Characterization of Anticipatory Postural Adjustments in Lateral Stepping: Impact of Footwear and Lower Limb Preference
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Instruments and Outcome Measures
2.4. Experimental Protocol
2.5. Data Analysis
2.6. Statistical Analysis
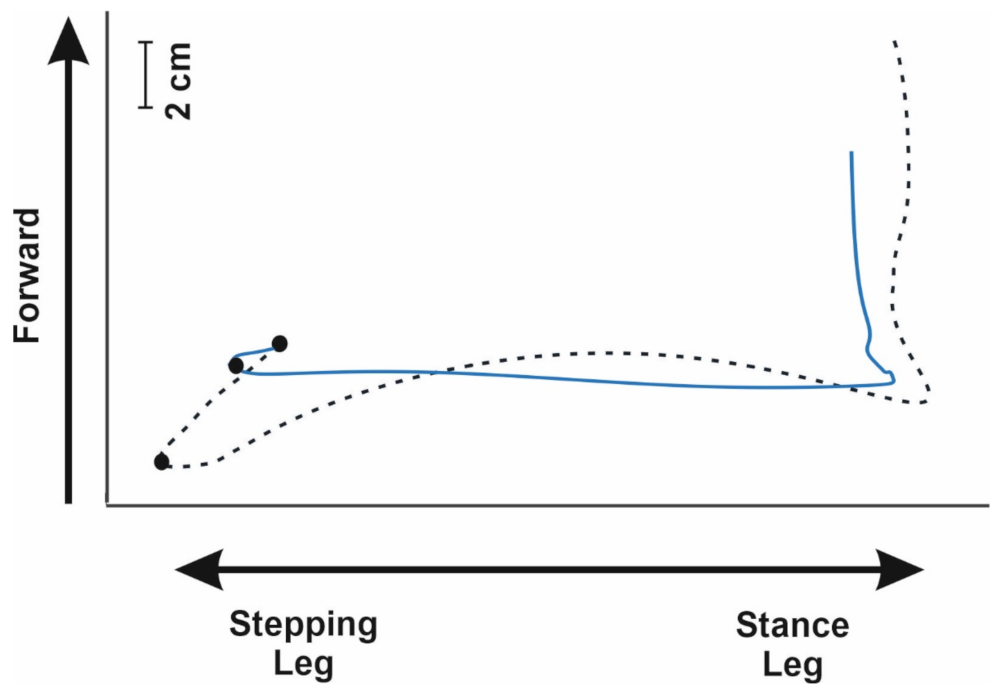
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouisset, S.; Do, M.-C. Posture, dynamic stability, and voluntary movement. Neurophysiol. Clin. Neurophysiol. 2008, 38, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, W. Effect of plantar desensitization on postural adjustments prior to step initiation. Gait Posture 2011, 34, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Mouchnino, L.; Blouin, J. When Standing on a Moving Support, Cutaneous Inputs Provide Sufficient Information to Plan the Anticipatory Postural Adjustments for Gait Initiation. PLoS ONE 2013, 8, e55081. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.-L. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Winter, D.; Ishac, M.; Gilchrist, L. Trajectory of the body COG and COP during initiation and termination of gait. Gait Posture 1993, 1, 9–22. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-047-054-914-8. [Google Scholar]
- Honeine, J.-L.; Schieppati, M.; Crisafulli, O.; Do, M.-C. The Neuro-Mechanical Processes That Underlie Goal-Directed Medio-Lateral APA during Gait Initiation. Front. Hum. Neurosci. 2016, 10, 445. [Google Scholar] [CrossRef]
- Lepers, R. The role of anticipatory postural adjustments and gravity in gait initiation. Exp. Brain Res. 1995, 107, 118–124. [Google Scholar] [CrossRef]
- Halliday, S.E.; Winter, D.A.; Frank, J.S.; Patla, A.E.; Prince, F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture 1998, 8, 8–14. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Horak, F.; Peterson, D. Anticipatory Postural Adjustment During Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients with Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1316–1324.e1. [Google Scholar] [CrossRef]
- Burleigh-Jacobs, A.; Horak, F.B.; Nutt, J.G.; Obeso, J.A. Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov. Disord. 1997, 12, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Remelius, J.G.; Hamill, J.; Kent-Braun, J.; Van Emmerik, R.E. Gait initiation in multiple sclerosis. Mot. Control. 2008, 12, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Crenna, P.; Frigo, C.A. A motor programme for the initiation of forward-oriented movements in humans. J. Physiol. 1991, 437, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Lyon, I.N.; Day, B.L. Control of frontal plane body motion in human stepping. Exp. Brain Res. 1997, 115, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Suzuki, T.; Yoshioka, S.; Fukashiro, S. Directional Control Mechanisms in Multidirectional Step Initiating Tasks. Front. Hum. Neurosci. 2020, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, P.; Anaka, E. Combined effects of speed and directional change on postural adjustments during gait initiation. J. Electromyogr. Kinesiol. 2011, 21, 734–741. [Google Scholar] [CrossRef]
