, W.; Nichthauser, J. Adsorption of alkylimidazolium and alkylpyridinium ionic liquids onto natural soils. Environ. Sci. Technol. 2007, 41, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, W.; Jungnickel, C.; Paszkiewicz, M.; Stepnowski, P. Interaction of novel ionic liquids with soils. Water Air Soil Pollut. 2013, 224, 1759. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef] [PubMed]
- Parolo, M.E.; Savini, M.C.; Loewy, R.M. Characterization of soil organic matter by FT-IR spectroscopy and its relationship with chlorpyrifos sorption. J. Environ. Manag. 2017, 196, 316–322. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. Analytical approaches for characterizing soil organic matter. Org. Geochem. 2000, 31, 609–625. [Google Scholar] [CrossRef]
- Heller, C.; Ellerbrock, H.; Roβkopf, N.; Klingenfuβ, C.; Zeitz, J. Soil organic matter characterization of temperate peatland soil with FTIR-spectroscopy: Effects of mire type and drainage intensity. Eur. J. Soil Sci. 2015, 66, 847–858. [Google Scholar] [CrossRef]
- Baczyński, T.; Małachowska-Jutsz, A.; Szalińska, A. Toxicological assessment of pesticide contaminated soils with use of biotests. Geol. Geophys. Environ. 2018, 44, 245–257. [Google Scholar] [CrossRef]
- Foucault, T.; Durand, M.-J.; Tack, K.; Schreck, E.; Geret, F.; Leveque, T.; Pradere, P.; Goix, S.; Dumat, C. Use of ecotoxicity test and ecoscores to improve the management of polluted soils: Case of a secondary lead smelter plant. J. Hazard. Mater 2013, 246, 291–297. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, V.I.H.; Adams, R.H.; Sánchez-Madrigal, F.; de Los S. Pascual-Chablé, J.; Gómez-Cruz, R. Soil contact bioassay for rapid determination of acute toxicity with Eisenia Foetida. Heliyon 2020, 6, e03131. [Google Scholar]
- Wieczerzak, M.; Kudłak, B.; Namieśnik, J. Study of the effect of residues of pharmaceuticals on the environment on the example of bioassay Microtox®. Mon. Für Chem. Chem. Mon. 2016, 147, 1455–1460. [Google Scholar] [CrossRef]
- Jaśkowiak, J.; Tkaczyk, O.; Słota, M.; Kwaśniewska, J.; Szarejko, I. Analysis of aluminum toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS ONE 2018, 13, e0193156. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R.; Pawłowska, B.; Pilis, W.; Szczegielniak, J.; Wróbel, J.; Telesiński, A. Phytotoxicity and effect of ionic liquids on antioxidant parameters in spring barley seedlings: The impact of exposure time. Processes 2020, 8, 1175. [Google Scholar] [CrossRef]
- PN-EN ISO 11269-2. Soil Quality–Determination of the Effects of Pollutants on Soil Flora–Part. 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants; Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- OECD/OCDE 208. Guidelines for the Testing of Chemical. Terrestrial Plant: Seedling Test: Seedlings Emergence and Seedling Growth Test; OECD/OCDE: Paris, France, 2006. [Google Scholar]
- Johnson, J.I.; Temple, K.I. Some variables affecting measurement of catalase activity in soil. Soil Sci. Soc. Am. Proc. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Casida, J.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Margesin, R.; Feller, G.; Hämmerle, M.; Stegner, U.; Schinner, F. A colorimetric method for the determination of lipase activity in soil. Biotechnol. Lett. 2002, 24, 27–33. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Browman, M.G.; Tabatabai, M.A. Phosphodiesterase activity of soils. Soil Sci. Soc. Am. J. 1978, 42, 284–290. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Abdelmagid, H.M.; Tabatabai, M.A. Nitrate reductase activity of soils. Soil Biol. Biochem. 1987, 19, 421–427. [Google Scholar] [CrossRef]
