A Radiometric Technique for Monitoring the Desulfurization Process of Blister Copper
Abstract
1. Introduction
Desulfurization of Blister Copper
2. Methods and Materials
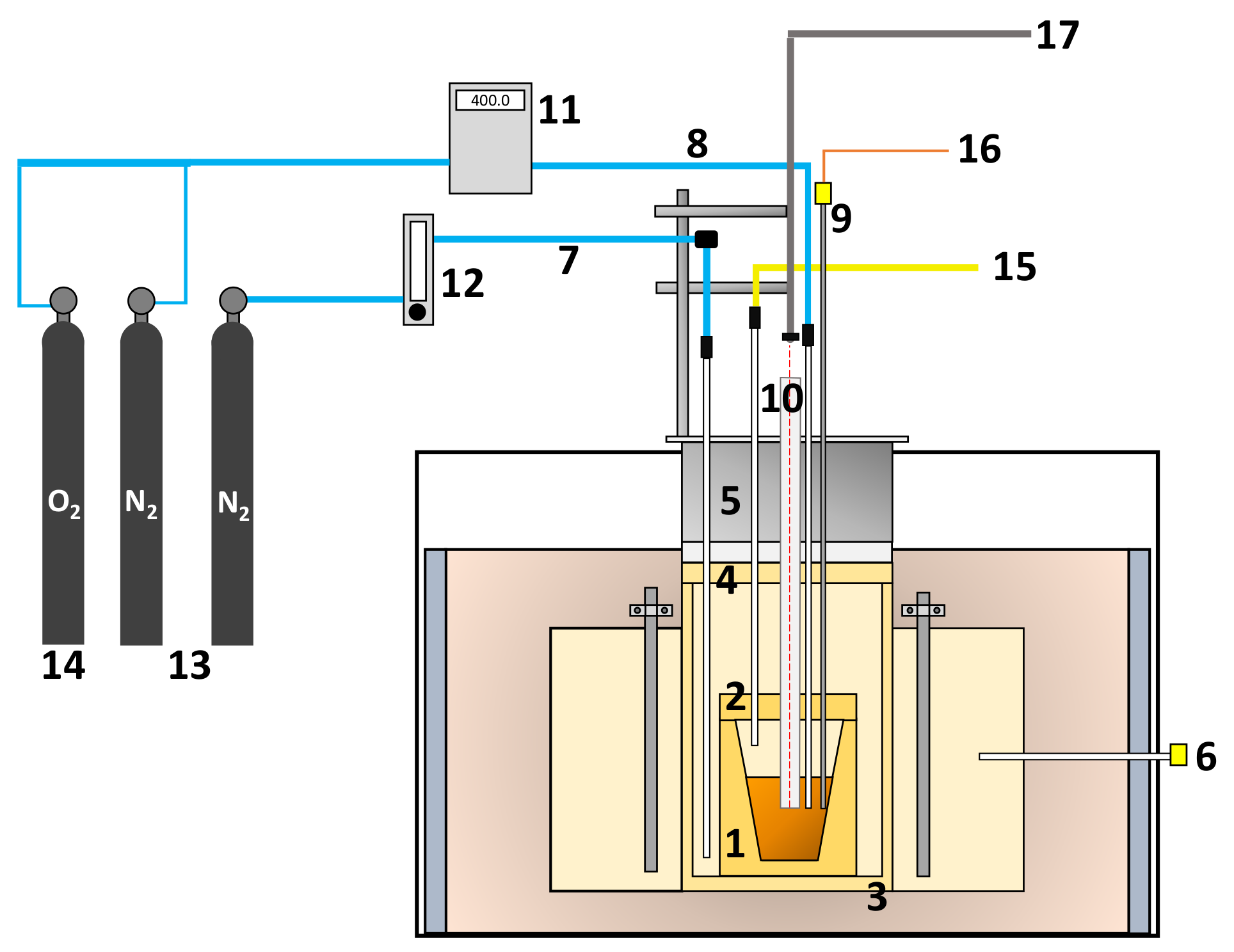
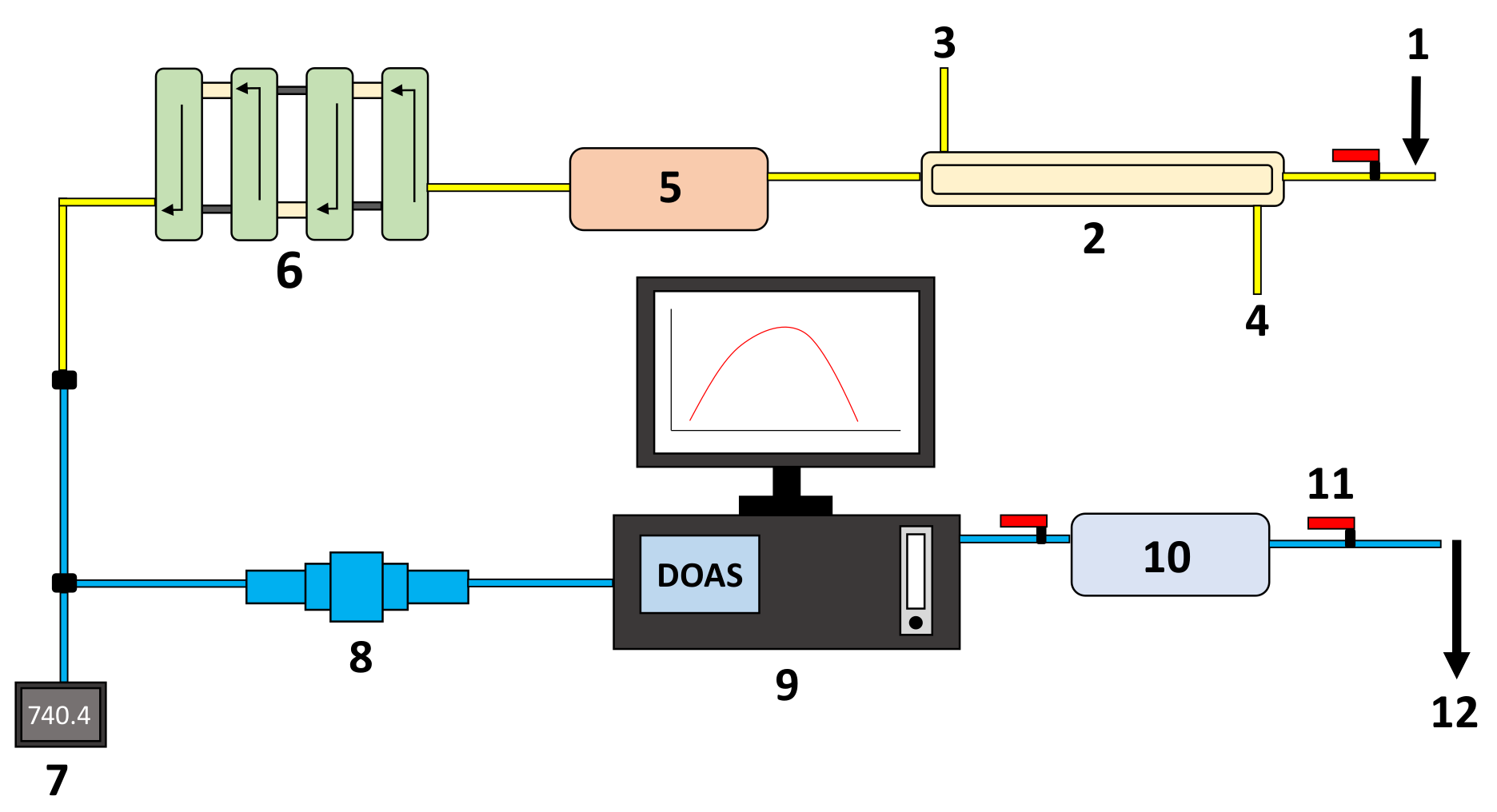
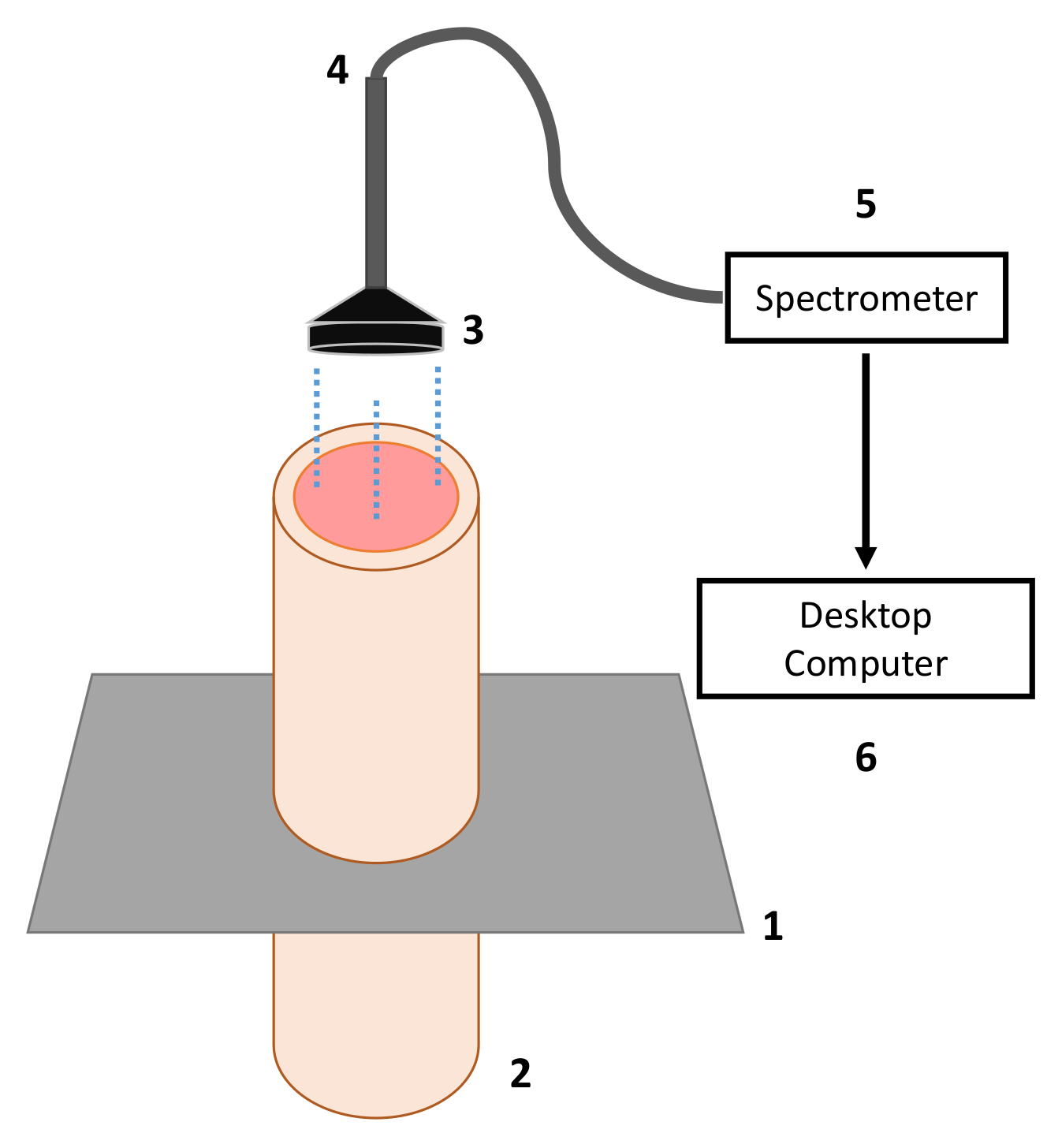
2.1. Experimental Setup
2.2. Experimental Design
2.3. Spectral Signal Processing
2.4. Error Sources and Limitations
3. Results and Discussion
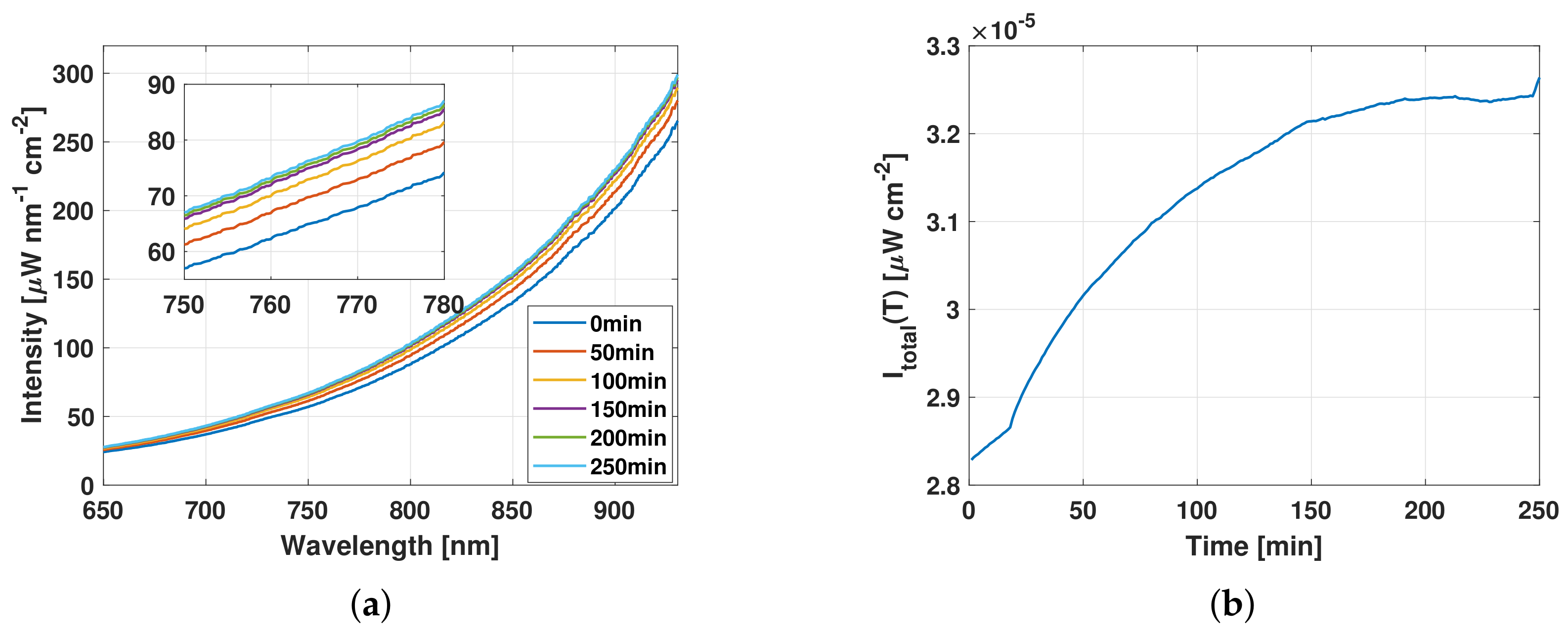
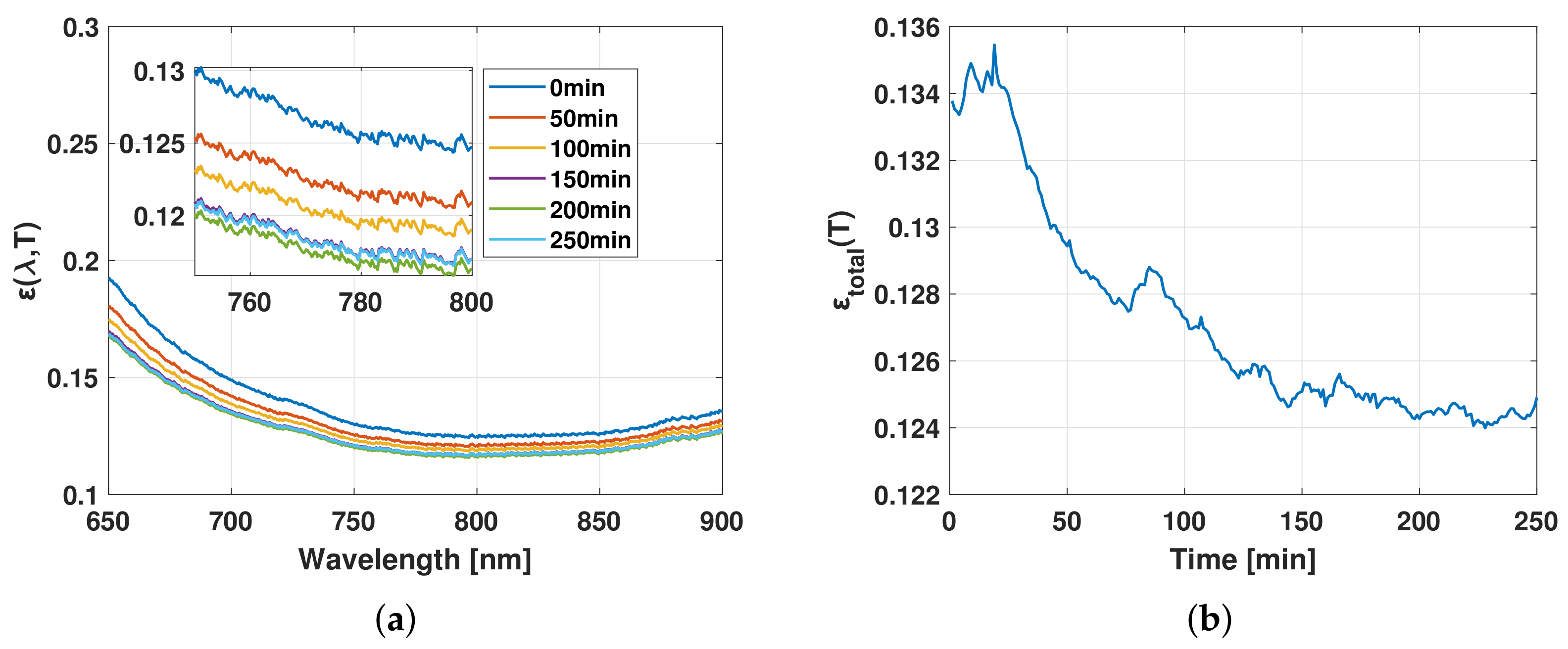
3.1. Results
3.2. Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moskalyk, R.; Alfantazi, A. Review of copper pyrometallurgical practice: Today and tomorrow. Miner. Eng. 2003, 16, 893–919. [Google Scholar] [CrossRef]
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until 2100 with regression and stock dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Wu, Y.; Li, L.; Li, B.; Pan, D.; Zuo, T. Environmental benefits of secondary copper from primary copper based on life cycle assessment in China. Resour. Conserv. Recycl. 2019, 146, 35–44. [Google Scholar] [CrossRef]
- Auping, W.; Pruyt, E.; Kwakkel, J.; Rademaker, M. Futures for Copper: Exploring Plausible Copper Price Scenarios and How to Act on Them; Report No 15/06/12; The Hague Center for Strategic Studies: The Hague, The Netherlands; Available online: https://www.researchgate.net/publication/256766279 (accessed on 10 January 2021).
