A Compact Multi-Distance DCS and Time Domain NIRS Hybrid System for Hemodynamic and Metabolic Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrument Description
2.2. DCS Data Analysis
2.3. Characterization Measurements
2.3.1. DCS System Behavior Over Time
- Stability: a long measurement, about 3 h, with integration time of 1 s, was performed on a liquid phantom to test the possible trend of variations in the retrieved and β parameters over time. The phantom was composed by distilled water, Intralipid and black India ink. The percentage of Intralipid and ink were selected to mimic cerebral optical parameters (nominal values = 10 cm−1, = 0.1 cm−1). The coefficient of variation (CV) was computed as the ratio between the standard deviation and the average value over all measurement times, for the two parameters and β.
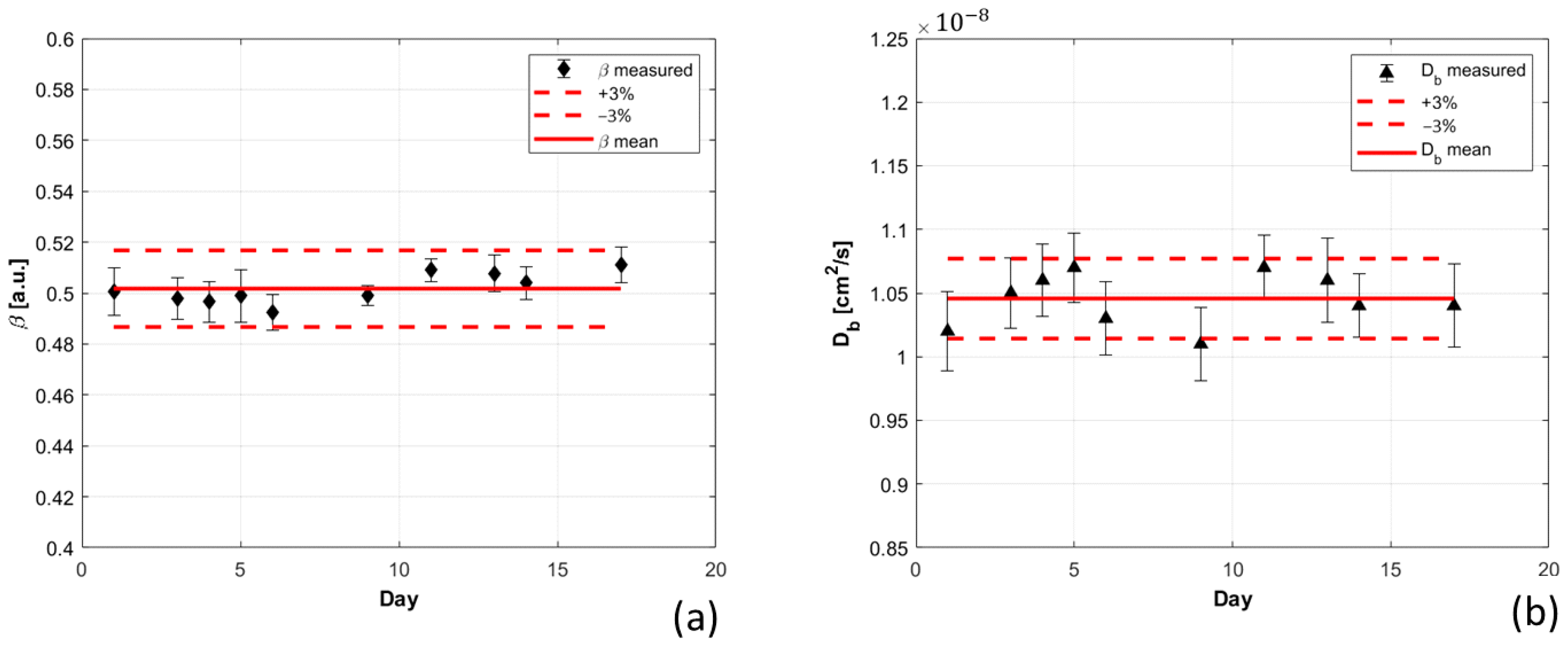
- Reproducibility: 30 measurements of 1 s integration time were repeated on the same liquid phantom, under the same experimental conditions, for 10 days. The liquid phantom used is a water-based solution of polydisperse microparticles (HemoPhotonics S.L., Barcelona, Spain) [21], it is not a biodegradable phantom, and therefore it does not change its composition over time, allowing us to reproduce the same measurements condition over different days. The was estimated using the mean optical parameters retrieved over the 10 days. To estimate the ability of our DCS system in reproducing similar results under the same experimental conditions, the CV was computed for the β and parameters retrieved on different days.