- Ladd, I.N.; Butler, J.H.A. Short-term assay of soil proteolytic enzyme activities using proteins and dipeptide derivates as substrates. Soil Biol. Biochem. 1972, 4, 19–39. [Google Scholar] [CrossRef]
- Alef, K.; Kleiner, D. Arginine ammonification, a simple method to estimate microbial activity potentials in soils. Soil Biol. Biochem. 1986, 18, 233–235. [Google Scholar] [CrossRef]
- Celi, L.; Schnitzer, M.; Nègre, M. Analysis of carboxyl groups in soil humic acids by wet chemical method, FTIR spectrometry and solution-state carbon-13 NMR.A comparative study. Soil Sci. 1997, 162, 189–197. [Google Scholar] [CrossRef]
- Bernier, M.H.; Levy, G.J.; Fine, P.; Borisover, M. Organic matter composition in soils irrigated with treated wastewater: FT-IR spectroscopic analysis of bulk soil samples. Geoderma 2013, 209, 233–240. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. Characterizing organic matter of soil aggregate coatings and biopores by Fourier transform infrared spectroscopy. Eur. J. Soil Sci. 2004, 55, 219–228. [Google Scholar] [CrossRef]
- Nadav, I.; Tarchitzky, J.; Chen, Y. Induction of soil water repellency following irrigation with treated wastewater: Effects of irrigation water quality and soil texture. Irrig. Sci. 2013, 31, 385–394. [Google Scholar] [CrossRef]
- Doherty, F.G. A review of the Microtox® toxicity test system for assessing the toxicity of sediments and soils. Water Qual. Res. J. 2001, 36, 475–518. [Google Scholar] [CrossRef]
- Różyło, K.; Bohacz, J. Microbial and enzyme analysis of soil after the agricultural utilization of biogas digestate and mineral mining waste. Int. J. Environ. Sci. Technol. 2020, 17, 1051–1062. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Curyło, K.; Telesiński, A.; Jarnuszewski, G.; Krzyśko-Łupicka, T.; Cybulska, K. Analysis of chemical and biochemical parameters of petrol-contaminated soil after biostimulation with an enzyme reagent. Processes 2020, 8, 949. [Google Scholar] [CrossRef]
- Płatkowski, M.; Telesiński, A. Response of soil phosphatases to glyphosate and its formulations–Roundup (Laboratory conditions). Plant Soil Environ. 2016, 62, 286–292. [Google Scholar] [CrossRef]
- Stręk, M.; Telesiński, A. Comparison of selenite (IV) and selenate (VI) effect on some oxidoreductive enzymes in soil contaminated with spent engine oil. Soil Plant. Environ. 2016, 62, 157–163. [Google Scholar]
- Curyło, K.; Telesiński, A. Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol. Open Life Sci. 2020, 15, 12–20. [Google Scholar] [CrossRef]
- Telesiński, A.; Krzyśko-Łupicka, T.; Cybulska, K.; Wróbel, J. Response of soil phosphatase activities to contamination with two types of tar oil. Environ. Sci. Pollut. Res. 2018, 25, 28642–28653. [Google Scholar] [CrossRef] [PubMed]
- Telesiński, A.; Pawłowska, B.; Pater, J.; Biczak, R.; Śnioszek, M. The role of anion in the impact of tetraethylammonium salts on soil phosphatase activities. Ecol. Quest. 2017, 28, 47–54. [Google Scholar] [CrossRef]