- Araneda, H. Desde el Cobre a la Innovación: Roadmap Tecnológico 2015–2035; Fundación Chile, 2016; ISBN 978-956-8200-30-5. Available online: https://consejominero.cl/wp-content/uploads/2019/04/Roadmap-miner (accessed on 10 January 2021).
- Coudurier, L.; Hopkins, D.W.; Wilkomirsky, I. Fundamentals of Metallurgical Processes: International Series on Materials Science and Technology, 2nd ed.; Pergamon Press: Oxford, UK, 1985; Volume 27. [Google Scholar]
- Habashi, F. Handbook of Extractive Metallurgy; Volume 2, Whiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Nertherlands, 2011. [Google Scholar]
- Turkdogan, E. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Marín, M.; Toro, C.; Arias, L.; Balladares, E. Estimation of Spectral Emissivity and S/Cu Ratio From Emissions of Copper Concentrates at the Flash Smelting Process. IEEE Access 2019, 7, 103346–103353. [Google Scholar] [CrossRef]
- Arias, L.; Torres, S.; Toro, C.; Balladares, E.; Parra, R.; Loeza, C.; Villagrán, C.; Coelho, P. Flash smelting copper concentrates spectral emission measurements. Sensors 2018, 18, 2009. [Google Scholar] [CrossRef]
