A Review of Insect Monitoring Approaches with Special Reference to Radar Techniques
Abstract
1. Introduction
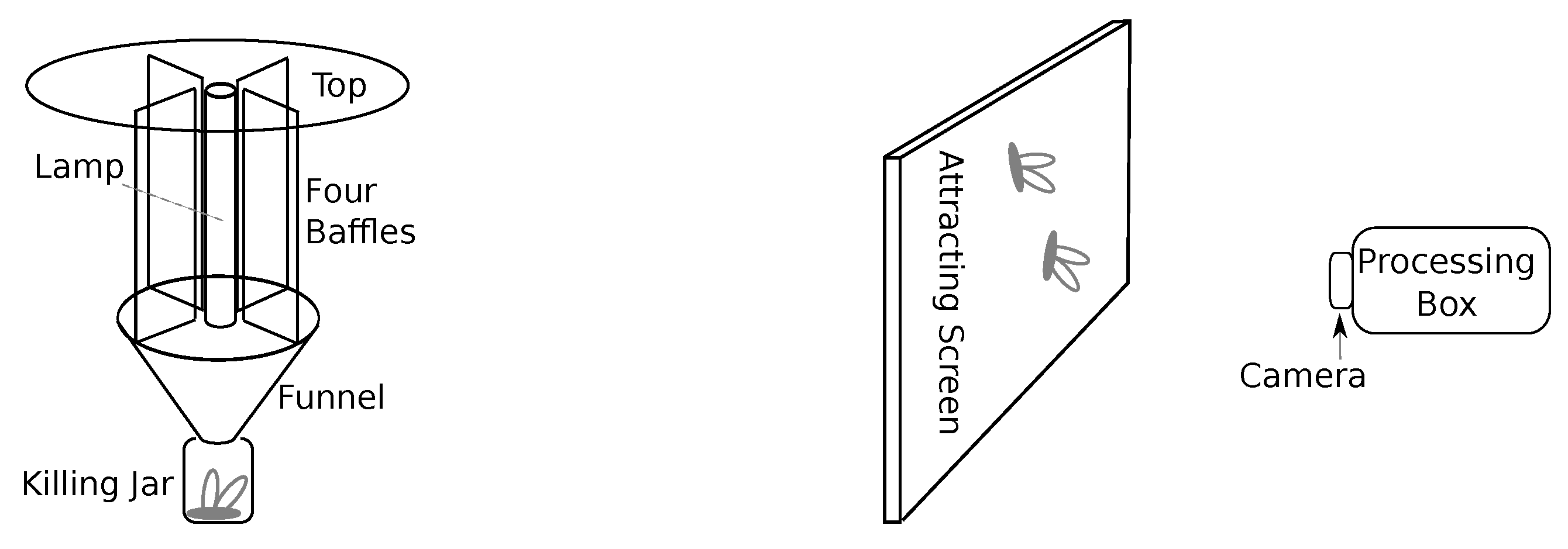
2. Insect Monitoring Methods
- a UV fluorescent tube,
- a light table (white screen, sometimes sprinkled with sugar water),
- a light ring (to ensure a diffuse foreground illumination of the insects),
- a web camera,
- a Raspberry Pi 4 computer configured for computer vision software, and
- a motion detection system allowing effective resource spending.
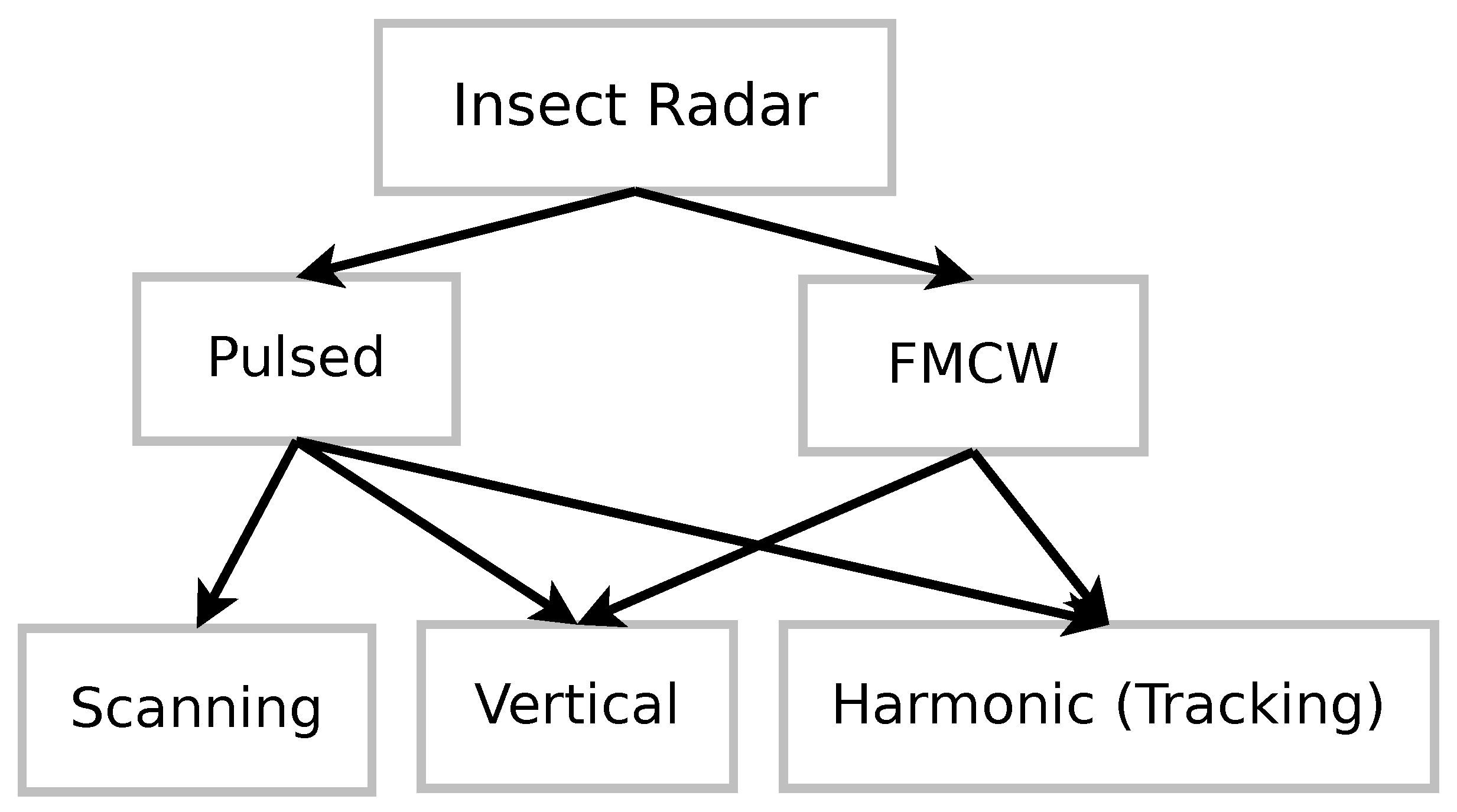
3. Insect Radar
- L-band (1–2 GHz, not utilized by insect radar),
- S-band (2–4 GHz),
- C-band (4–8 GHz),
- X-band (8–12 GHz),
- Ku-band (12–18 GHz), and
- Ka-band (26–40 GHz).
3.1. Pulsed Scanning Radar
3.2. Vertical-Looking Flight Observations
3.3. Low-Altitude Observations
3.4. Frequency Modulated Continuous Wave Radar
3.5. Tendencies and Classification
4. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKibben, B. The End of Nature, 2nd ed.; Bloomsbury Publishing: New York, NY, USA, 2003. [Google Scholar]
- Groom, M.; Meffe, G.; Carroll, C. Principles of Conservation Biology; Sinauer: Sunderland, MA, USA, 2012. [Google Scholar]
- Samways, M.J. Insect Diversity Conservation; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Lockwood, J.A. The Moral Standing of Insects and the Ethics of Extinction. Fla. Entomol. 1987, 70, 70–89. [Google Scholar] [CrossRef]
- New, T.R. Forests and Insect Conservation in Australia; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- McLean Ian, F.G.; Key, R.S. A History of Invertebrate Conservation in the British Statutory Conservation Agencies. In Insect Conservation: Past, Present and Prospects; New, T.R., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 41–74. [Google Scholar] [CrossRef]
- Pyle, R.M. The Origins and History of Insect Conservation in the United States. In Insect Conservation: Past, Present and Prospects; New, T.R., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 157–170. [Google Scholar] [CrossRef]
- Watts, C.; Stringer, I.; Gibbs, G. Insect Conservation in New Zealand: An Historical Perspective. In Insect Conservation: Past, Present and Prospects; Springer: Dordrecht, The Netherlands, 2012; pp. 213–243. [Google Scholar] [CrossRef]
- Spitzer, K. Insect Conservation Developments in Central Europe. In Insect Conservation: Past, Present and Prospects; Springer: Dordrecht, The Netherlands, 2012; pp. 303–315. [Google Scholar] [CrossRef]
- Ishii, M.; Nakamura, Y. Development and Future of Insect Conservation in Japan. InInsect Conservation: Past, Present and Prospects; Springer: Dordrecht, The Netherlands, 2012; pp. 339–357. [Google Scholar] [CrossRef]
- New, T.R. Insect Species Conservation; Ecology, Biodiversity and Conservation; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- McGrath, M. Global Insect Decline May See ‘Plague of Pests’. 2019. Available online: https://www.bbc.com/news/science-environment-47198576 (accessed on 10 February 2021).