- Kalam, A.; Tah, J.; Mukherjee, A.K. Pesticide effects on microbial population and soil enzyme activities during vermicomposting of agricultural waste. J. Environ. Biol. 2004, 25, 201–208. [Google Scholar]
- Roco, C.C.; Bergaust, L.L.; Shapleigh, J.P.; Yavitt, J.B. Reduction of nitrate to nitrite by microbes under oxic conditions. Soil Biol. Biochem. 2016, 100, 1–8. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, L.; Wang, J.; Wang, J.; Su, B.; Liu, T.; Zhang, C.; Gao, C.; Shao, Y. Toxic effects of ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate on soil enzyme activity and soil microbial community diversity. Ecotoxicol. Environ. Saf. 2017, 135, 201–208. [Google Scholar] [CrossRef]
- Cheng, C.; Ma, J.; Wang, J.; Du, Z.; Li, B.; Wang, J.; Gao, C.; Zhu, L. Toxicity comparison of three imidazolium bromide ionic liquids to soil microorganisms. Environ. Pollut. 2019, 255, 113321. [Google Scholar] [CrossRef]
- Telesiński, A. Response of soil peroxidases to 1-alkyl-3-methylimidazolium ionic liquids with tetrafluoroborate anion. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Et Zootech. 2017, 338, 217–226. [Google Scholar] [CrossRef]
- Telesiński, A.; Pawłowska, B.; Biczak, R.; Pater, J. Activity of dehydrogenases in clay soil exposed to quaternary ammonium salts with the iodine anion. Pol. J. Soil Sci. 2017, 50, 189–196. [Google Scholar] [CrossRef]
- Telesiński, A.; Biczak, R.; Stręk, M.; Płatkowski, M.; Pawłowska, B.; Emin, M. A study on the fluoride content and the enzymatic activity in soil exposed to inorganic ammonium salt and quaternary ammonium salts with hexafluorophosphate anions. Fluoride 2018, 51, 206–219. [Google Scholar]
- Demberelnyamba, D.; Kim, K.-S.; Choi, S.; Park, S.-Y.; Lee, H.; Kom, C.-J.; Yoo, I.-D. Synthesis and antimicrobial properties of imidazolium and pyrolidinonium salts. Bioorg. Med. Chem. 2004, 15, 853–857. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, Y. Toxicity and antimicrobial activities of ionic liquids with halogen anions. J. Environ. Prot. 2011, 2, 298–303. [Google Scholar] [CrossRef]
- Docherty, K.M.; Kulpa, C.F. Toxic and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185–189. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-miceobial activities of ionic liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Ganske, F.; Bornscheuer, U.T. Growth Escherichia coli, Pichia pastoris and Bacillus cereus in the presence of the ionic liquids [BMIM][BF4] and [BMIM][PF6] and organic solvents. Biotechnol. Lett. 2006, 28, 465–469. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Tojo, J.; Rodrigez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, Z.; Li, B.; Sun, X.; Wang, J.; Wang, J.; Zhu, L. Evaluating toxicity of 1-octyl-3-methylimidazolium hexafluorophosphate to microorganisms in soil. Chemosphere 2018, 210, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Sydow, M.; Owsianiak, M.; Framski, G.; Woźniak-Karczewska, M.; Piotrowska-Cyplik, A.; Ławniczak, Ł.; Szulc, A.; Zgoła-Grześkowiak, A.; Heipieper, H.J.; Chrzanowski, Ł. Biodiversity of soil bacteria exposed to sub-lethal concentrations of phosphonium-based ionic liquids: Effects of toxicity and biodegradation. Ecotoxicol. Environ. Saf. 2018, 147, 157–164. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhu, L.; Du, Z.; Wang, J.; Sun, X.; Zhou, T. Effects of 1-octyl-3-methylimidazolium nitrate on the microbes in brown soil. J. Environ. Sci. 2018, 67, 249–259. [Google Scholar] [CrossRef]