2.3.2. Variations with Optical Properties, Temperature, Viscosity, and Count Rate
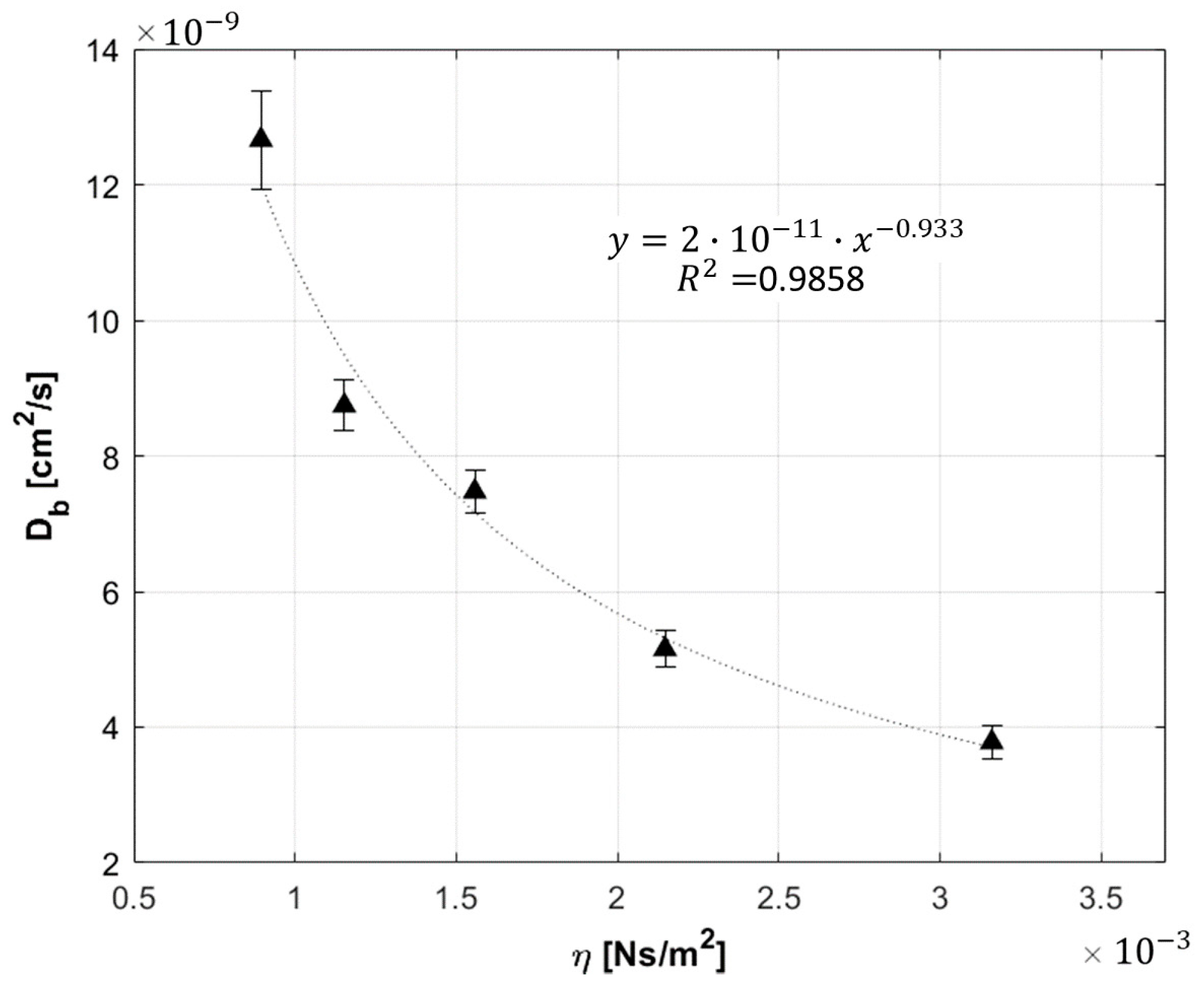
- Phantom viscosity: five liquid phantoms, made up of distilled water, lipofundin, ink, and glycerol were made. The glycerol concentration varied from 0 to 40% [24] in step of 10. Increasing glycerol concentration, the viscosity of the phantom became higher, and from Einstein equation (Equation (2)), we expect a decreasing . The inter-fiber distance was 1 cm, and only one detection channel was exploited.
- Phantom optical parameters: eight liquid phantoms were prepared with different concentrations of Intralipid and black India ink, to change and , respectively. Measurements were first analyzed considering the average values of optical properties over all the phantoms, and then reanalyzed using the proper optical properties measured for each phantom with TD-NIRS instrument. The two results were compared, highlighting the errors done in the estimation of the parameter when wrong optical coefficients were used.
- Phantom temperature: the liquid phantom, made up of distilled water, Intralipid, and black India ink (0% glycerol), was heated up from 20 to 40 °C (in step of 10 °C). was estimated using the optical properties measured at each temperature. From Einstein relation (Equation (2)), we expect it to increase with temperature.