- Hochkirch, A. The insect crisis we can’t ignore. Nature 2016, 539, 141. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Bruch, O. Ermittlung der Biomassen flugaktiver Insekten im Naturschutzgebiet Orbroicher Bruch mit Malaise Fallen in den Jahren 1989 und 2013. Mitteilungen Entomologischen Verein Krefeld 2013, 1, 1–5. [Google Scholar]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Leather, S. “Ecological Armageddon”—More evidence for the drastic decline in insect numbers. Ann. Appl. Biol. 2017, 172, 1–3. [Google Scholar] [CrossRef]
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Cardim Ferreira Lima, M.; Damascena de Almeida Leandro, M.E.; Valero, C.; Pereira Coronel, L.C.; Gonçalves Bazzo, C.O. Automatic Detection and Monitoring of Insect Pests—A Review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Stepanian, P.M.; Reynolds, D.R.; Reynolds, A.M. Investigating vertical motion of small insects in atmospheric boundary layer using millimetre-wavelength radar and Doppler LIDAR. J. Eng. 2019, 2019, 6906–6909. [Google Scholar] [CrossRef]
- Dwivedi, M.; Shadab, M.H.; Santosh, V.R. Insect Pest Detection, Migration and Monitoring Using Radar and LiDAR Systems. In Innovative Pest Management Approaches for the 21st Century; Springer: Singapore, 2020; pp. 61–76. [Google Scholar] [CrossRef]
- Weisser, W.; Siemann, E. Insects and Ecosystem Function; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Medeiroa, M.J.; Eiben, J.A.; Haines, W.P.; Kaholoaa, R.; King, C.; Krushelnycky, P.D.; Magnacca, K.N.; Rubinoff, D.; Starr, F.; Starr, K. The importance of insect monitoring to conservation actions in Hawaii. Proc. Hawaii. Ent. Soc. 2013, 45, 149–166. [Google Scholar]
- Sakai, A.K.; Wagner, W.L.; Mehrhoff, L.A. Patterns of Endangerment in the Hawaiian Flora. Syst. Biol. 2002, 51, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.J.; Fancy, S.G. Timing of Breeding and Molting in Six Species of Hawaiian Honeycreepers. Condor 1994, 96, 151–161. [Google Scholar] [CrossRef]
- Henneman, M.L.; Memmott, J. Infiltration of a Hawaiian Community by Introduced Biological Control Agents. Science 2001, 293, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, M.A.; Sithole, H.; Samways, M.J.; Simaika, J.P.; Pryke, J.S.; Picker, M.; Uys, C.; Armstrong, A.J.; Dippenaar-Schoeman, A.S.; Engelbrecht, I.A.; et al. Conservation and monitoring of invertebrates in terrestrial protected areas. Koedoe 2011, 53, 131–143. [Google Scholar] [CrossRef]
- Basset, Y.; Mavoungou, J.F.; Mikissa, J.B.; Missa, O.; Miller, S.E.; Kitching, R.L.; Alonso, A. Discriminatory power of different arthropod data sets for the biological monitoring of anthropogenic disturbance in tropical forests. Biodivers. Conserv. 2004, 13, 709–732. [Google Scholar] [CrossRef]
- McGeoch, M.A. The selection, testing and application of terrestrial insects as bioindicators. Biol. Rev. 1998, 73, 181–201. [Google Scholar] [CrossRef]
- Pimentel, D. Pesticides and Pest Control. In Integrated Pest Management: Innovation-Development Process: Volume 1; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 83–87. [Google Scholar] [CrossRef]
- Petrovskii, S.; Petrovskaya, N.; Bearup, D. Multiscale approach to pest insect monitoring: Random walks, pattern formation, synchronization, and networks. Phys. Life Rev. 2014, 11, 467–525. [Google Scholar] [CrossRef]
- Malaise, R. A new insect-trap. Entomol. Tidskrift 1937, 58, 148–160. [Google Scholar]
- Williams, C.B. An Analysis of Four Years Captures of Insects in a Light Trap. Part II. The Effect of Weather Conditions on Insect Activity; and the Estimation and Forecasting of Changes in the Insect Population. Trans. R. Entomol. Soc. Lond. 1940, 90, 227–306. [Google Scholar] [CrossRef]
- Frost, S.W. The Pennsylvania Insect Light Trap1. J. Econ. Entomol. 1957, 50, 287–292. [Google Scholar] [CrossRef]
- Matthews, R.W.; Matthews, J.R. The Malaise Trap: Its Utility and Potential for Sampling Insect Populations. Great Lakes Entomol. 1971, 4, 117–122. [Google Scholar]
- Gressitt, J.L.; Gressitt, M.K. An improved Malaise trap. Pac. Insects 1962, 4, 87–90. [Google Scholar]
- Southwood, E. Ecological Methods with Particular Reference to the Study of Insect Populations; Barnes and Noble: New York, NY, USA, 1966. [Google Scholar]
- Gunstream, S.E.; Chew, R.M. A Comparison of Mosquito Collection by Malaise and Miniature Light Traps1. J. Med. Entomol. 1967, 4, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.F. Species diversity and seasonal abundance in tropical Sphingidae (Lepidoptera). Proc. R. Entomol. Soc. Lond. Ser. A Gen. Entomol. 1969, 44, 162–168. [Google Scholar] [CrossRef]
- Cho, J.; Choi, J.; Qiao, M.; Ji, C.W.; Kim, H.Y.; Uhm, K.B.; Chon, T.S. Automatic identification of whiteflies, aphids and thrips in greenhouse based on image analysis. Int. J. Math. Comput. Simul. 2007, 346, 244. [Google Scholar]
- Bechar, I.; Moisan, S.; Thonnat, M.; Bremond, F. On-Line Video Recognition and Counting of Harmful Insects. In Proceedings of the 2010 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010. [Google Scholar] [CrossRef]
- Shariff, A.R.M.; Aik, Y.Y.; Hong, W.T.; Mansor, S.; Mispan, R. Automated Identification and Counting of Pests in the Paddy Fields Using Image Analysis. In Computers in Agriculture and Natural Resources, Orlando, FL, USA, 23–25 July 2006; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar] [CrossRef]
- Park, Y.S.; Han, M.W.; Kim, H.Y.; Uhm, K.B.; Park, C.G.; Lee, J.; Chon, T.S. Density estimation of rice planthoppers using digital image processing algorithm. Korean J. Appl. Entomol. 2003, 42, 57–63. [Google Scholar]
- Ridgway, C.; Davies, E.; Chambers, J.; Mason, D.; Bateman, M. Rapid machine vision method for the detection of insects and other particulate bio-contaminants of bulk grain in transit. Biosyst. Eng. 2002, 83, 21–30. [Google Scholar] [CrossRef]
- Zayas, I.Y.; Flinn, P.W. Detection of Insects in Bulkwheat Samples with Machine Vision. Trans. ASAE 1998, 41, 883–888. [Google Scholar] [CrossRef]