- Smernik, R.J.; Kookana, R.S. The effects of organic matteremineral interactions and organic matter chemistry on diuron sorption across a diverse range of soils. Chemosphere 2015, 119, 99–104. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity–benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Studzińska, S.; Kowalkowski, T.; Buszewski, B. Study of ionic liquid cations transport in soil. J. Hazard. Mater. 2009, 168, 1542–1547. [Google Scholar] [CrossRef]
- Gorman-Lewis, D.J.; Fein, J.B. Experimental study of the adsorption of an ionic liquid onto bacterial and mineral surfaces. Environ. Sci. Technol. 2004, 38, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, W.; Kotłowska, A.; Kamysz, W.; Stepnowski, P. Sorption of ionic liquids onto soils: Experimental and chemometric studies. Chemosphere 2012, 88, 1202–1207. [Google Scholar] [CrossRef]
- Matzke, M.; Thiele, K.; Müller, A.; Filser, J. Sorption and desorption of imidazolium based ionic liquids in different soil types. Chemosphere 2009, 74, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.; Stolte, S.; Arning, J.; Uebers, U.; Filser, J. Imidazolium based ionic liquids in soil: Effects of the side chain length on wheat (Triticum aestivum) and cress (Lepidium sativum) as affected by different clays and organic matter. Green Chem. 2008, 10, 584–591. [Google Scholar] [CrossRef]
- Stolte, S.; Matzke, M.; Arning, J.; Böschen, A.; Pitner, W.R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007, 9, 1170–1179. [Google Scholar] [CrossRef]
- Studzińska, S.; Sprynskyy, M.; Buszewski, B. Study in sorption kinetics of some ionic liquids on different soil types. Chemosphere 2008, 71, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.; Markowska, A.; Hupka, J.; Aranowski, R.; Jungnickel, C. Sorption of ionic liquids. Environ. Protect. Eng. 2009, 35, 53–64. [Google Scholar]
- Kowalska, D.; Maculewicz, J.; Stepnowski, P.; Dołżonek, J. Ionic liquids as environmental hazards–Crucial data in view of future PBT and PMT assessment. J. Hazard. Mater. 2021, 403, 123896. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, J.; Ma, Z.; Du, Z.; Zhang, C.; Zhu, L.; Wang, J. Effects of 1-alkyl-3-methylimidazolium nitrate on soil physical and chemical properties and microbial biomass. Arch. Environ. Contam. Toxicol. 2018, 74, 577–586. [Google Scholar] [CrossRef]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nałęcz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Urbaniak, M.; Baran, A.; Szara, M.; Mierzejewska, E.; Lee, S.; Takazawa, M.; Kannan, K. Evaluation of ecotoxicological and chemical properties of soil amended with Hudson River (New York, USA) sediment. Environ. Sci. Pollut. Res. 2020, 27, 7388–7397. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Háhn, J.; Kriszt, B.; Szaboszlay, S. Acute and chronic toxicity and their mixtures measured by Aliivibrio fischeri ecotoxicological assay. Ecotoxicol. Environ. Saf. 2019, 185, 109702. [Google Scholar] [CrossRef] [PubMed]