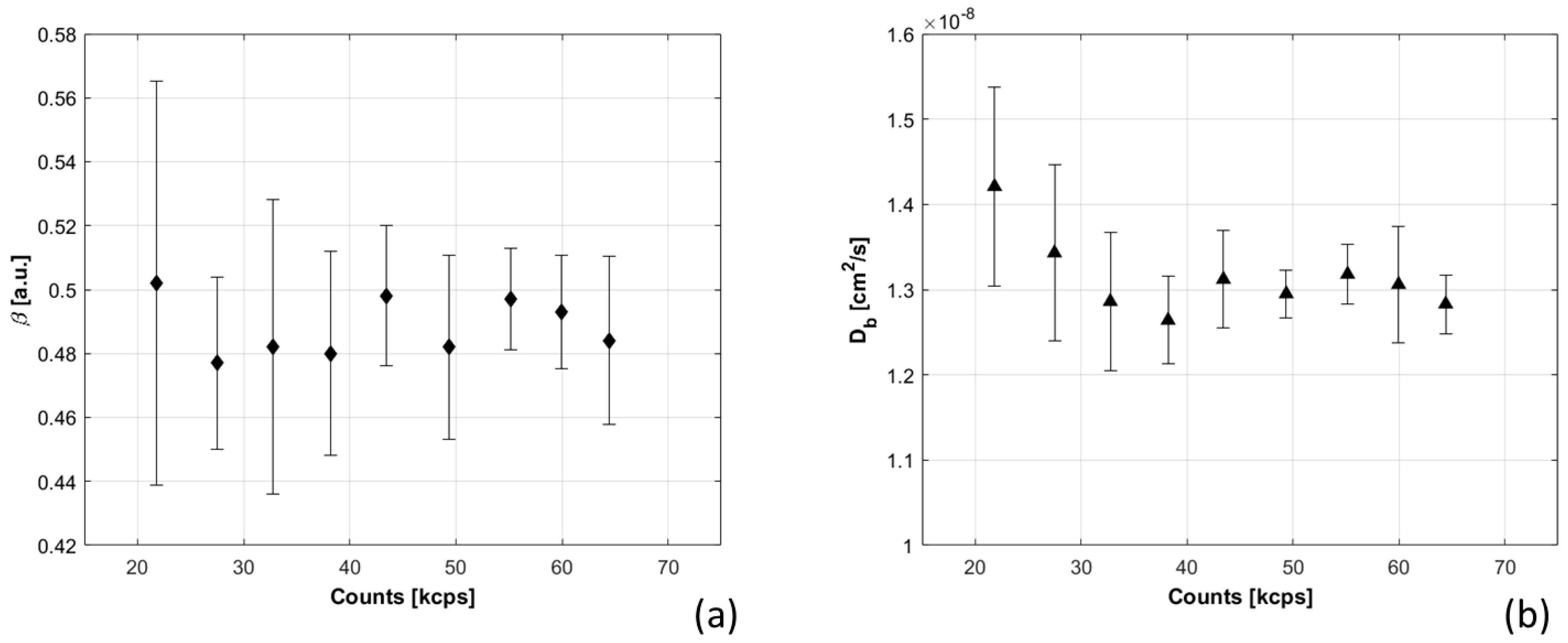
- Count rates: the probe was positioned on a liquid phantom, and measurements were acquired changing the power emitted by the laser. The total count rate for each detection channel was increased, and the correspondent estimated.
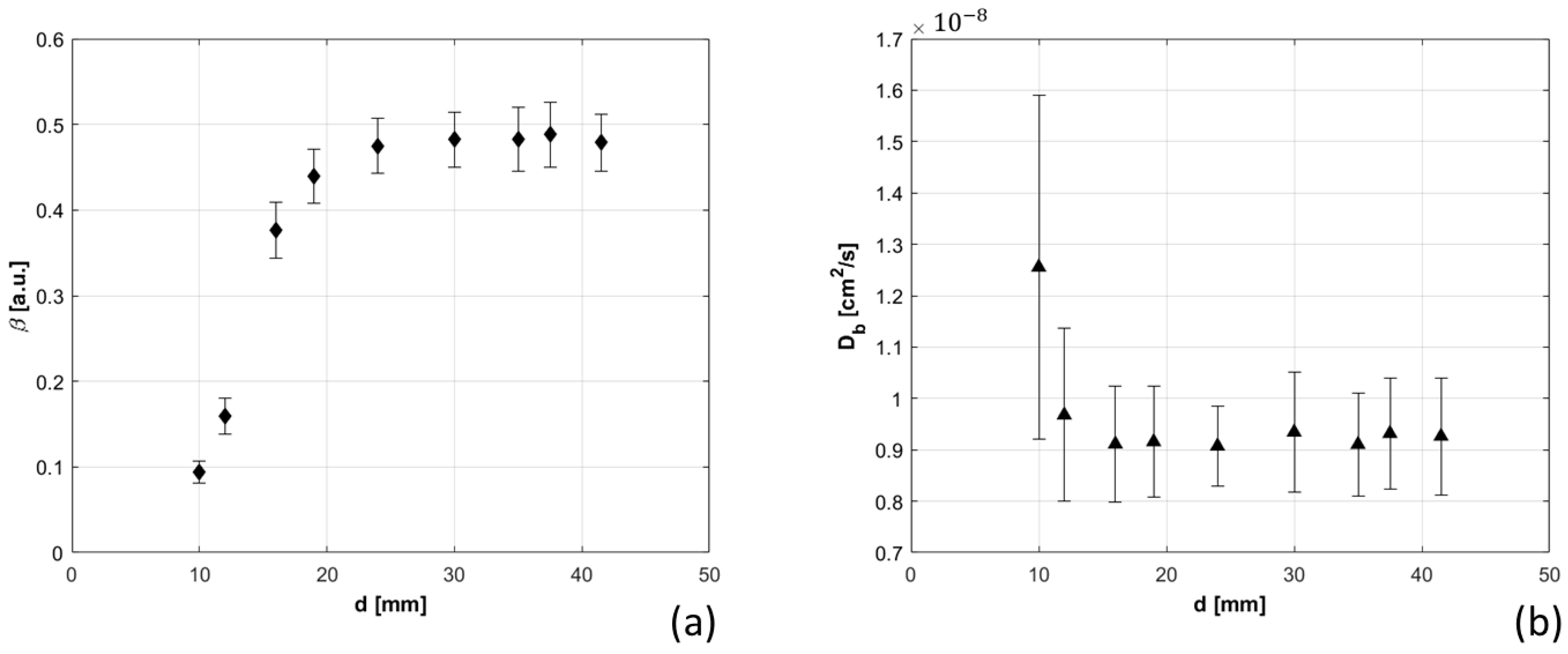
2.3.3. Bilayer Measurements
2.3.4. TD-NIRS Signal Crosstalk
2.4. In Vivo Measurements
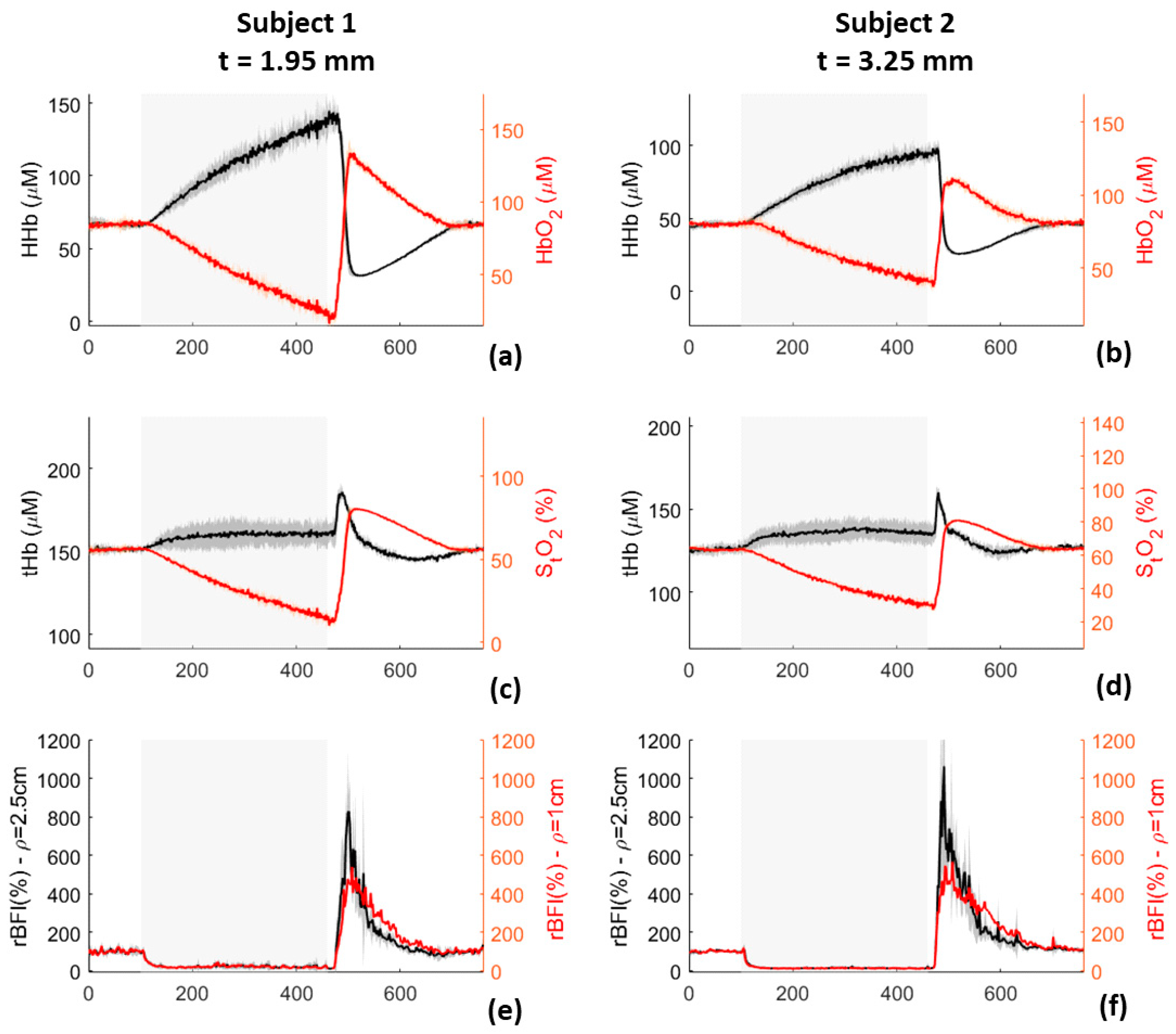
2.4.1. Venous Occlusion
2.4.2. Arterial Oclusion
3. Results
3.1. Characterization Measurements
3.1.1. Stability
3.1.2. Reproducibility
3.1.3. Phantom Viscosity
3.1.4. Phantom Optical Parameters
3.1.5. Phantom Temperature
3.1.6. Total Count Rates
3.1.7. Bilayer Measurements
3.1.8. Influence of TD-NIRS Signal
3.1.9. Venous Occlusion
3.1.10. Arterial Occlusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Carli, A.; Andresen, B.; Giovannella, M.; Durduran, T.; Contini, D.; Spinelli, L.; Weigel, U.M.; Passera, S.; Pesenti, N.; Mosca, F.; et al. Cerebral oxygenation and blood flow in term infants during postnatal transition: BabyLux project. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F648–F653. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.; Contini, D.; Pifferi, A.; Caffini, M.; Re, R.; Zucchelli, L.; Spinelli, L. Time domain functional NIRS imaging for human brain mapping. Neuroimage 2014, 85, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pirovano, I.; Contini, D.; Spinelli, L.; Torricelli, A. Time domain near infrared spectroscopy device for monitoring muscle oxidative metabolism: Custom probe and in vivo applications. Sensors 2018, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S.; Sassaroli, A. Near-infrared optical mammography for breast cancer detection with intrinsic contrast. Ann. Biomed. Eng. 2012, 40, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef]