- Ashaghathra, S.M.; Weckler, P.; Solie, J.; Stone, M.; Wayadande, A. Identifying Pecan Weevils through Image Processing Techniques Based on Template Matching. In Proceedings of the 2007 ASAE Annual Meeting, Minneapolis, MI, USA, 17–20 June 2007. [Google Scholar] [CrossRef]
- Wen, C.; Guyer, D.E.; Li, W. Local feature-based identification and classification for orchard insects. Biosyst. Eng. 2009, 104, 299–307. [Google Scholar] [CrossRef]
- Yao, Q.; Lv, J.; Liu, Q.J.; Diao, G.Q.; Yang, B.J.; Chen, H.M.; Tang, J. An Insect Imaging System to Automate Rice Light-Trap Pest Identification. J. Integr. Agric. 2012, 11, 978–985. [Google Scholar] [CrossRef]
- Shimoda, M.; Honda, K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Peitsch, D.; Fietz, A.; Hertel, H.; de Souza, J.; Ventura, D.F.; Menzel, R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 1992, 170, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Von Helversen, O. Zur spektralen Unterschiedsempfindlichkeit der Honigbiene. J. Comp. Physiol. 1972, 80, 439–472. [Google Scholar] [CrossRef]
- Koshitaka, H.; Kinoshita, M.; Vorobyev, M.; Arikawa, K. Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc. R. Soc. B Biol. Sci. 2008, 275, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Hardie, J. Spectral specificity for targeted flight in the black bean aphid, Aphis fabae. J. Insect Physiol. 1989, 35, 619–626. [Google Scholar] [CrossRef]
- Yang, E.C.; Lee, D.W.; Wu, W.Y. Action spectra of phototactic responses of the flea beetle, Phyllotreta striolata. Physiol. Entomol. 2003, 28, 362–368. [Google Scholar] [CrossRef]
- Reisenman, C.E.; Lazzari, C.R.; Giurfa, M. Circadian control of photonegative sensitivity in the haematophagous bug Triatoma infestans. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1998, 183, 533–541. [Google Scholar] [CrossRef]
- Kim, M.G.; Yang, J.Y.; Lee, H.S. Phototactic behavior: Repellent effects of cigarette beetle, Lasioderma serricorne (Coleoptera: Anobiidae), to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 331–333. [Google Scholar] [CrossRef]
- Post, C.T.; Goldsmith, T.H. Pigment Migration and Light-Adaptation in the Eye of the Moth, Galleria Mellonella. Biol. Bull. 1965, 128, 473–487. [Google Scholar] [CrossRef]
- Walcott, B. Movement of retinula cells in insect eyes on light adaptation. Nature 1969, 223, 971–972. [Google Scholar] [CrossRef]
- Bateman, M.A. The Ecology of Fruit Flies. Annu. Rev. Entomol. 1972, 17, 493–518. [Google Scholar] [CrossRef]
- Pittendrigh, C.S. Temporal Organization: Reflections of a Darwinian Clock-Watcher. Annu. Rev. Entomol. 1993, 55, 17–54. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.; Collins, G. Photoperiodic Regulation of Insect and Molluscan Hormones; Novartis Foundation Symposia; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Saunders, D.S. Insect photoperiodism: Seeing the light. Physiol. Entomol. 2012, 37, 207–218. [Google Scholar] [CrossRef]
- Meyer-Rochow, V. Fine structural changes in dark-light adaptation in relation to unit studies of an insect compound eye with a crustacean-like rhabdom. J. Insect Physiol. 1974, 20, 573–589. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Meng, J.Y.; Wang, X.P.; Zhu, F.; Lei, C.L. Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera, and on the development of its F1 generation. Insect Sci. 2011, 18, 697–702. [Google Scholar] [CrossRef]
- Antignus, Y. Manipulation of wavelength-dependent behaviour of insects: An IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res. 2000, 71, 213–220. [Google Scholar] [CrossRef]
- Legarrea, S.; Karnieli, A.; Fereres, A.; Weintraub, P.G. Comparison of UV-absorbing Nets in Pepper Crops: Spectral Properties, Effects on Plants and Pest Control. Photochem. Photobiol. 2010, 86, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.C. Aspects of Flight Mechanics in Anisopterous Dragonflies. J. Exp. Biol. 1960, 37, 631–656. [Google Scholar]
- Goodman, L.J. The Role of Certain Optomotor Reactions in Regulating Stability in the Rolling Plane During Flight in the Desert Locust, Schistocerca Gregaria. J. Exp. Biol. 1965, 42, 385–407. [Google Scholar]
- White, P.J.T.; Glover, K.; Stewart, J.; Rice, A. The Technical and Performance Characteristics of a Low-Cost, Simply Constructed, Black Light Moth Trap. J. Insect Sci. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Bjerge, K.; Nielsen, J.B.; Videbæk Sepstrup, M.; Helsing-Nielsen, F.; Høye, T.T. A light trap and computer vision system to detect and classify live moths (Lepidoptera) using tracking and deep learning. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ruczyński, I.; Hałat, Z.; Zegarek, M.; Borowik, T.; Dechmann, D.K.N. Camera transects as a method to monitor high temporal and spatial ephemerality of flying nocturnal insects. Methods Ecol. Evol. 2019, 11, 294–302. [Google Scholar] [CrossRef]
- Pádua, L.; Adão, T.; Sousa, A.; Peres, E.; Sousa, J.J. Individual Grapevine Analysis in a Multi-Temporal Context Using UAV-Based Multi-Sensor Imagery. Remote Sens. 2020, 12, 139. [Google Scholar] [CrossRef]
- Song, B.; Park, K. Verification of Accuracy of Unmanned Aerial Vehicle (UAV) Land Surface Temperature Images Using In-Situ Data. Remote Sens. 2020, 12, 288. [Google Scholar] [CrossRef]
- Brydegaard, M. Towards Quantitative Optical Cross Sections in Entomological Laser Radar–Potential of Temporal and Spherical Parameterizations for Identifying Atmospheric Fauna. PLoS ONE 2015, 10, e0135231. [Google Scholar] [CrossRef]
- Malmqvist, E.; Jansson, S.; Török, S.; Brydegaard, M. Effective Parameterization of Laser Radar Observations of Atmospheric Fauna. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 327–334. [Google Scholar] [CrossRef]
- Brydegaard, M.; Gebru, A.; Kirkeby, C.; Akesson, S.; Smith, H. Daily Evolution of the Insect Biomass Spectrum in an Agricultural Landscape Accessed with Lidar. EPJ Web Conf. 2016, 119, 22004. [Google Scholar] [CrossRef]