- Antonkiewicz, A.; Baran, A.; Pełka, R.; Wisła-Świder, A.; Nowak, E.; Konieczka, P. A mixture of cellulose production waste with municipal sewage as new material for ecological management of wastes. Ecotoxicol. Environ. Saf. 2019, 169, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Cuske, M.; Karczewska, A.; Gałka, B.; Matyja, K. Would forest litter cause a risk of increased copper solubility and toxicity in polluted soils remediated via phytostabilization? Pol. J. Environ. Stud. 2017, 26, 419–423. [Google Scholar] [CrossRef]
- Butarewicz, A.; Wrzaszcz, E.; Rosochacki, S. Toxicity of sewage from industrial wastewater treatment plants. J. Ecol. Eng. 2019, 20, 191–199. [Google Scholar] [CrossRef]
- Ranke, J.; Molter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1cell viability assays. Ecotoxicol. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef]
- Matzke, M.; Stolte, S.; Thiele, K.; Juffernholz, T.; Arning, J.; Ranke, J.; Welz-Biermann, U.; Jastorff, B. The influence of anion species on the toxicity of 1-alkyl-3- methylimidazolium ionic liquids observed in an (eco)toxicological test battery. Green Chem. 2007, 9, 1198–1207. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Bayo, J.; Pérez de los Ríos, A.; Vicente, M.A.; Bernal, F.J.; Quesada-Medina, J. Discovering less toxic ionic liquids by using the Microtox® toxicity test. Ecotoxicol. Environ. Saf. 2015, 116, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.; Monsalvo, V.M.; Lopez, J.; Mena, I.F.; Palomar, J.; Rodriguez, J.J.; Mohedano, A.F. Assessment the ecotoxicity and inhibition of imidazolium ionic liquids by respiration inhibition assays. Ecotoxicol. Environ. Saf. 2018, 162, 29–34. [Google Scholar] [CrossRef]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Bhavya, V.P.; Anil Kumar, S.; Shivanna, M.; Shivakumar, K.M. Ashok Alur, Effect of organic matter on soil enzyme activity, organic carbon and microbial activity under different land use systems. Int. J. Chem. Stud. 2017, 5, 301–305. [Google Scholar]
- Masciandaro, G.; Macci, C.; Doni, S.; Maserti, B.E.; Leo, A.C.B.; Ceccanti, B.; Wellington, E. Comparison of extraction methods for recovery of extracellular β-glucosidase in two different forest soils. Soil Biol. Biochem. 2008, 40, 2156–2161. [Google Scholar] [CrossRef]
- Štursová, M.; Baldrian, P. Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 2011, 338, 99–110. [Google Scholar] [CrossRef]
- Li, J.; Nie, M.; Pendall, E. Soil physico-chemical properties are more important than microbial diversity and enzyme activity in controlling carbon and nitrogen stocks near Sydney, Australia. Geoderma 2020, 366, 114201. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Baum, C.; Eckhardt, K.-U.; Kandeler, E. Stability and composition of soil organic matter control respiration and soil enzyme activities. Soil Biol. Biochem. 2008, 40, 1496–1505. [Google Scholar] [CrossRef]
- Olagoke, F.K.; Kalbitz, K.; Vogel, C. Control of soil extracellular enzyme activities by clay minerals–perspectives on microbial responses. Soil Syst. 2019, 3, 64. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Ahn, M.-Y. Organo-mineral–enzyme interaction and soil enzyme activity. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 271–292. [Google Scholar]


| Enzyme | Buffer | Substrate | Incubation Temperature/Time | Wavelength | References |
|---|---|---|---|---|---|
| Carbon cycle | |||||
| DHA | 0.1 M Tris buffer, pH 7.6 | 1% 2,3,5-triphenyltetrazolium chloride | 25 °C/24 h | 485 nm | [33] |