- Glaister, B.C.; Bernatz, G.C.; Klute, G.; Orendurff, M.S. Video task analysis of turning during activities of daily living. Gait Posture 2007, 25, 289–294. [Google Scholar] [CrossRef]
- Vaugoyeau, M.; Viallet, F.; Mesure, S.; Massion, J. Coordination of axial rotation and step execution: Deficits in Parkinson’s disease. Gait Posture 2003, 18, 150–157. [Google Scholar] [CrossRef]
- Rogers, M.W.; Mille, M.-L. Lateral Stability and Falls in Older People. Exerc. Sport Sci. Rev. 2003, 31, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. The Role of Limb Movements in Maintaining Upright Stance: The “Change-in-Support” Strategy. Phys. Ther. 1997, 77, 488–507. [Google Scholar] [CrossRef] [PubMed]
- Patla, A.; Frank, J.; Winter, D.; Rietdyk, S.; Prentice, S.; Prasad, S. Age-related changes in balance control system: Initiation of stepping. Clin. Biomech. 1993, 8, 179–184. [Google Scholar] [CrossRef]
- King, L.; Horak, F.B. Lateral Stepping for Postural Correction in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2008, 89, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Luchies, C.W.; Wallace, D.; Pazdur, R.; Young, S.; DeYoung, A.J. Effects of Age on Balance Assessment Using Voluntary and Involuntary Step Tasks. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1999, 54, M140–M144. [Google Scholar] [CrossRef] [PubMed]
- Gray, V.L.; Yang, C.-L.; Fujimoto, M.; Waller, S.M.; Rogers, M.W. Stepping characteristics during externally induced lateral reactive and voluntary steps in chronic stroke. Gait Posture 2019, 71, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Brenière, Y.; Do, M.C.; Bouisset, S. Are Dynamic Phenomena Prior to Stepping Essential to Walking? J. Mot. Behav. 1987, 19, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Massion, J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 1992, 38, 35–56. [Google Scholar] [CrossRef]
- Sparto, P.J.; Jennings, J.R.; Furman, J.M.; Redfern, M.S. Lateral step initiation behavior in older adults. Gait Posture 2014, 39, 799–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lira, J.L.O.; Ugrinowitsch, C.; Coelho, D.B.; Teixeira, L.A.; De Lima-Pardini, A.C.; Magalhães, F.H.; Barbosa, E.R.; Horak, F.B.; Silva-Batista, C. Loss of presynaptic inhibition for step initiation in parkinsonian individuals with freezing of gait. J. Physiol. 2020, 598, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Delval, A.; Tard, C.; Defebvre, L. Why we should study gait initiation in Parkinson’s disease. Neurophysiol. Clin. Neurophysiol. 2014, 44, 69–76. [Google Scholar] [CrossRef]
- Massot, C.; Simoneau-Buessinger, E.; Agnani, O.; Donze, C.; Leteneur, S. Anticipatory postural adjustment during gait initiation in multiple sclerosis patients: A systematic review. Gait Posture 2019, 73, 180–188. [Google Scholar] [CrossRef]
- Dessery, Y.; Barbier, F.; Gillet, C.; Corbeil, P. Does lower limb preference influence gait initiation? Gait Posture 2011, 33, 550–555. [Google Scholar] [CrossRef]
- Vieira, M.F.; Sacco, I.D.C.N.; Nora, F.G.D.S.A.; Rosenbaum, D.; da Costa, P.H.L. Footwear and Foam Surface Alter Gait Initiation of Typical Subjects. PLoS ONE 2015, 10, e0135821. [Google Scholar] [CrossRef]