- Toro, C.; Arias, L.; Torres, S.; Sbarbaro, D. Flame spectra-temperature estimation based on a color imaging camera and a spectral reconstruction technique. Appl. Opt. 2014, 53, 6351–6361. [Google Scholar] [CrossRef]
- Díaz, W.; Toro, C.; Balladares, E.; Parra, V.; Coelho, P.; Reyes, G.; Parra, R. Spectral Characterization of Copper and Iron Sulfide Combustion: A Multivariate Data Analysis Approach for Mineral Identification on the Blend. Metals 2019, 9, 1017. [Google Scholar] [CrossRef]
- Parra, R.; Parra Sánchez, V.R.; Balladares, E.; Villagrán-Flores, C.; Loeza-Arqueros, C.; Pérez-Tello, M.; Torres-Inostroza, S.; Arias-Parada, L.; Sbárbaro-Hofer, D. Experimental study of molten matte spectrum and its relationship with composition. In Proceedings of the International Copper Conference, Kobe, Japan, 13–16 November 2016. [Google Scholar]
- Yañez, J.; Torres, S.; Sbarbaro, D.; Parra, R.; Saavedra, C. Analytical instrumentation for copper pyrometallurgy: Challenges and opportunities. IFAC-PapersOnLine 2018, 51, 251–256. [Google Scholar] [CrossRef]