- Buckley, E.M.; Parthasarathy, A.B.; Grant, P.E.; Yodh, A.G.; Franceschini, M.A. Diffuse correlation spectroscopy for measurement of cerebral blood flow: Future prospects. Neurophotonics 2014, 1, 011009. [Google Scholar] [CrossRef]
- Busch, D.R.; Balu, R.; Baker, W.B.; Guo, W.; He, L.; Diop, M.; Milej, D.; Kavuri, V.; Amendolia, O.; Lawrence, K.S.; et al. Detection of Brain Hypoxia Based on Noninvasive Optical Monitoring of Cerebral Blood Flow with Diffuse Correlation Spectroscopy. Neurocrit. Care 2019, 30, 72–80. [Google Scholar] [CrossRef]
- Giovannella, M.; Andresen, B.; Andersen, J.B.; El-Mahdaoui, S.; Contini, D.; Spinelli, L.; Torricelli, A.; Greisen, G.; Durduran, T.; Weigel, U.M.; et al. Validation of diffuse correlation spectroscopy against 15O-water PET for regional cerebral blood flow measurement in neonatal piglets. J. Cereb. Blood Flow Metab. 2020, 40, 2055–2065. [Google Scholar] [CrossRef]
- Diop, M.; Verdecchia, K.; Lee, T.-Y.; Lawrence, K.S. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed. Opt. Express 2011, 2, 2068–2081. [Google Scholar] [CrossRef]
- Carp, S.A.; Farzam, P.; Redes, N.; Hueber, D.M.; Franceschini, M.A. Combined multi-distance frequency domain and diffuse correlation spectroscopy system with simultaneous data acquisition and real-time analysis. Biomed. Opt. Express 2017, 89, 3993–4006. [Google Scholar] [CrossRef]
- Cortese, L.; Presti, G.L.; Zanoletti, M.; Buttafava, M.; Renna, M.; Contini, D.; Mora, A.D.; Pifferi, A.; Taroni, P.; Tosi, A.; et al. LUCA device: A multi-wavelength time-resolved spectroscopy and diffuse correlation spectroscopy device with an integrated clinical ultrasound module/probe. In Biophotonics Congress: Biomedical Optics 2020 (Translational, Microscopy, OCT, OTS, BRAIN); OSA Technical Digest, Paper SW4D.7; Optical Society of America: Washington, DC, USA, 2020. [Google Scholar]