- Brydegaard, M.; Malmqvist, E.; Jansson, S.; Larsson, J.; Török, S.; Zhao, G. The Scheimpflug lidar method. In Lidar Remote Sensing for Environmental Monitoring 2017; Singh, U.N., Ed.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2017; Volume 10406, pp. 104–120. [Google Scholar] [CrossRef]
- Zhao, G.; Lian, M.; Li, Y.; Duan, Z.; Zhu, S.; Mei, L.; Svanberg, S. Mobile lidar system for environmental monitoring. Appl. Opt. 2017, 56, 1506–1516. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, B.; Feng, H.; Zhu, S.; Hu, L.; Brydegaard, M.; Li, Y.; Jansson, S.; Malmqvist, E.; Svanberg, K.; et al. Application of lidar remote sensing of insects in agricultural entomology on the Chinese scene. J. Appl. Entomol. 2020, 144, 161–169. [Google Scholar] [CrossRef]
- Zhu, S.; Malmqvist, E.; Li, W.; Jansson, S.; Li, Y.; Duan, Z.; Svanberg, K.; Feng, H.; Song, Z.; Zhao, G.; et al. Insect abundance over Chinese rice fields in relation to environmental parameters, studied with a polarization-sensitive CW near-IR lidar system. Appl. Phys. B 2017, 123. [Google Scholar] [CrossRef]
- Jansson, S.; Brydegaard, M. Passive kHz lidar for the quantification of insect activity and dispersal. Anim. Biotelemetry 2018, 6. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Quintero-Torres, R.; Brick, R.; Sokolov, A.V.; Scully, M.O. Insect flight velocity measurement with a CW near-IR Scheimpflug lidar system. Opt. Express 2020, 28, 21891. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, B.K.; Jansson, S.; Brydegaard, M.; Zoueu, J.T. Entomological Scheimpflug lidar for estimating unique insect classes in-situ field test from Ivory Coast. OSA Contin. 2020, 3, 2362–2371. [Google Scholar] [CrossRef]
- Kirkeby, C.; Wellenreuther, M.; Brydegaard, M. Observations of movement dynamics of flying insects using high resolution lidar. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S.; Atkinson, P.; Ignell, R.; Brydegaard, M. First Polarimetric Investigation of Malaria Mosquitoes as Lidar Targets. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Brydegaard, M.; Jansson, S. Advances in entomological laser radar. J. Eng. 2019, 2019, 7542–7545. [Google Scholar] [CrossRef]
- Crawford, A. Radar reflections in the lower atmosphere. Proc. Inst. Radio Eng. 1949, 37, 404–405. [Google Scholar]
- Plank, V.G. Atmospheric angels mimic radar echoes. Electronics 1958, 31, 140–144. [Google Scholar]
- Tolbert, C.; Straiton, A.; Britt, C. Phantom radar targets at millimeter radio wavelengths. IRE Trans. Antennas Propag. 1958, 6, 380–384. [Google Scholar] [CrossRef]
- Riley, J. Radar as an Aid to the Study of Insect Flight. In A Handbook on Biotelemetry and Radio Tracking; Pergamon: Oxford, UK, 1980; pp. 131–140. [Google Scholar] [CrossRef]
- Schaefer, G. Radar observations of insect flight. In Symposia of the Royal entomological Society of London; Blackwell: Oxford, UK, 1976. [Google Scholar]
- Riley, J. Quantitative Analysis of Radar Returns from Insects; Great Malvern: Worcestershire, UK, 1979. [Google Scholar]
- Battan, L. Radar Observation of the Atmosphere; University of Chicago Press: Chicago, IL, USA, 1973. [Google Scholar]
- Greneker, E.F. Radar Reflectivity of Airborne Insects; Technical Report; Georgia Institute of Technology: Atlanta, GA, USA, 1978. [Google Scholar]
- Drake, V.; Reynolds, D. Radar Entomology: Observing Insect Flight and Migration; CAB International: Wallingford, UK, 2012. [Google Scholar]
- Bottigliero, S.; Milanesio, D.; Saccani, M.; Maggiora, R.; Viscardi, A.; Gallesi, M.M. An innovative harmonic radar prototype for miniaturized lightweight passive tags tracking. In Proceedings of the 2019 IEEE Radar Conference (RadarConf), Boston, MA, USA, 22–26 April 2019; pp. 1–6. [Google Scholar]
- Hao, Z.; Drake, V.A.; Taylor, J.R. Resolving the heading direction ambiguity in vertical beam radar observations of migrating insects. Ecol. Evol. 2019, 9, 6003–6013. [Google Scholar] [CrossRef]
- Lavrenko, A.; Pawson, S.; Cavers, J. On the Use of Additional Transmitters for Increasing Detection Range in Harmonic Radar. In Proceedings of the 2019 13th International Conference on Signal Processing and Communication Systems (ICSPCS), Gold Coast, QLD, Australia, 16–18 December 2019. [Google Scholar] [CrossRef]
- Li, W.; Hu, C.; Wang, R.; Liu, C.; Li, W. Experimental validations of insect orientation extraction based on fully polarimetric measurement. J. Eng. 2019, 2019, 7954–7957. [Google Scholar] [CrossRef]
- Kong, S.; Wang, R.; Lang, T.; Liu, C. Measurement of insect mass based on ellipsoid scattering model. J. Eng. 2019, 2019, 7455–7458. [Google Scholar] [CrossRef]
- Mirkovic, D.; Stepanian, P.M.; Wainwright, C.E.; Reynolds, D.R.; Menz, M.H.M. Characterizing animal anatomy and internal composition for electromagnetic modelling in radar entomology. Remote Sens. Ecol. Conserv. 2019, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, W.; Wang, R.; Long, T.; Drake, V.A. Discrimination of Parallel and Perpendicular Insects Based on Relative Phase of Scattering Matrix Eigenvalues. IEEE Trans. Geosci. Remote. Sens. 2020, 58, 3927–3940. [Google Scholar] [CrossRef]
- Rogers, R.M.; Buler, J.J.; Wainwright, C.E.; Campbell, H.A. Opportunities and challenges in using weather radar for detecting and monitoring flying animals in the Southern Hemisphere. Austral Ecol. 2019, 45, 127–136. [Google Scholar] [CrossRef]
- Stepanian, P.M.; Entrekin, S.A.; Wainwright, C.E.; Mirkovic, D.; Tank, J.L.; Kelly, J.F. Declines in an abundant aquatic insect, the burrowing mayfly, across major North American waterways. Proc. Natl. Acad. Sci. USA 2020, 117, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fu, X.; Zhao, S.; Shen, X.; Wyckhuys, K.A.G.; Wu, K. Long-term shifts in abundance of (migratory) crop-feeding and beneficial insect species in northeastern Asia. J. Pest Sci. 2020, 93, 583–594. [Google Scholar] [CrossRef]
- Montgomery, G.A.; Dunn, R.R.; Fox, R.; Jongejans, E.; Leather, S.R.; Saunders, M.E.; Shortall, C.R.; Tingley, M.W.; Wagner, D.L. Is the insect apocalypse upon us? How to find out. Biol. Conserv. 2020, 241, 108327. [Google Scholar] [CrossRef]