- Bohnes, S.; Scherer, V.; Linka, S.; Neuroth, M. Spectral emissivity measurements of single mineral phases and ash deposits. In Proceedings of the ASME 2005 Summer Heat Transfer Conference collocated with the ASME 2005 Pacific Rim Technical Conference and Exhibition on Integration and Packaging of MEMS, NEMS, and Electronic Systems, American Society of Mechanical Engineers Digital Collection, San Francisco, CA, USA, 17–22 July 2005; pp. 175–182. [Google Scholar]
- Więcek, B.; Pacholski, K.; Świątczak, T.; Wittchen, W.; Borecki, M. Multispectral system for measuring the radiation parameters of steel slag during the discharge of steelworks furnace. In Proceedings of the 11th International Conference On Quantitative Infrared Thermography, QIRT, Quebec, QC, Canada, 24–29 June 2010. [Google Scholar]
- Strąkowski, R.; Pacholski, K.; Więcek, B.; Olbrycht, R.; Wittchen, W.; Borecki, M. Radiative parameters of steel slag for FeO content estimation using multispectral thermography system. Quant. Infrared Thermogr. J. 2014, 11, 222–232. [Google Scholar] [CrossRef]
- Strąkowski, R.; Pacholski, K.; Więcek, B.; Olbrycht, R.; Wittchen, W.; Borecki, M. Estimation of FeO content in the steel slag using infrared imaging and artificial neural network. Measurement 2018, 117, 380–389. [Google Scholar] [CrossRef]
- Sugiura, M.; Ootani, Y.; Nakashima, M. Radiation thermometry for high-temperature liquid stream at blast furnace. In Proceedings of the SICE Annual Conference 2011, Tokyo, Japan, 13–18 September 2011; pp. 472–475. [Google Scholar]