- Giovannella, M.; Contini, D.; Pagliazzi, M.; Pifferi, A.; Spinelli, L.; Erdmann, R.; Donat, R.; Rocchetti, I.; Rehberger, M.; König, N.; et al. BabyLux device: A diffuse optical system integrating diffuse correlation spectroscopy and time-resolved near-infrared spectroscopy for the neuromonitoring of the premature newborn brain. Neurophotonics 2019, 6, 025007. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, K.; Diop, M.; Lee, T.-Y.; Lawrence, K.S. Quantifying the cerebral metabolic rate of oxygen by combining diffuse correlation spectroscopy and time-resolved near-infrared spectroscopy. J. Biomed. Opt. 2013, 18, 027007. [Google Scholar] [CrossRef] [PubMed]
- Lacerenza, M.; Buttafava, M.; Renna, M.; Mora, A.D.; Spinelli, L.; Zappa, F.; Pifferi, A.; Torricelli, A.; Tosi, A.; Contini, D. Wearable and wireless time-domainnear-infrared spectroscopy system for brainand muscle hemodynamic monitoring. Biomed. Opt. Express 2020, 11, 5934–5949. [Google Scholar] [CrossRef] [PubMed]
- Buttafava, M.; Martinenghi, E.; Tamborini, D.; Contini, D.; Mora, A.D.; Renna, M.; Torricelli, A.; Pifferi, A.; Zappa, F.; Tosi, A. A Compact Two-Wavelength Time-Domain NIRS System Based on SiPM and Pulsed Diode Lasers. IEEE Photon. J. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Amendola, C.; Pirovano, I.; Lacerenza, M.; Contini, D.; Spinelli, L.; Cubeddu, R.; Torricelli, A.; Re, R. Use of 3D printed PLA for diffuse optics. In Proceedings of the Optics InfoBase Conference Papers, Washington, DC, United States, 20–23 April 2020; p. JTh2A.16. [Google Scholar]
- Rice, S.O. Mathematical Analysis of Random Noise. Bell Syst. Tech. J. 1945, 23, 282–332. [Google Scholar] [CrossRef]