| LIP | 100 mM NaH2PO4/NaOH buffer, pH 7.25 | 100 mM p-nitrophenyl butyrate | 20 °C/10 min | 400 nm | [34] |
| GLU | modified universal buffer *, pH 6.0 | 25 mM p-nitrophenyl-β-D-glucopyranoside | 37 °C/1 h | 400 nm | [35] |
| Phosphorus cycle | |||||
| ACP | modified universal buffer *, pH 6.5 | 115 mM p-nitrophenyl phosphate hexahydrate | 37 °C/1 h | 400 nm | [36] |
| ALP | modified universal buffer *, pH 11.0 | ||||
| PD | 0.05 M Tris buffer, pH 8.0 | 5 mM bis(p-nitrophenyl) phosphate | 37 °C/1 h | 400 nm | [37] |
| PT | modified universal buffer *, pH 10.0 | 23 mg tris(p-nitrophenyl) phosphate ** | 37 °C/1 h | 400 nm | [38] |
| Nitrogen cycle | |||||
| URE | 0.1 M borate buffer, pH 10.0 | 79.9 mM urea | 37 °C/2 h | 660 nm | [39] |
| NR | 0.19 M ammonium chloride buffer, pH 8.5 | 25 mM KNO3 | 25 °C/24 h | 520 nm | [40] |
| PROT | 0.05 M Tris buffer. pH 8.1 | 2% casein | 50 °C/2 h | 700 nm | [41] |
| ArgD | water | 11.5 M L-arginine | 37 °C/3 h | 630 nm | [42] |
| Enzyme | Time of Exposition (days) | |||
|---|---|---|---|---|
| 1 | 7 | 14 | 21 | |
| Carbon cycle | ||||
| DHA (mg TPF kg−1 DM h−1) | 1.65 ± 0.06 a | 1.62 ± 0.11 a | 1.64 ± 0.06 a | 1.61 ± 0.07 a |
| CAT (mg H2O2 kg−1 DM h−1) | 8.51 ± 0.31 a | 8.34 ± 0.25 a | 84.0 ± 0.31 a | 84.2 ± 0.20 a |
| LIP (mg p-NP kg−1 DM h−1) | 23.24 ± 1.06 a | 25.17 ± 1.87 a | 24.62 ± 0.92 a | 25.77 ± 1.39 a |
| GLU (mg p-NP kg−1 DM h−1) | 86.79 ± 1.97 a | 91.58 ± 6.04 a | 89.81 ± 1.53 a | 90.98 ± 0.97 a |
| Phosphorus cycle | ||||
| ACP (mg p-NP kg−1 DM h−1) | 75.60 ± 2.65 a | 75.56 ± 3.92 a | 77.14 ± 3.42 a | 77.68 ± 4.02 a |
| ALP (mg p-NP kg−1 DM h−1) | 128.27 ± 6.27 a | 129.95 ± 3.45 a | 134.48 ± 4.39 a | 129.47 ± 1.84 a |
| PD (mg p-NP kg−1 DM h−1) | 12.54 ± 0.86 a | 11.54 ± 0.54 a | 13.27 ± 1.28 a | 12.67 ± 1.20 a |
| PT (mg p-NP kg−1 DM h−1) | 8.59 ± 0.46 a | 9.30 ± 0.80 a | 8.53 ± 0.47 a | 8.80 ± 0.36 a |
| Nitrogen cycle | ||||
| URE (mg N-NH4 kg−1 DM h−1) | 73.68 ± 3.49 a | 74.75 ± 2.84 a | 77.09 ± 3.59 a | 75.92 ± 3.13 a |
| NR (mg N-NO2− kg−1 DM h−1) | 2.59 ± 0.11 a | 2.56 ± 0.10 a | 2.64 ± 0.15 a | 2.64 ± 0.16 a |
| PROT (mg Tyr kg−1 DM h−1) | 26.37 ± 1.46 a | 26.86 ± 1.01 a | 26.06 ± 1.87 a | 26.19 ± 2.08 a |
| ArgD (mg N-NH4 kg−1 DM h−1) | 3.28 ± 0.21 a | 3.32 ± 0.10 a | 3.43 ± 0.19 a | 3.56 ± 0.13 a |
| Variable Factors | Carbon Cycle | Phosphorus Cycle | Nitrogen Cycle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA | CAT | LIP | GLU | ACP | ALP | PD | PT | URE | NR | PROT | ArgD | |
| D | 67.64 | 78.63 | 87.23 | 15.02 | 62.58 | 74.30 | 35.43 | 18.54 | 64.10 | 81.81 | 73.86 | 85.77 |
| ET | 20.82 | 16.61 | 2.36 | 69.32 | 26.09 | 16.37 | 59.16 | 36.25 | 32.05 | 5.19 | 20.87 | 8.74 |
| D × ET | 7.67 | 3.97 | 8.97 | 9.89 | 6.78 | 8.62 | 4.40 | 10.15 | 3.37 | 6.08 | 4.94 | 4.68 |
| Error | 3.86 | 0.79 | 1.44 | 5.78 | 4.56 | 0.71 | 1.01 | 35.06 | 0.49 | 6.93 | 0.33 | 0.81 |
| Dose of [TBP][Br] (mg kg−1 DM) | A/C | B/C | A/B |
|---|---|---|---|
| Day 1 | |||
| 0 (control) | 0.516 ± 0.023 b | 3.696 ± 0.128 ab | 0.137 ± 0.009 h |
| 1 | 0.501 ± 0.019 b | 3.676 ± 0.110 abc | 0.134 ± 0.006 h |
| 10 | 0.508 ± 0.017 b | 3.825 ± 0.157 ab | 0.139 ± 0.004 gh |
| 100 | 0.478 ± 0.039 b | 3.777 ± 0.293 ab | 0.139 ± 0.005 gh |
| 1000 | 0.486 ± 0.034 b | 3.965 ± 0.164 a | 0.143 ± 0.005 fgh |