- Becciu, P.; Menz, M.H.M.; Aurbach, A.; Cabrera-Cruz, S.A.; Wainwright, C.E.; Scacco, M.; Ciach, M.; Pettersson, L.B.; Maggini, I.; Arroyo, G.M.; et al. Environmental effects onflying migrants revealed by radar. Ecography 2019, 42, 942–955. [Google Scholar] [CrossRef]
- Souza Cunha, A.E. Evaluating a Doppler Radar Monitor for Assessing Honey Bee Colony Health; The Honors College at the University of Maine: Orono, ME, USA, 2019. [Google Scholar]
- Litman, J.R. Under the radar: Detection avoidance in brood parasitic bees. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180196. [Google Scholar] [CrossRef] [PubMed]
- Souza Cunha, A.; Rose, J.; Prior, J.; Aumann, H.; Emanetoglu, N.; Drummond, F. A novel non-invasive radar to monitor honey bee colony health. Comput. Electron. Agric. 2020, 170, 105241. [Google Scholar] [CrossRef]
- Wittman, J.T.; Nicoll, R.A.; Myers, S.W.; Chaloux, P.H.; Aukema, B.H. Characterizing and Simulating the Movement of Late-Instar Gypsy Moth (Lepidoptera: Erebidae) to Evaluate the Effectiveness of Regulatory Practices. Environ. Entomol. 2019, 48, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, D.M.; Acebes-Doria, A.L.; Rice, K.B.; Short, B.D.; Adams, C.G.; Gut, L.J.; Leskey, T.C. Estimating Monitoring Trap Plume Reach and Trapping Area for Nymphal and Adult Halyomorpha halys (Hemiptera: Pentatomidae) in Crop and Non-crop Habitats. Environ. Entomol. 2019, 48, 1104–1112. [Google Scholar] [CrossRef]
- Krishnasamy, V.; Sundaraguru, R.; Amala, U. Emerging vistas of Remote Sensing Tools in Pollination Studies. Sociobiology 2019, 66, 394. [Google Scholar] [CrossRef]
- Zhang, Z.; Zha, Y.; Cai, S.; Hong, C.; Liang, P.; Chen, J. Application of harmonic radar to analyze dispersal behavior of the Japanese pine sawyer beetle, Monochamus alternatus (Coleoptera: Cerambycidae). Entomol. Res. 2020, 50, 50–58. [Google Scholar] [CrossRef]
- Drake, V.A.; Hatty, S.; Symons, C.; Wang, H. Insect Monitoring Radar: Maximizing Performance and Utility. Remote Sens. 2020, 12, 596. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Reynolds, D.R.; Reynolds, A.M. Linking Small-Scale Flight Manoeuvers and Density Profiles to the Vertical Movement of Insects in the Nocturnal Stable Boundary Layer. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Hu, C.; Wang, R.; Zhang, F.; Wang, L.; Long, T.; Wu, K. Insect Multifrequency Polarimetric Radar Cross Section: Experimental Results and Analysis. IEEE Trans. Geosci. Remote Sens. 2020, 1–13. [Google Scholar] [CrossRef]
- Wang, R.; Cai, J.; Hu, C.; Zhou, C.; Zhang, T. A Novel Radar Detection Method for Sensing Tiny and Maneuvering Insect Migrants. Remote Sens. 2020, 12, 3238. [Google Scholar] [CrossRef]
- Alzaabi, O.S. Airborne Insect Radar Scattering Characterization Using Electromagnetic Modeling. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2019. [Google Scholar]
- Agency, T.E.S. Satellite Frequency Bands. Available online: https://www.esa.int/Applications/Telecommunications_Integrated_Applications/Satellite_frequency_bands (accessed on 10 February 2021).
- Cheng, H.; Linlin, F.; Rui, W.; Chao, Z.; Weidong, L.; ZHANG, F.; Tianjiao, L.; Teng, L. Analysis of Insect RCS Characteristics. J. Electron. Inf. Technol. 2020, 42, 140–153. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, X.; Hu, C.; Wang, R.; Liu, C.; Li, W. Insect wing-beat frequency automatic extraction and experimental verification with a Ku-band insect radar system. J. Eng. 2019, 2019, 7973–7976. [Google Scholar] [CrossRef]
- Cai, J.; Yuan, Q.; Wang, R.; Liu, C.; Zhang, T. Insect detection and density estimation based on a Ku-band scanning entomological radar. J. Eng. 2019, 2019, 7636–7639. [Google Scholar] [CrossRef]
- Hu, C.; Li, W.; Wang, R.; Zhang, T.; Li, W. Insect speed extraction method based on a high resolution and full polarisation radar with vertical-looking mode. J. Eng. 2019, 2019, 5889–5892. [Google Scholar] [CrossRef]
- Hu, C.; Li, W.; Wang, R.; Long, T.; Liu, C.; Drake, V.A. Insect Biological Parameter Estimation Based on the Invariant Target Parameters of the Scattering Matrix. IEEE Trans. Geosci. Remote Sens. 2019, 57, 6212–6225. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, C.; Rui, W.; Cheng, H. RCS Feature-aided Insect Target Tracking Algorithm. J. Radars 2019, 8, 598–605. [Google Scholar] [CrossRef]
- Mao, H.; Wang, R.; Hu, C.; Yang, J. Fully Polarimetric Radar Observing Insects Flight. In Proceedings of the 2019 IEEE International Conference on Signal, Information and Data Processing (ICSIDP), Chongqing, China, 11–13 December 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Kranstauber, B.; Bouten, W.; Leijnse, H.; Wijers, B.C.; Verlinden, L.; Shamoun-Baranes, J.; Dokter, A.M. High-Resolution Spatial Distribution of Bird Movements Estimated from a Weather Radar Network. Remote Sens. 2020, 12, 635. [Google Scholar] [CrossRef]
- Jatau, P.; Melnikov, V. Classifying Bird and Insect Radar Echoes at S Band. In Proceedings of the 99th American Meteorological Society Annual Meeting, Phoenix, AZ, USA, 6–10 January 2019. [Google Scholar]
- Zulkifli, S.; Balleri, A. FMCW Radar Prototype Development for Detection and Classification of Nano-Targets. In Proceedings of the 2020 IEEE International Radar Conference (RADAR), Washington, DC, USA, 28–30 April 2020; pp. 738–743. [Google Scholar] [CrossRef]
- Young, J.; Garratt, M. Drones become even more insect-like. Science 2020, 368, 586–587. [Google Scholar] [CrossRef]
- Ray, J.D.; Stepanian, P.; Kelly, J. Evaluation of NEXRAD Radar as a Tool for Monitoring Monarch Butterflies; Technical Report; Pantex Plant (PTX): Amarillo, TX, USA, 2019. [Google Scholar]
- Satterfield, D.A.; Sillett, T.S.; Chapman, J.W.; Altizer, S.; Marra, P.P. Seasonal insect migrations: Massive, influential, and overlooked. Front. Ecol. Environ. 2020, 18, 335–344. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Zhang, H.; Liu, J.; Jiang, Y.; Wyckhuys, K.A.G.; Wu, K. Global warming modifies long-distance migration of an agricultural insect pest. J. Pest Sci. 2020, 93, 569–581. [Google Scholar] [CrossRef]