- Boas, D.A.; Yodh, A.G. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J. Opt. Soc. Am. A 1997, 14, 192–215. [Google Scholar] [CrossRef]
- Ackerson, B.J.; Dougherty, R.L.; Reguigui, N.M.; Nobbmann, U. Correlation transfer: Application of radiative transfer solution methods to photon correlation problems. J. Thermophys. Heat Transf. 1992, 6, 577–588. [Google Scholar] [CrossRef]
- Zerbini, P.E.; Vanoli, M.; Rizzolo, A.; Grassi, M.; Pimentel, R.M.D.; Spinelli, L.; Torricelli, A. Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 2015, 101, 58–65. [Google Scholar] [CrossRef]
- Pagliazzi, M.; Giovannella, M.; Weigel, U.M.; Pifferi, A.; Torricelli, A.; Durduran, T. Long-lasting, liquid phantom for diffuse optical and correlation spectroscopies. In Proceedings of the Biomedical Optics, Fort Lauderdale, FL, USA, 25–28 April 2016; p. JTu3A.25. [Google Scholar]
- Cheung, C.; Culver, J.P.; Takahashi, K.; Greenberg, J.H.; Yodh, A.G. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys. Med. Biol. 2001, 46, 2053–2065. [Google Scholar] [CrossRef]
- Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 1905, 322, 549–560. [Google Scholar] [CrossRef]
- Cortese, L.; Lo Presti, G.; Pagliazzi, M.; Contini, D.; Dalla Mora, A.; Pifferi, A.; Sekar, S.K.V.; Spinelli, L.; Taroni, P.; Zanoletti, M.; et al. Liquid phantoms for near-infrared and diffuse correlation spectroscopies with tunable optical and dynamic properties. Biomed. Opt. Express 2018, 9, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Del Bianco, S.; Martelli, F.; Cignini, F.; Zaccanti, G.; Pifferi, A.; Torricelli, A.; Bassi, A.; Taroni, P.; Cubeddu, R. Liquid phantom for investigating light propagation through layered diffusive media. Opt. Express 2004, 12, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.N.; Kumar, M. Experimental determination of the kinematic viscosity of glycerol-water mixtures. Proc. R. Soc. Lond. A 1994, 444, 573–581. [Google Scholar]