- Sugiura, M.; Otani, Y.; Nakashima, M.; Omoto, N. Continuous temperature measurement of liquid iron and slag tapped from a blast furnace. SICE J. Control Meas. Syst. Integr. 2014, 7, 147–151. [Google Scholar] [CrossRef][Green Version]
- Prietl, T.; Filzwieser, A.; Wallner, S. Productivity increase in a Peirce-Smith converter using the COP KIN and OPC system. In Converter and Fire Refining Practices, Proceedings of the Annual TMS Meeting, San Francisco, CA, USA, 13–17 February 2005; The Minerals, Metals, & Materials Society: Warrendale, PA, USA, 2005; pp. 177–190. [Google Scholar]
- Howell, J.R.; Menguc, M.P.; Siegel, R. Thermal Radiation Heat Transfer; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Cengel, Y. Heat and Mass Transfer: Fundamentals and Applications; McGraw-Hill Higher Education: New York, NY, USA, 2011. [Google Scholar]
- Medina, M.A.S.; Imris, I. Pirometalurgia del Cobre y Comportamiento de Sistemas Fundidos; Departamento de Ingeniería Metalúrgica, Universidad de Concepción: Concepción, Chile, 2002. [Google Scholar]
- Jalkanen, H. Phenomenology of the oxidation kinetics of molten cuprous sulphide and copper. Scand. J. Metall. 1981, 10, 257–262. [Google Scholar]