- Russo, Y.; Vannozzi, G. Anticipatory postural adjustments in forward and backward single stepping: Task variability and effects of footwear. J. Biomech. 2021, 122, 110442. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Stergiou, N. Does Footwear affect ankle Coordination Strategies? J. Am. Podiatr. Med. Assoc. 2004, 94, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hollander, K.; Petersen, E.; Zech, A.; Hamacher, D. Effects of barefoot vs. shod walking during indoor and outdoor conditions in younger and older adults. Gait Posture 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Cudejko, T.; Gardiner, J.; Akpan, A.; D’Août, K. Minimal footwear improves stability and physical function in middle-aged and older people compared to conventional shoes. Clin. Biomech. 2020, 71, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cudejko, T.; Gardiner, J.; Akpan, A.; D’Août, K. Minimal shoes improve stability and mobility in persons with a history of falls. Sci. Rep. 2020, 10, 21755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Paquette, M.R.; Zhang, S. A comparison of gait biomechanics of flip-flops, sandals, barefoot and shoes. J. Foot Ankle Res. 2013, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Menant, J.C.; Steele, J.; Menz, H.; Munro, B.J.; Lord, S.R. Effects of Footwear Features on Balance and Stepping in Older People. Gerontology 2008, 54, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Menant, J.C.; Steele, J.R.; Menz, H.B.; Munro, B.J.; Lord, S.R. Optimizing footwear for older people at risk of falls. J. Rehabil. Res. Dev. 2008, 45, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Õunpuu, S.; Winter, D.A. Bilateral electromyographical analysis of the lower limbs during walking in normal adults. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 429–438. [Google Scholar] [CrossRef]
- Yiou, E.; Do, M. Control of mediolateral stability during rapid step initiation with preferred and non-preferred leg: Is it symmetrical? Gait Posture 2010, 32, 145–147. [Google Scholar] [CrossRef]
- Riddick, D.A.; Jorge, M. Footwear: Foundation for Lower Extremity Orthoses. In Orthotics and Prosthetics in Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 164–182. [Google Scholar]
- Madigan, M.L.; Lloyd, E.M. Age and Stepping Limb Performance Differences During a Single-Step Recovery from a Forward Fall. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2005, 60, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.L.; Lloyd, E.M. Age-Related Differences in Peak Joint Torques During the Support Phase of Single-Step Recovery from a Forward Fall. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2005, 60, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Shirota, C.; Simon, A.M.; Kuiken, T.A. Trip recovery strategies following perturbations of variable duration. J. Biomech. 2014, 47, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Caderby, T.; Yiou, E.; Peyrot, N.; Bonazzi, B.; Dalleau, G. Detection of swing heel-off event in gait initiation using force-plate data. Gait Posture 2013, 37, 463–466. [Google Scholar] [CrossRef]