- De Blasi, R.A.; Ferrari, M.; Natali, A.; Conti, G.; Mega, A.; Gasparetto, A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J. Appl. Physiol. 1994, 76, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Baker, W.B.; Parthasarathy, A.B.; Ko, T.S.; Wang, D.; Schenkel, S.; Durduran, T.; Li, G.; Yodh, A.G. Calibration of diffuse correlation spectroscopy blood flow index with venous-occlusion diffuse optical spectroscopy in skeletal muscle. J. Biomed. Opt. 2015, 20, 125005. [Google Scholar] [CrossRef]
- He, L.; Baker, W.B.; Milej, D.; Kavuri, V.C.; Mesquita, R.C.; Busch, D.R.; Abramson, K.; Jiang, J.Y.; Diop, M.; Lawrence, K.S.; et al. Noninvasive continuous optical monitoring of absolute cerebral blood flow in critically ill adults. Neurophotonics 2018, 5, 045006. [Google Scholar]
- Milej, D.; Abdalmalak, A.; Rajaram, A.; Lawrence, K.S. Direct assessment of extracerebral signal contamination on optical measurements of cerebral blood flow, oxygenation, and metabolism. Neurophotonics 2020, 7, 045002. [Google Scholar] [CrossRef]
- Khalid, M.; Milej, D.; Rajaram, A.; Abdalmalak, A.; Morrison, L.; Diop, M.; Lawrence, K.S. Development of a stand-alone DCS system for monitoring absolute cerebral blood flow. Biomed. Opt. Express 2019, 10, 4607–4620. [Google Scholar] [CrossRef]
- Irwin, D.; Dong, L.; Shang, Y.; Cheng, R.; Kudrimoti, M.; Stevens, S.D.; Yu, G. Influences of tissue absorption and scattering on diffuse correlation spectroscopy blood flow measurements. Biomed. Opt. Express 2011, 2, 1969–1985. [Google Scholar] [CrossRef]