- Riley, J.R.; Reynolds, D.R.; Rainey, R.C. Radar-based studies of the migratory flight of grasshoppers in the middle Niger area of Mali. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 1979, 204, 67–82. [Google Scholar] [CrossRef]
- Riley, J.R. Radar cross section of insects. Proc. IEEE 1985, 73, 228–232. [Google Scholar] [CrossRef]
- Mueller, E.A.; Larkin, R.P. Insects Observed Using Dual-Polarization Radar. J. Atmos. Ocean. Technol. 1985, 2, 49–54. [Google Scholar] [CrossRef]
- Drake, V.A.; Helm, K.F.; Readshaw, J.L.; Reid, D.G. Insect migration across Bass Strait during spring: A radar study. Bull. Entomol. Res. 1981, 71, 449–466. [Google Scholar] [CrossRef]
- Vaughn, C.R. Birds and insects as radar targets: A review. Proc. IEEE 1985, 73, 205–227. [Google Scholar] [CrossRef]
- Larkin, R.P. Flight speeds observed with radar, a correction: Slow birds are insects. Behav. Ecol. Sociobiol. 1991, 29, 221–224. [Google Scholar] [CrossRef]
- Zrnic, D.S.; Ryzhkov, A.V. Observations of insects and birds with a polarimetric radar. IEEE Trans. Geosci. Remote Sens. 1998, 36, 661–668. [Google Scholar] [CrossRef]
- Rennie, S.; Rikus, L.; Eizenberg, N.; Steinle, P.; Krysta, M. Impact of Doppler Radar Wind Observations on Australian High-Resolution Numerical Weather Prediction. Weather Forecast. 2020, 35, 309–324. [Google Scholar] [CrossRef]
- Gauthreaux, S.; Diehl, R. Discrimination of Biological Scatterers in Polarimetric Weather Radar Data: Opportunities and Challenges. Remote Sens. 2020, 12, 545. [Google Scholar] [CrossRef]
- Cui, K.; Hu, C.; Wang, R.; Sui, Y.; Mao, H.; Li, H. Deep-learning-based extraction of the animal migration patterns from weather radar images. Sci. China Inf. Sci. 2020, 63. [Google Scholar] [CrossRef]
- Hu, C.; Li, S.; Wang, R.; Cui, K.; Wu, D.; Ma, S. Extracting animal migration pattern from weather radar observation based on deep convolutional neural networks. J. Eng. 2019, 2019, 6541–6545. [Google Scholar] [CrossRef]
- Hu, X.; Ge, J.; Du, J.; Li, Q.; Huang, J.; Fu, Q. A robust low-level cloud and clutter discrimination method for ground-based millimeter-wavelength cloud radar. Atmos. Meas. Tech. Discuss. 2020, 2020, 1–36. [Google Scholar] [CrossRef]
- Cui, K.; Hu, C.; Wang, R.; Li, S.; Wu, D.; Ma, S. Quantifying insect migration across Bohai strait using weather radar. J. Eng. 2019, 2019, 6095–6098. [Google Scholar] [CrossRef]
- Shamoun-Baranes, J.; Nilsson, C.; Bauer, S.; Chapman, J. Taking radar aeroecology into the 21st century. Ecography 2019, 42, 847–851. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef]
- Hobbs, S. A radar signal processor for biological applications. Meas. Sci. Technol. 1991, 2, 415. [Google Scholar] [CrossRef]
- Beerwinkle, K.; Lopez, J., Jr.; Schleider, P.; Lingren, P. Annual patterns of aerial insect densities at altitudes from 500 to 2400 meters in east-central Texas indicated by continuously-operating vertically-oriented radar. Southwest. Entomol. Suppl. (USA) 1995, 18, 63–79. [Google Scholar]
- Perry, J.N.; Woiwod, I.P.; Hanski, I. Using Response-Surface Methodology to Detect Chaos in Ecological Time Series. Oikos 1993, 68, 329–339. [Google Scholar] [CrossRef]
- Woiwod, I.P.; Hanski, I. Patterns of Density Dependence in Moths and Aphids. J. Anim. Ecol. 1992, 61, 619–629. [Google Scholar] [CrossRef]
- Halbert, S.; Jennings, M.; Cogan, C.; Quisenberry, S.; Johnson, J. Potential use of suction trap collections of aphids as indicators of plant biodiversity. Insects Chang. Environ. 1995, 504, 499–504. [Google Scholar]
- Fleming, R.; Tatchell, G. Shifts in the Flight Periods of British Aphids: A Response to Climate Warming; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Tatchell, G. Monitoring and forecasting aphid problems. In Proceedings of the Conference on Aphid–Plant Interactions: Populations to Molecules; Peters, D., Webster, J., Chlouber, C., Eds.; Experiment Station Miscellaneous Publication No. 132. USDA–Agricultural Research Service and Oklahoma State University: Stillwater, OK, USA, 1991; pp. 215–231. [Google Scholar]
- Smith, A.; Riley, J. Signal processing in a novel radar system for monitoring insect migration. Comput. Electron. Agric. 1996, 15, 267–278. [Google Scholar] [CrossRef]
- Chapman, J.; Smith, A.; Woiwod, I.; Reynolds, D.; Riley, J. Development of vertical-looking radar technology for monitoring insect migration. Comput. Electron. Agric. 2002, 35, 95–110. [Google Scholar] [CrossRef]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent Insights from Radar Studies of Insect Flight. Annu. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D. Vertical-Looking Radar: A New Tool for Monitoring High-Altitude Insect Migration. BioScience 2003, 53, 503–511. [Google Scholar] [CrossRef]
- Reynolds, D.R.; Riley, J.R. Flight Behaviour and Migration of Insect Pests. Radar Studies in Developing Countries; Number No. 71; Natural Resources Institute (NRI): Chatham, UK, 1997. [Google Scholar]
- Reynolds, D.; Chapman, J.; Edwards, A.; Smith, A.; Wood, C.; Barlow, J.; Woiwod, I. Radar studies of the vertical distribution of insects migrating over southern Britain: The influence of temperature inversions on nocturnal layer concentrations. Bull. Entomol. Res. 2005, 95, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Harman, I.; Drake, V. Insect monitoring radar: Analytical time-domain algorithm for retrieving trajectory and target parameters. Comput. Electron. Agric. 2004, 43, 23–41. [Google Scholar] [CrossRef]
- Hobbs, S.; Aldhous, A. Insect ventral radar cross-section polarisation dependence measurements for radar entomology. IEE Proc. Radar Sonar Navig. 2006, 153, 502. [Google Scholar] [CrossRef]
- Aldhous, A.C. An Investigation of the Polarisation Dependence of Insect Radar cross Sections at Constant Aspect. Ph.D. Thesis, Ecological Physics Research Group, Cranfield CERES, Cranfield, UK, 1989. [Google Scholar]
- Wang, H.; Drake, V. Insect monitoring radar: Retrieval of wingbeat information from conical-scan observation data. Comput. Electron. Agric. 2004, 43, 209–222. [Google Scholar] [CrossRef]