- Delval, A.; Moreau, C.; Bleuse, S.; Tard, C.; Ryckewaert, G.; Devos, D.; Defebvre, L. Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin. Neurophysiol. 2014, 125, 1675–1681. [Google Scholar] [CrossRef]
- Nurse, M.A.; Nigg, B. The effect of changes in foot sensation on plantar pressure and muscle activity. Clin. Biomech. 2001, 16, 719–727. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Peterson, D.; Mancini, M. The effect of tactile feedback on gait initiation in people with Parkinson’s disease: A pilot study. Gait Posture 2020, 80, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Morio, C.; Lake, M.J.; Gueguen, N.; Rao, G.; Baly, L. The influence of footwear on foot motion during walking and running. J. Biomech. 2009, 42, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E. Adaptability of anticipatory postural adjustments associated with voluntary movement. World J. Orthop. 2012, 3, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E.; Fourcade, P.; Artico, R.; Caderby, T. Influence of temporal pressure constraint on the biomechanical organization of gait initiation made with or without an obstacle to clear. Exp. Brain Res. 2016, 234, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Kukkar, K.K.; Aruin, A.S. Support surface related changes in feedforward and feedback control of standing posture. J. Electromyogr. Kinesiol. 2013, 24, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.; Waked, E.; Mcclaran, J. Proprioception and Stability: Foot Position Awareness as a Function of Age and Footware. Age Ageing 1995, 24, 67–72. [Google Scholar] [CrossRef] [PubMed]
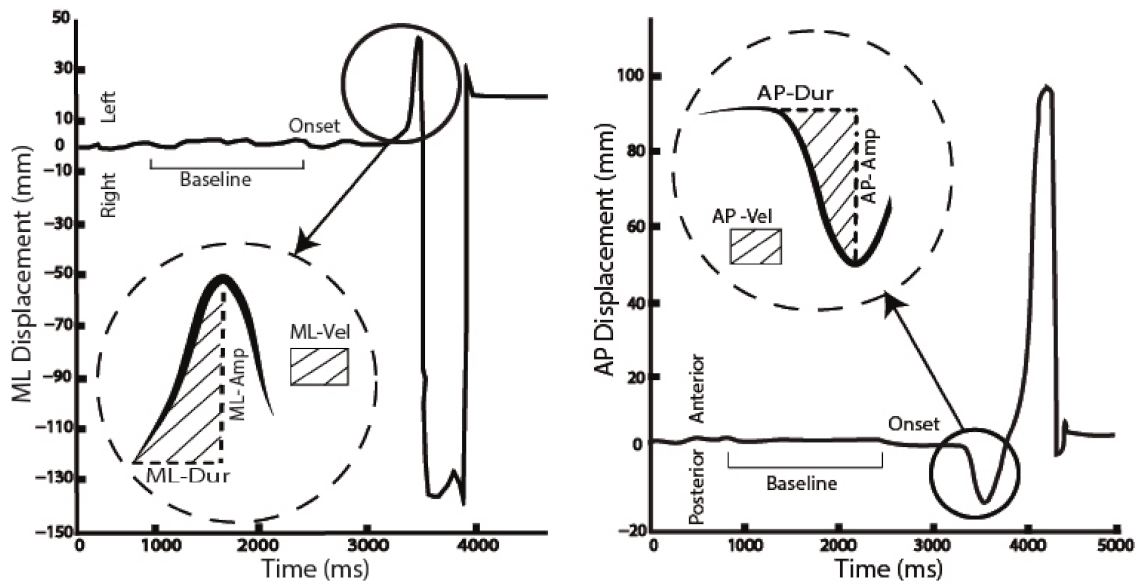
| APA | Preferred Limb | Non-Preferred Limb | |||
|---|---|---|---|---|---|
| Barefoot | Shod | Barefoot | Shod | ||
| Duration (ms) | ML | 420 ± 55 | 443 ± 80 | 413 ± 104 | 432 ± 67 |
| AP | 553 ± 149 | 538 ± 127 | 564 ± 141 | 531 ± 135 | |
| Amplitude (mm) | ML | 12.3 ± 5.9 | 15.8 ± 7.5 * | 12.5 ±6.9 | 14.6 ± 5.8 * |
| AP | 12.7 ± 4.2 | 12.5 ± 5.4 | 12.2 ± 3.9 | 11.6 ± 4.2 | |
| Velocity (m/s) | ML | 0.030 ± 0.015 | 0.037 ± 0.016 * | 0.032 ± 0.016 | 0.036 ± 0.015 * |
| AP | 0.025 ± 0.008 | 0.024 ± 0.007 | 0.023 ± 0.007 | 0.024 ± 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, Y.; Marinkovic, D.; Obradovic, B.; Vannozzi, G. Characterization of Anticipatory Postural Adjustments in Lateral Stepping: Impact of Footwear and Lower Limb Preference. Sensors 2021, 21, 8244. https://doi.org/10.3390/s21248244
Russo Y, Marinkovic D, Obradovic B, Vannozzi G. Characterization of Anticipatory Postural Adjustments in Lateral Stepping: Impact of Footwear and Lower Limb Preference. Sensors. 2021; 21(24):8244. https://doi.org/10.3390/s21248244
Chicago/Turabian StyleRusso, Yuri, Dragan Marinkovic, Borislav Obradovic, and Giuseppe Vannozzi. 2021. "Characterization of Anticipatory Postural Adjustments in Lateral Stepping: Impact of Footwear and Lower Limb Preference" Sensors 21, no. 24: 8244. https://doi.org/10.3390/s21248244
APA StyleRusso, Y., Marinkovic, D., Obradovic, B., & Vannozzi, G. (2021). Characterization of Anticipatory Postural Adjustments in Lateral Stepping: Impact of Footwear and Lower Limb Preference. Sensors, 21(24), 8244. https://doi.org/10.3390/s21248244