- Fukunaka, Y.; Nishikawa, K.; Sohn, H.; Asaki, Z. Desulfurization kinetics of molten copper by gas bubbling. Metall. Trans. B 1991, 22, 5–11. [Google Scholar] [CrossRef]
- Elliott, J.F. Phase relationships in the pyrometallurgy of copper. Metall. Mater. Trans. B 1976, 7, 17–33. [Google Scholar] [CrossRef]
- Mackey, P. The physical chemistry of copper smelting slags—A review. Can. Metall. Q. 1982, 21, 221–260. [Google Scholar] [CrossRef]
- Nagata, K.; Nagane, T.; Susa, M. Measurement of normal spectral emissivity of liquid copper. ISIJ Int. 1997, 37, 399–403. [Google Scholar] [CrossRef]
- Singham, J. Tables of emissivity of surfaces. Int. J. Heat Mass Transf. 1962, 5, 67–76. [Google Scholar] [CrossRef]
- Stubbs, C.M. A Spectro-Photometric Comparison of the Emissivity of Solid and Liquid Copper and of Liquid Silver at High Temperatures with that of a Full Radiator. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1913, 88, 195–205. [Google Scholar]
- Bidwell, C.C. A comparison of actual and black-body temperatures. Phys. Rev. 1914, 3, 439. [Google Scholar] [CrossRef]
- Burgess, G.K.; Waltenberg, R.G. The Emissivity of Metals and Oxides; US Government Printing Office: Washington, DC, USA, 1915.