- Drake, V.A.; Chapman, J.W.; Lim, K.S.; Reynolds, D.R.; Riley, J.R.; Smith, A.D. Ventral-aspect radar cross sections and polarization patterns of insects at X band and their relation to size and form. Int. J. Remote Sens. 2017, 38, 5022–5044. [Google Scholar] [CrossRef]
- Loper, G.M.; Wolf, W.W.; Taylor, O.R. Radar detection of drones responding to honeybee queen pheromone. J. Chem. Ecol. 1993, 19, 1929–1938. [Google Scholar] [CrossRef]
- Mascanzoni, D.; Wallin, H. The harmonic radar: A new method of tracing insects in the field. Ecol. Entomol. 1986, 11, 387–390. [Google Scholar] [CrossRef]
- Fuks, P. Harmonic Radar, a Modern Method for Location of Avalanche Victims; Division of Electromagnetic Theory, Royal Inst. of Technology: Stockholm, Sweden, 1981. [Google Scholar]
- Bingham, R.P. Harmonics-Understanding the Facts; Dranetz Technologies: Edison, NJ, USA, 1994. [Google Scholar]
- Riley, J.R.; Smith, A.D.; Reynolds, D.R.; Edwards, A.S.; Osborne, J.L.; Williams, I.H.; Carreck, N.L.; Poppy, G.M. Tracking bees with harmonic radar. Nature 1996, 379, 29–30. [Google Scholar] [CrossRef]
- Roland, J.; McKinnon, G.; Backhouse, C.; Taylor, P.D. Even smaller radar tags on insects. Nature 1996, 381, 120. [Google Scholar] [CrossRef]
- Lövei, G.L.; Stringer, I.A.; Devine, C.D.; Cartellieri, M. Harmonic Radar—A Method Using Inexpensive Tags to Study Invertebrate Movement on Land. N. Z. J. Ecol. 1997, 21, 187–193. [Google Scholar]
- Reynolds, D.; Riley, J. Remote-sensing, telemetric and computer-based technologies for investigating insect movement: A survey of existing and potential techniques. Comput. Electron. Agric. 2002, 35, 271–307. [Google Scholar] [CrossRef]
- Riley, J.; Smith, A. Design considerations for an harmonic radar to investigate the flight of insects at low altitude. Comput. Electron. Agric. 2002, 35, 151–169. [Google Scholar] [CrossRef]
- Colpitts, B.; Boiteau, G. Harmonic Radar Transceiver Design: Miniature Tags for Insect Tracking. IEEE Trans. Antennas Propag. 2004, 52, 2825–2832. [Google Scholar] [CrossRef]
- Psychoudakis, D.; Moulder, W.; Chen, C.; Zhu, H.; Volakis, J.L. A Portable Low-Power Harmonic Radar System and Conformal Tag for Insect Tracking. IEEE Antennas Wirel. Propag. Lett. 2008, 7, 444–447. [Google Scholar] [CrossRef]
- Kim, J.; Jung, M.; Kim, H.G.; Lee, D.H. Potential of harmonic radar system for use on five economically important insects: Radar tag attachment on insects and its impact on flight capacity. J. Asia-Pac. Entomol. 2016, 19, 371–375. [Google Scholar] [CrossRef]
- Boiteau, G.; Meloche, F.; Vincent, C.; Leskey, T.C. Effectiveness of Glues Used for Harmonic Radar Tag Attachment and Impact on Survival and Behavior of Three Insect Pests. Environ. Entomol. 2009, 38, 168–175. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, C.G.; Seo, B.Y.; Boiteau, G.; Vincent, C.; Leskey, T.C. Detectability of Halyomorpha halys (Hemiptera: Pentatomidae) by Portable Harmonic Radar in Agricultural Landscapes. Fla. Entomol. 2014, 97, 1131–1138. [Google Scholar] [CrossRef]
- Hsu, M.L.; Jan, S.J.; Tsai, Z.M.; Wang, H.; Chang, F.R.; Jau, P.H.; Lin, K.Y.; Yang, E.C. Portable 9.4/18.8 GHz harmonic radar system using pulse Pseudorandom code principle. In Proceedings of the 2015 European Microwave Conference (EuMC), Paris, France, 6–11 September 2015. [Google Scholar] [CrossRef]
- He, Z.Z.; Luo, J.; Gui, L.Y.; Hua, D.K.; Du, T.H.; Wang, F.L.; Liang, P.; Shi, Y.F.; Yang, X. Tracking the movement trajectory of newly emerged adult Chinese citrus flies with insect harmonic radar. J. Asia-Pac. Entomol. 2019, 22, 853–859. [Google Scholar] [CrossRef]
- Kho, J.W.; Jung, M.; Lee, D.H. Evaluating the efficacy of two insect detection methods with Riptortus pedestris (Hemiptera: Alydidae): Portable harmonic radar system and fluorescent marking system. Pest Manag. Sci. 2019, 75, 224–233. [Google Scholar] [CrossRef]
- Milanesio, D.; Bottigliero, S.; Saccani, M.; Maggiora, R.; Viscardi, A.; Gallesi, M.M. An harmonic radar prototype for insect tracking in harsh environments. In Proceedings of the 2020 IEEE International Radar Conference (RADAR), Washington, DC, USA, 27 April–27 May 2020; pp. 648–653. [Google Scholar] [CrossRef]
- Mumtaz, F.; Ram, S.S.; Purandare, S. Development of Harmonic Radar for Insect Detection. Ph.D. Thesis, IIIT-Delhi, Indraprastha Institute of Information Technology Delhi, New Delhi, India, 2019. [Google Scholar]
- Lavrenko, A.; Litchfield, B.; Woodward, G.; Pawson, S. Design and Evaluation of a Compact Harmonic Transponder for Insect Tracking. IEEE Microw. Wirel. Components Lett. 2020, 30, 445–448. [Google Scholar] [CrossRef]
- Lavrenko, A.; Cavers, J. Two-region model for harmonic radar transponders. Electron. Lett. 2020, 56, 835–838. [Google Scholar] [CrossRef]
- Metcalf, J.I. Microstructure of Radar Echo Layers in the Clear Atmosphere. J. Atmos. Sci. 1975, 32, 362. [Google Scholar] [CrossRef][Green Version]
- Eaton, F.D.; McLaughlin, S.A.; Hines, J.R. A new frequency-modulated continuous wave radar for studying planetary boundary layer morphology. Radio Sci. 1995, 30, 75–88. [Google Scholar] [CrossRef]
- Dekker, P.L.; Bajaj, A.N.; Frasier, S. Radar and acoustic observations during VTMX fieldcampaign. In Proceedings of the 10th Conference on Mountain Meteorology, Park City, UT, USA, 17–21 June 2002. [Google Scholar]
- Richter, J.H.; Jensen, D.R.; Noonkester, V.R.; Kreasky, J.B.; Stimmann, M.W.; Wolf, W.W. Remote Radar Sensing: Atmospheric Structure and Insects. Science 1973, 180, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.; Bowers, J.; Laufenberg, E.; Storwold, D.; Mclaughlin, S. Possible detection of insects in an urban environment by a frequency modulated-continuous wave radar. In Proceedings of the 5th Symposium on the Urban Environment, Vancouver, BC, Canada, 23–26 August 2004; pp. 347–353. [Google Scholar]
- Contreras, R.F.; Frasier, S.J. Return from insects in the clear-air convective boundary layer. In Proceedings of the 2007 IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007. [Google Scholar] [CrossRef]