| Day 7 | |||
| 0 (control) | 0.492 ± 0.092 b | 3.711 ± 0.218 abc | 0.139 ± 0.005 gh |
| 1 | 0.494 ± 0.016 b | 3.763 ± 0.357 ab | 0.135 ± 0.005 h |
| 10 | 0.543 ± 0.036 b | 3.384 ± 0.297 abcd | 0.161 ± 0.006 fgh |
| 100 | 0.658 ± 0.045 a | 3.170 ± 0.137 bcde | 0.208 ± 0.019 e |
| 1000 | 0.694 ± 0.013 a | 2.692 ± 0.142 de | 0.258 ± 0.015 bc |
| Day 14 | |||
| 0 (control) | 0.491 ± 0.014 b | 3.639 ± 0.313 abc | 0.137 ± 0.003 h |
| 1 | 0.489 ± 0.030 b | 3.834 ± 0.308 ab | 0.137 ± 0.005 h |
| 10 | 0.547 ± 0.048 b | 3.248 ± 0.306 abcd | 0.169 ± 0.006 f |
| 100 | 0.686 ± 0.032 a | 3.001 ± 0.090 cde | 0.229 ± 0.017 de |
| 1000 | 0.694 ± 0.036 a | 2.456 ± 0.186 e | 0.283 ± 0.015 ab |
| Day 21 | |||
| 0 (control) | 0.496 ± 0.015 b | 3.655 ± 0.349 abc | 0.137 ± 0.004 h |
| 1 | 0.500 ± 0.029 b | 3.869 ± 0.359 a | 0.136 ± 0.003 h |
| 10 | 0.546 ± 0.036 b | 3.257 ± 0.187 abcd | 0.168 ± 0.002 fg |
| 100 | 0.683 ± 0.009 a | 2.819 ± 0.107 de | 0.243 ± 0.007 cd |
| 1000 | 0.709 ± 0.020 a | 2.475 ± 0.178 e | 0.287 ± 0.015 a |
| Variable Factor | A/C | B/C | A/B |
|---|---|---|---|
| D | 52.48 | 39.10 | 61.48 |
| ET | 18.65 | 20.82 | 17.32 |
| D × ET | 22.06 | 23.35 | 19.28 |
| Error | 6.81 | 16.73 | 1.92 |
| Enzyme | A/C | B/C | A/B | %T |
|---|---|---|---|---|
| Carbon cycle | ||||
| DHA | 0.955 * | −0.902 * | 0.934 * | 0.684 * |
| CAT | −0.884 * | 0.853 * | −0.911 * | −0.755 * |
| LIP | 0.280 | −0.122 | 0.151 | 0.068 |
| GLU | −0.276 | 0.243 | −0.259 | −0.134 |
| Phosphorus cycle | ||||
| ACP | 0.097 | 0.022 | −0.052 | −0.152 |
| ALP | −0.352 | 0.188 | −0.212 | −0.052 |
| PD | −0.814 * | 0.806 * | −0.851 * | −0.541 * |
| PT | −0.033 | 0.097 | −0.091 | −0.217 |
| Nitrogen cycle | ||||
| URE | −0.823 * | 0.828 * | −0.897 * | −0.790 * |
| NR | 0.832 * | −0.839 * | 0.907 * | 0.879 * |
| PROT | 0.843 * | −0.763 * | 0.811 * | 0.588 * |
| ArgD | −0.801 * | 0.843 * | −0.894 * | −0.833 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telesiński, A.; Pawłowska, B.; Biczak, R.; Śnieg, M.; Wróbel, J.; Dunikowska, D.; Meller, E. Enzymatic Activity and Its Relationship with Organic Matter Characterization and Ecotoxicity to Aliivibrio fischeri of Soil Samples Exposed to Tetrabutylphosphonium Bromide. Sensors 2021, 21, 1565. https://doi.org/10.3390/s21051565
Telesiński A, Pawłowska B, Biczak R, Śnieg M, Wróbel J, Dunikowska D, Meller E. Enzymatic Activity and Its Relationship with Organic Matter Characterization and Ecotoxicity to Aliivibrio fischeri of Soil Samples Exposed to Tetrabutylphosphonium Bromide. Sensors. 2021; 21(5):1565. https://doi.org/10.3390/s21051565
Chicago/Turabian StyleTelesiński, Arkadiusz, Barbara Pawłowska, Robert Biczak, Marek Śnieg, Jacek Wróbel, Dorota Dunikowska, and Edward Meller. 2021. "Enzymatic Activity and Its Relationship with Organic Matter Characterization and Ecotoxicity to Aliivibrio fischeri of Soil Samples Exposed to Tetrabutylphosphonium Bromide" Sensors 21, no. 5: 1565. https://doi.org/10.3390/s21051565
APA StyleTelesiński, A., Pawłowska, B., Biczak, R., Śnieg, M., Wróbel, J., Dunikowska, D., & Meller, E. (2021). Enzymatic Activity and Its Relationship with Organic Matter Characterization and Ecotoxicity to Aliivibrio fischeri of Soil Samples Exposed to Tetrabutylphosphonium Bromide. Sensors, 21(5), 1565. https://doi.org/10.3390/s21051565