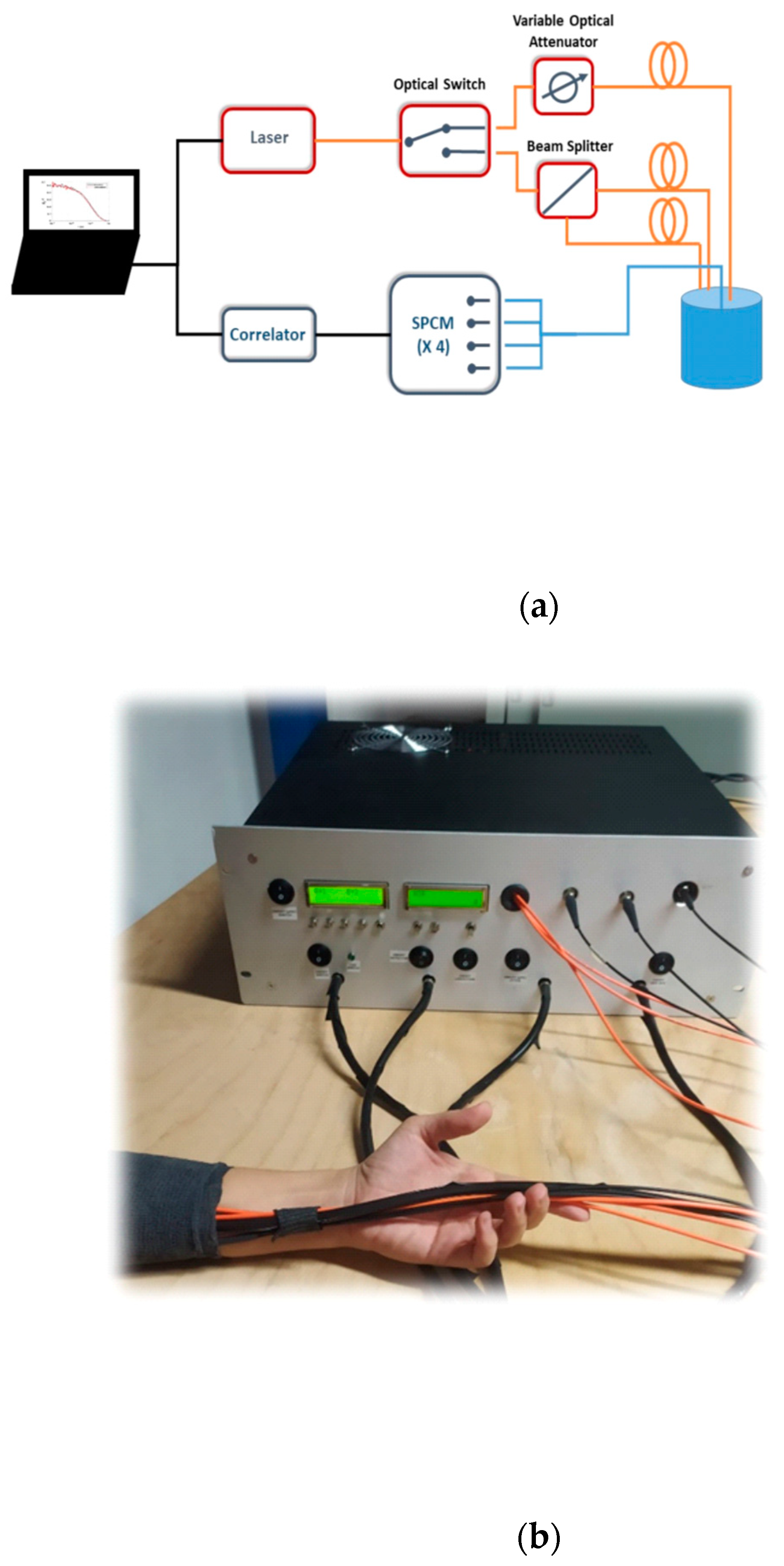
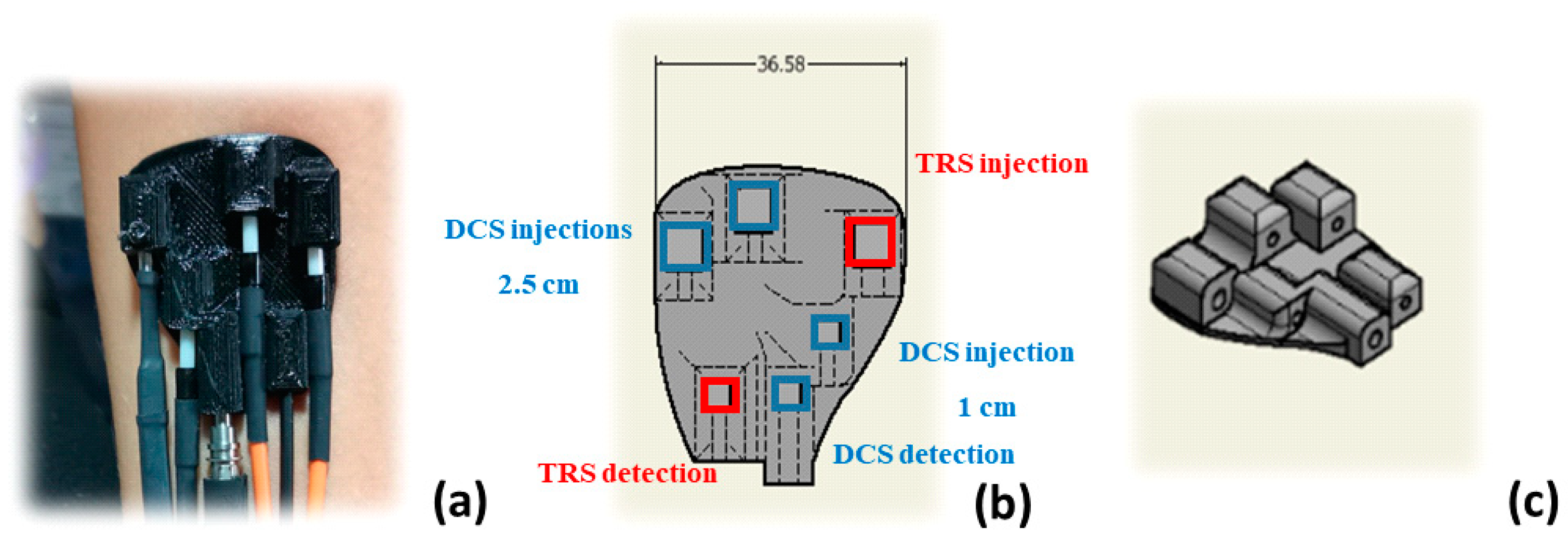

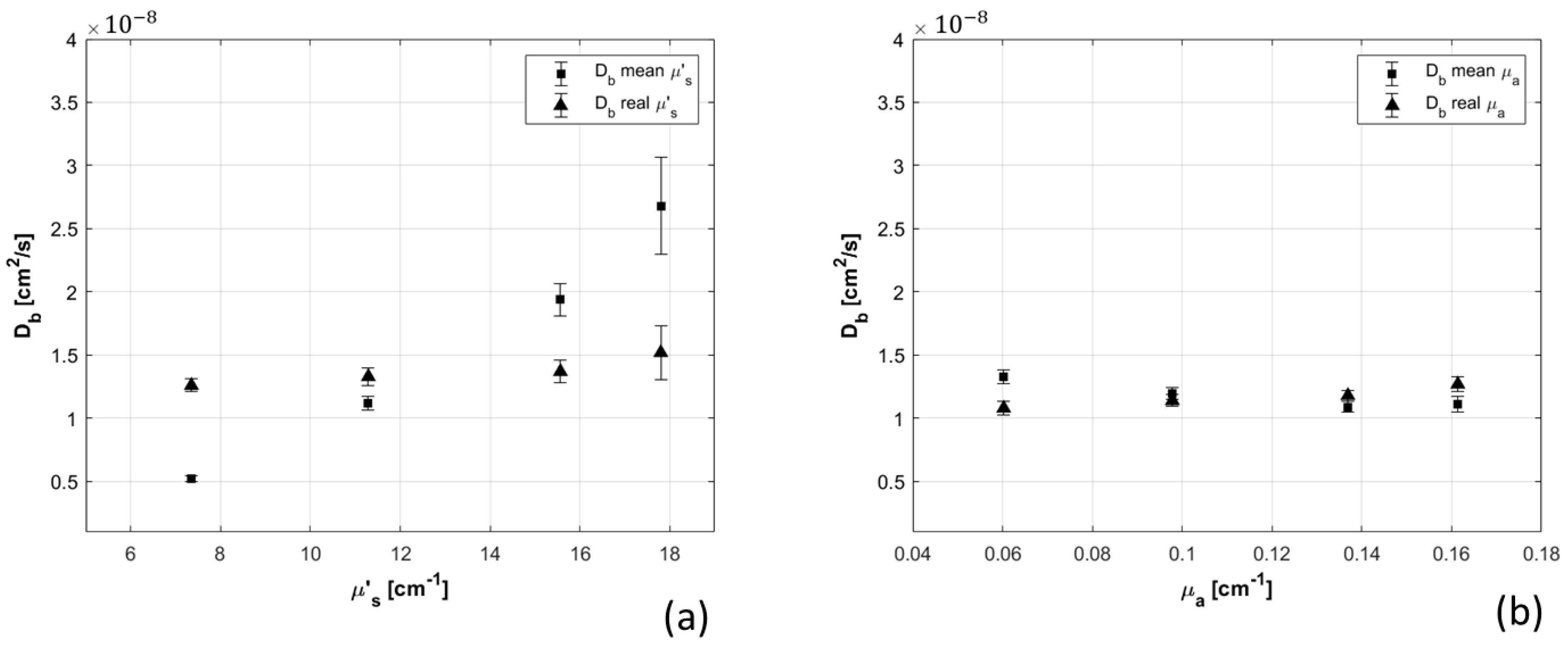



| Phantom # | µ’s [cm−1] | µa [cm−1] | Error µ’s [%] | Average Optical Values [cm−1] | Error µa [%] | Error [%] |
|---|---|---|---|---|---|---|
| 0 | 10.91 | 0.047 | 0 | 0.11 | −45 | −23 |
| 1 | 10.83 | 0.089 | 0 | 0.11 | −11 | −4 |
| 2 | 10.84 | 0.130 | 0 | 0.11 | 23 | 7 |
| 3 | 10.85 | 0.159 | 0 | 0.11 | 45 | 12 |
| 4 | 6.84 | 0.094 | −41 | 12.58 | 0 | 58 |
| 5 | 10.79 | 0.088 | 10 | 12.58 | 0 | 16 |
| 6 | 15.22 | 0.096 | 23 | 12.58 | 0 | −41 |
| 7 | 17.72 | 0.097 | 41 | 12.58 | 0 | −76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amendola, C.; Lacerenza, M.; Buttafava, M.; Tosi, A.; Spinelli, L.; Contini, D.; Torricelli, A. A Compact Multi-Distance DCS and Time Domain NIRS Hybrid System for Hemodynamic and Metabolic Measurements. Sensors 2021, 21, 870. https://doi.org/10.3390/s21030870
Amendola C, Lacerenza M, Buttafava M, Tosi A, Spinelli L, Contini D, Torricelli A. A Compact Multi-Distance DCS and Time Domain NIRS Hybrid System for Hemodynamic and Metabolic Measurements. Sensors. 2021; 21(3):870. https://doi.org/10.3390/s21030870
Chicago/Turabian StyleAmendola, Caterina, Michele Lacerenza, Mauro Buttafava, Alberto Tosi, Lorenzo Spinelli, Davide Contini, and Alessandro Torricelli. 2021. "A Compact Multi-Distance DCS and Time Domain NIRS Hybrid System for Hemodynamic and Metabolic Measurements" Sensors 21, no. 3: 870. https://doi.org/10.3390/s21030870
APA StyleAmendola, C., Lacerenza, M., Buttafava, M., Tosi, A., Spinelli, L., Contini, D., & Torricelli, A. (2021). A Compact Multi-Distance DCS and Time Domain NIRS Hybrid System for Hemodynamic and Metabolic Measurements. Sensors, 21(3), 870. https://doi.org/10.3390/s21030870