- Tahir, N.; Brooker, G. Recent developments and recommendations for improving harmonic radar tracking systems. In Proceedings of the 5th European Conference on Antennas and Propagation (EUCAP), Rome, Italy, 11–15 April 2011; pp. 1531–1535. [Google Scholar]
- Tahir, N.; Brooker, G. Toward the Development of Millimeter Wave Harmonic Sensors for Tracking Small Insects. IEEE Sens. J. 2015, 15, 5669–5676. [Google Scholar] [CrossRef]
- Storz, G.; Lavrenko, A. Compact Low-cost FMCW Harmonic Radar for Short Range Insect Tracking. In Proceedings of the 2020 IEEE International Radar Conference, Washington, DC, USA, 28–30 April 2020. [Google Scholar]
- Aljaser, S. Miniaturization of a Low Power Harmonic Radar for UAV Use; Student Paper; Lund University Libraries: Lund, Sweden, 2019. [Google Scholar]
- Yang, J.; Shen, Y.; Cai, L.; Tong, K.F.; Lim, K.S.; Reynolds, A.; Rawlings, C. Development of Millimeter-wave FMCW Vertical-looking Entomological Radar System. In Proceedings of the 2019 International Workshop on Electromagnetics: Applications and Student Innovation Competition (iWEM), Qingdao, China, 18–20 September 2019; pp. 1–2. [Google Scholar]
- Noskov, A.; Zipf, A. Backend and frontend strategies for deployment of WebGIS services. In Proceedings of the Sixth International Conference on Remote Sensing and Geoinformation of the Environment (RSCy2018), Paphos, Cyprus, 26–29 March 2018. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Pollut. Res. 2020, 27, 33503–33515. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Reynolds, D.; Smith, A. Migratory and foraging movements in beneficial insects: A review of radar monitoring and tracking methods. Int. J. Pest Manag. 2004, 50, 225–232. [Google Scholar] [CrossRef]
- Hollaus, M.; Vreugdenhil, M. Radar Satellite Imagery for Detecting Bark Beetle Outbreaks in Forests. Curr. For. Rep. 2019, 5, 240–250. [Google Scholar] [CrossRef]
- Elis, V.R.; Almeida, E.R.; Porsani, J.L.; Stangari, M.C. Ground-penetrating radar, resistivity, and induced polarization applied in forensic research in tropical soils. In Proceedings of the 18th International Conference on Ground Penetrating Radar, Golden, CO, USA, 14–19 June 2020; pp. 224–227. [Google Scholar] [CrossRef]
- Wood, C.R.; O’Connor, E.J.; Hurley, R.A.; Reynolds, D.R.; Illingworth, A.J. Cloud-radar observations of insects in the UK convective boundary layer. Meteorol. Appl. 2009, 16, 491–500. [Google Scholar] [CrossRef]
- Chandra, A.S.; Kollias, P.; Giangrande, S.E.; Klein, S.A. Long-Term Observations of the Convective Boundary Layer Using Insect Radar Returns at the SGP ARM Climate Research Facility. J. Clim. 2010, 23, 5699–5714. [Google Scholar] [CrossRef]
- Egli, S.; Maier, F.; Bendix, J.; Thies, B. Vertical distribution of microphysical properties in radiation fogs—A case study. Atmos. Res. 2015, 151, 130–145. [Google Scholar] [CrossRef]
- Huggard, P.G.; Oldfield, M.L.; Moyna, B.P.; Ellison, B.N.; Matheson, D.N.; Bennett, A.J.; Gaffard, C.; Oakley, T.; Nash, J. 94 GHz FMCW cloud radar. Millimetre Wave Terahertz Sens. Technol. 2008, 7117. [Google Scholar] [CrossRef]
- Bennett, A.; Gaffard, C.; Oakley, T.; Huggard, P.; Moyna, B. Cloud Radar- Initial Measurements from the 94GHz FMCW Radar. In Proceedings of the 8th International Symposium on Tropospheric Profiling, Delft, The Netherlands, 19–23 October 2009. [Google Scholar]
- Luke, E.P.; Kollias, P.; Johnson, K.L.; Clothiaux, E.E. A Technique for the Automatic Detection of Insect Clutter in Cloud Radar Returns. J. Atmos. Ocean. Technol. 2008, 25, 1498–1513. [Google Scholar] [CrossRef]
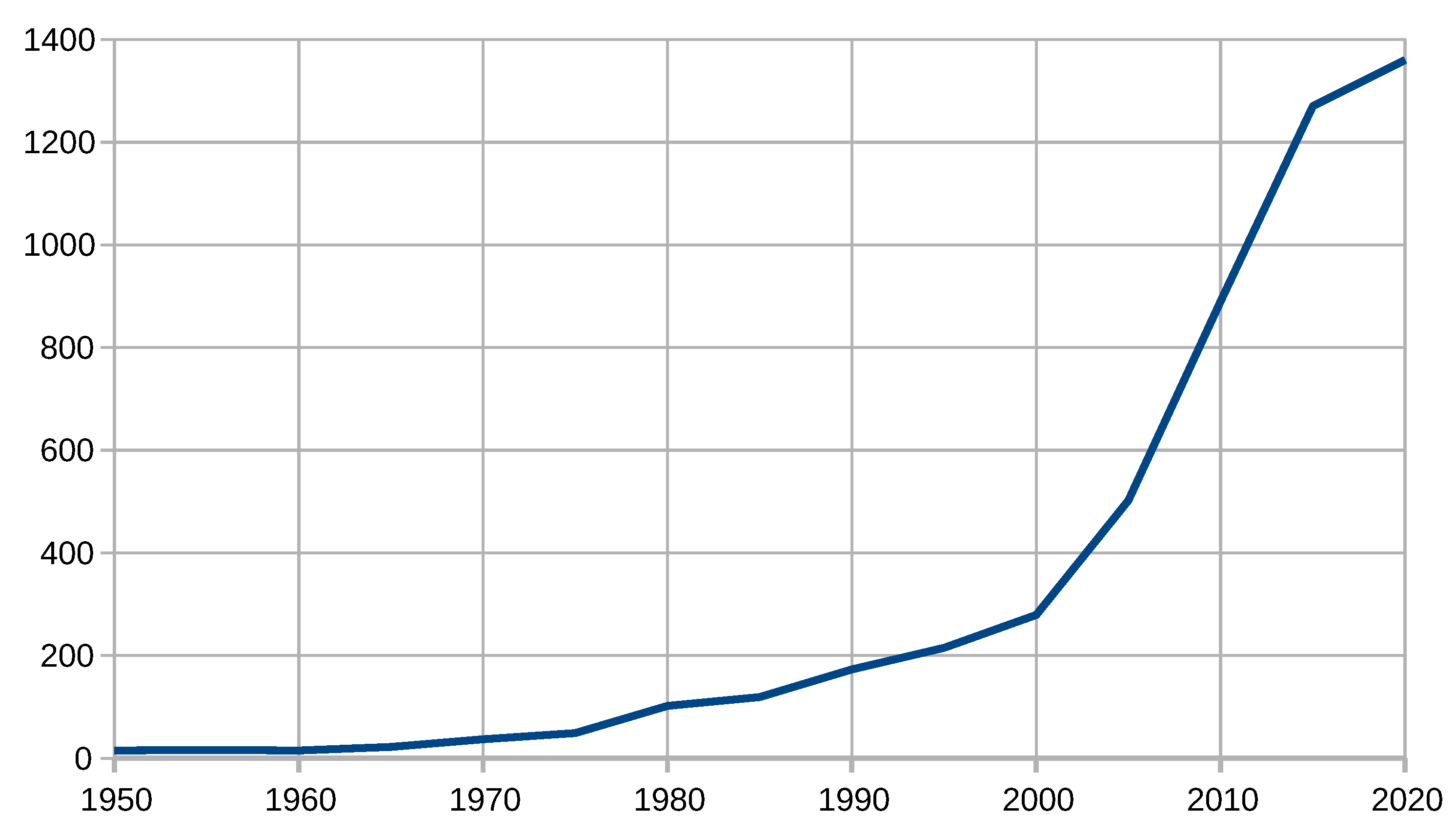
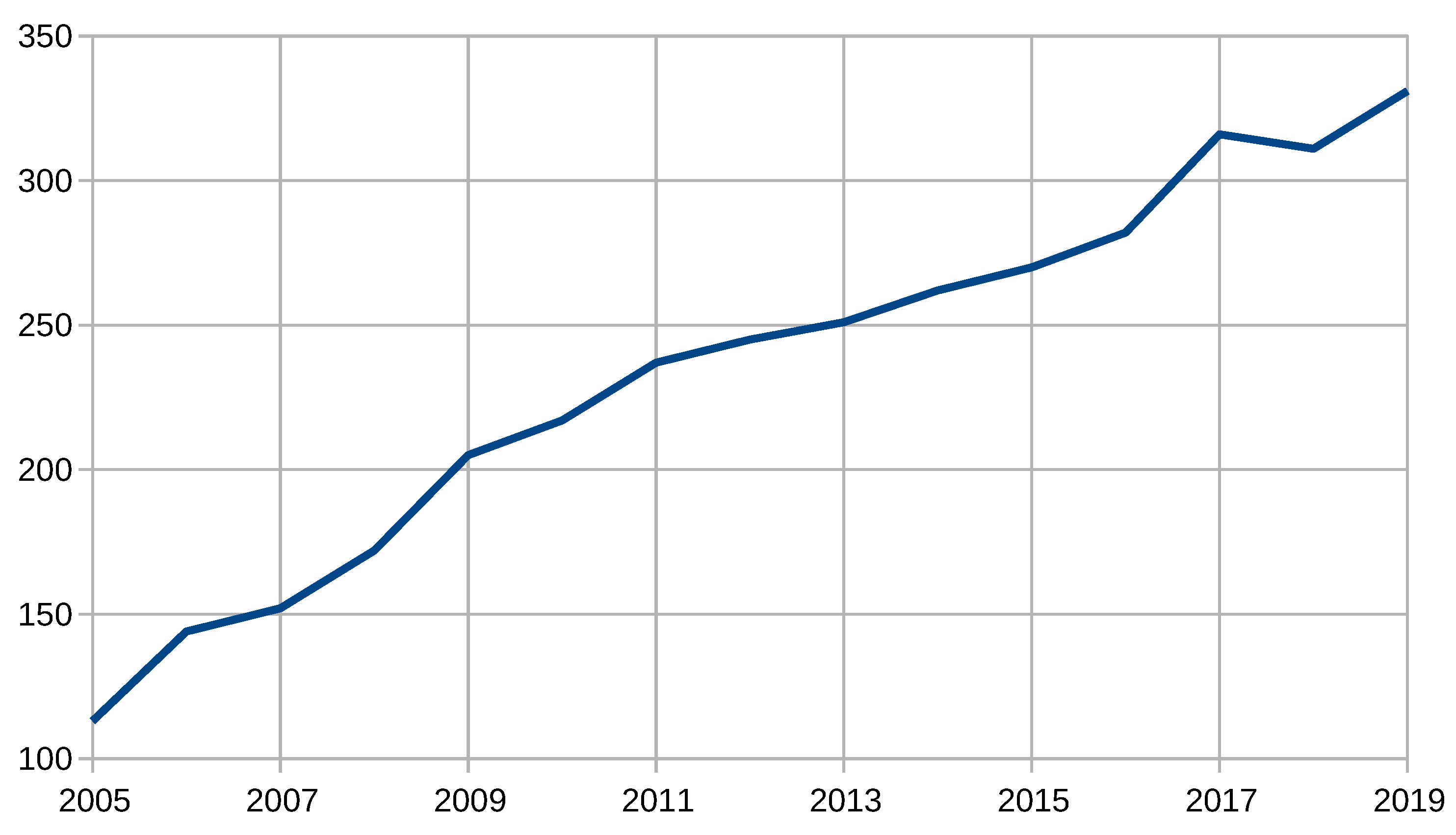
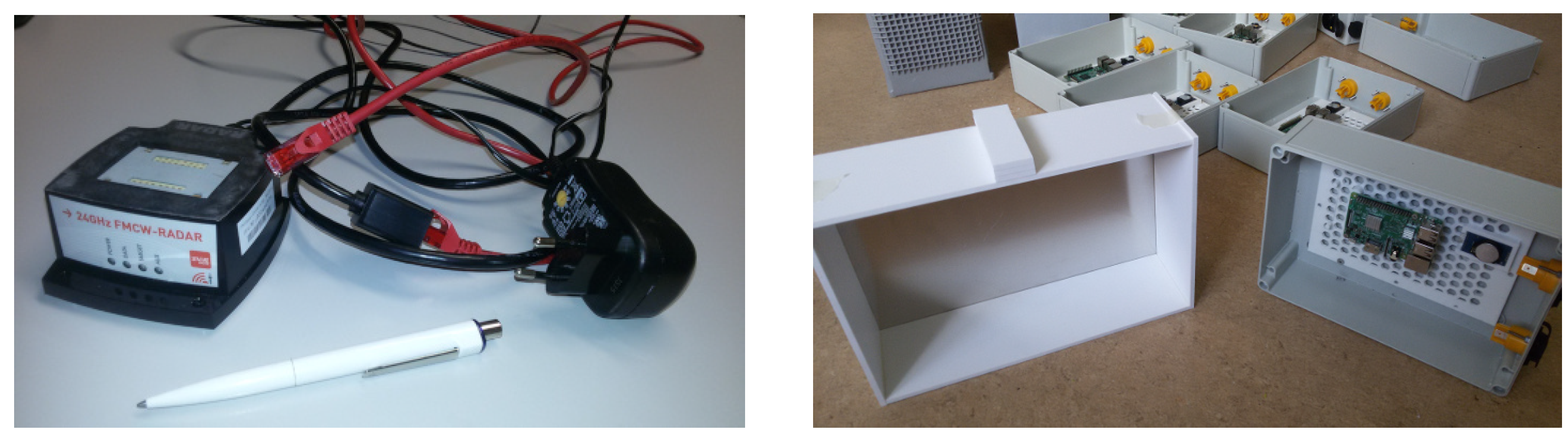
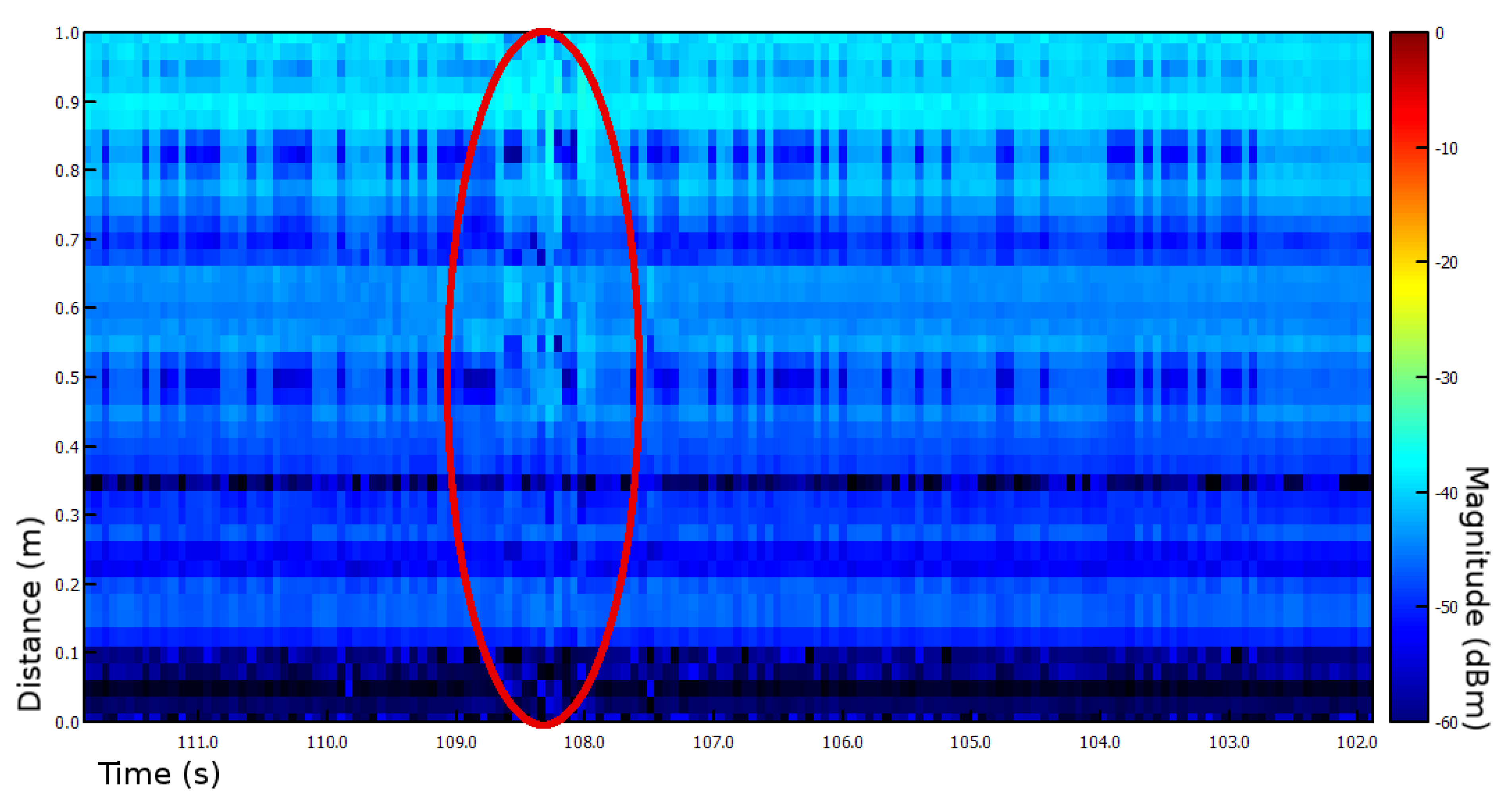
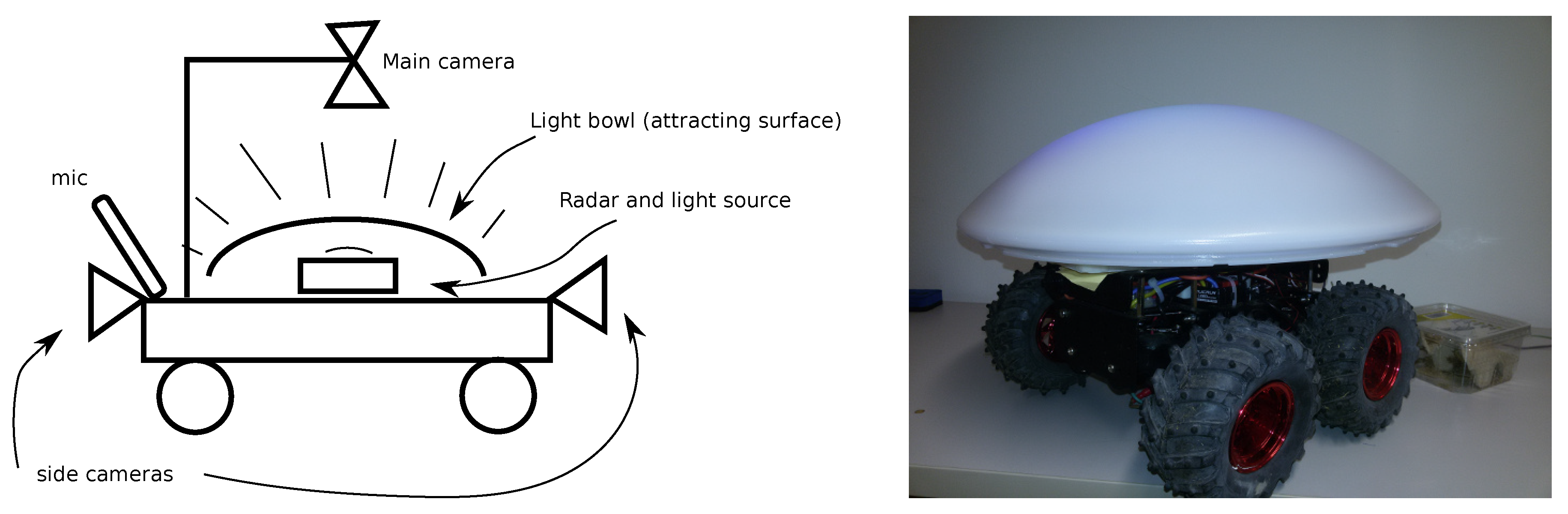
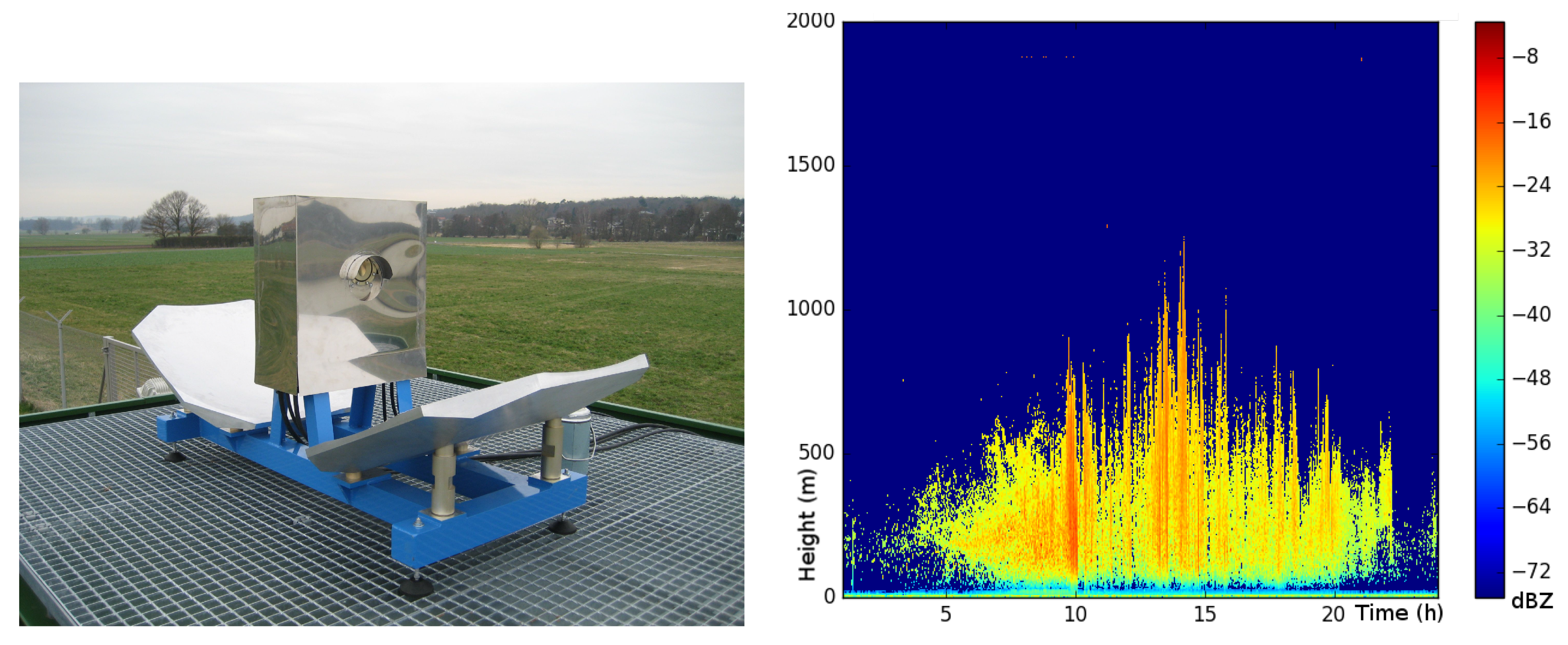
| Years (1946–2020) | Amount | Years (2005–2019) | Amount |
|---|---|---|---|
| 1946–1950 | 15 | 2005 | 113 |
| 1951–1955 | 16 | 2006 | 144 |
| 1956–1960 | 15 | 2007 | 152 |
| 1961–1965 | 22 | 2008 | 172 |
| 1966–1970 | 37 | 2009 | 205 |
| 1971–1975 | 49 | 2010 | 217 |
| 1976–1980 | 102 | 2011 | 237 |
| 1981–1985 | 119 | 2012 | 245 |
| 1986–1990 | 173 | 2013 | 251 |
| 1991–1995 | 215 | 2014 | 262 |
| 1996–2000 | 279 | 2015 | 270 |
| 2001–2005 | 502 | 2016 | 282 |
| 2006–2010 | 890 | 2017 | 316 |
| 2011–2015 | 1270 | 2018 | 311 |
| 2016–2020 | 1360 | 2019 | 331 |
| Radar Orientation | Pulsed | FMCW |
|---|---|---|
| High-Altitude: Scanning Large Areas | [90,93,137,138,139,140,141] | |
| High-Altitude: Vertically Oriented (including VLR) | [135,150,151,157,158,160,163,164,166] | [192,193,194,199,205,206] |
| Low-Altitude: Harmonic | [169,172,173,174,175,176,177,178,179,180,181,183] | [178,182,195,196,197] |
| Pulsed | FMCW | Scanning | Vertical | Harmonic | C-Band | S-Band | X-Band | Ku-Band | Ka-Band | |
|---|---|---|---|---|---|---|---|---|---|---|
| All-time | 5100 | 50 | 29,900 | 500 | 2890 | 440 | 210 | 290 | 20 | 30 |
| Since 2015 | 1240 | 20 | 8331 | 140 | 1010 | 110 | 80 | 90 | 10 | 10 |
| % since 2015 | 24 | 40 | 28 | 28 | 35 | 25 | 38 | 31 | 50 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noskov, A.; Bendix, J.; Friess, N. A Review of Insect Monitoring Approaches with Special Reference to Radar Techniques. Sensors 2021, 21, 1474. https://doi.org/10.3390/s21041474
Noskov A, Bendix J, Friess N. A Review of Insect Monitoring Approaches with Special Reference to Radar Techniques. Sensors. 2021; 21(4):1474. https://doi.org/10.3390/s21041474
Chicago/Turabian StyleNoskov, Alexey, Joerg Bendix, and Nicolas Friess. 2021. "A Review of Insect Monitoring Approaches with Special Reference to Radar Techniques" Sensors 21, no. 4: 1474. https://doi.org/10.3390/s21041474
APA StyleNoskov, A., Bendix, J., & Friess, N. (2021). A Review of Insect Monitoring Approaches with Special Reference to Radar Techniques. Sensors, 21(4), 1474. https://doi.org/10.3390/s21041474