- Burgess, G. The Estimation of Temperature of Copper by Means of Optical Pyrometers. Bull. Bur. Stand. 1909, 6, 111–119. [Google Scholar] [CrossRef]
- Otter, M. Temperaturabhängigkeit der optischen konstanten massiver metalle. Z. für Physik 1961, 161, 539–549. [Google Scholar] [CrossRef]
- Lange, K.W.; Schenck, H. Messung des spektralen Emissionsgrades von Metallen und Metallegierungen. Arch. für das Eisenhüttenwesen 1968, 39, 611–615. [Google Scholar] [CrossRef]
- Menzel, E.; Stahlknecht, J. Optical and thermo-electric thermometry of small metal crystals. Z. Angew. Physik. 1969, 26, 216–218. [Google Scholar]
- Dokko, W.; Bautista, R.G. The normal spectral emissivity measurements on liquid copper. Metall. Trans. B 1979, 10, 450–453. [Google Scholar] [CrossRef]
- Touloukian, Y.S.; Dewitt, D. Thermal Radiative Properties: Metallic Elements and Alloys; The TPRC Data Series IfI/Plenum Press: New York, NY, USA, 1970; Volume 7. [Google Scholar]

| Variable | Value | Unit |
|---|---|---|
| Blister copper mass | 3.67 | kg |
| Oxidizing gas flow | 0.42 | L/min |
| Nitrogen flow * | 10.00 | L/min |
| Suction flow | 4.00 | L/min |
| Temperature | 1225 | °C |
| Parameter | Value | Unit |
|---|---|---|
| Integration time of the spectrometer | 10 | ms |
| Spectrometer sampling time | 60 | s |
| Effective spectral range | 650–900 | nm |
| Collection area of the fiber probe | 7.50 × 10−1 | cm2 |
| Distance of the collimator to the melt | 68 | cm |
| Temperature sampling time | 60 | s |
| DOAS sampling time | 60 | s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, A.; Pérez, F.; Roa, M.; Sanhueza, I.; Rojas, H.; Parra, V.; Balladares, E.; Parra, R.; Torres, S. A Radiometric Technique for Monitoring the Desulfurization Process of Blister Copper. Sensors 2021, 21, 842. https://doi.org/10.3390/s21030842
Vásquez A, Pérez F, Roa M, Sanhueza I, Rojas H, Parra V, Balladares E, Parra R, Torres S. A Radiometric Technique for Monitoring the Desulfurization Process of Blister Copper. Sensors. 2021; 21(3):842. https://doi.org/10.3390/s21030842
Chicago/Turabian StyleVásquez, Alejandro, Francisco Pérez, Maximiliano Roa, Ignacio Sanhueza, Hugo Rojas, Victor Parra, Eduardo Balladares, Roberto Parra, and Sergio Torres. 2021. "A Radiometric Technique for Monitoring the Desulfurization Process of Blister Copper" Sensors 21, no. 3: 842. https://doi.org/10.3390/s21030842
APA StyleVásquez, A., Pérez, F., Roa, M., Sanhueza, I., Rojas, H., Parra, V., Balladares, E., Parra, R., & Torres, S. (2021). A Radiometric Technique for Monitoring the Desulfurization Process of Blister Copper. Sensors, 21(3), 842. https://doi.org/10.3390/s21030842










