Modifications in Prefrontal Cortex Oxygenation in Linear and Curvilinear Dual Task Walking: A Combined fNIRS and IMUs Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. The fNIRS Data Acquisition and Processing
2.4. IMUs Data Acquisition and Processing
- Normalized Root Mean Square (nRMS), a measure of acceleration dispersion and computed as follows:
- Attenuation Coefficients (AC) between pelvis (P) and sternum (S) for each acceleration component (j) [29], defined as:
2.5. Statistical Analysis
3. Results
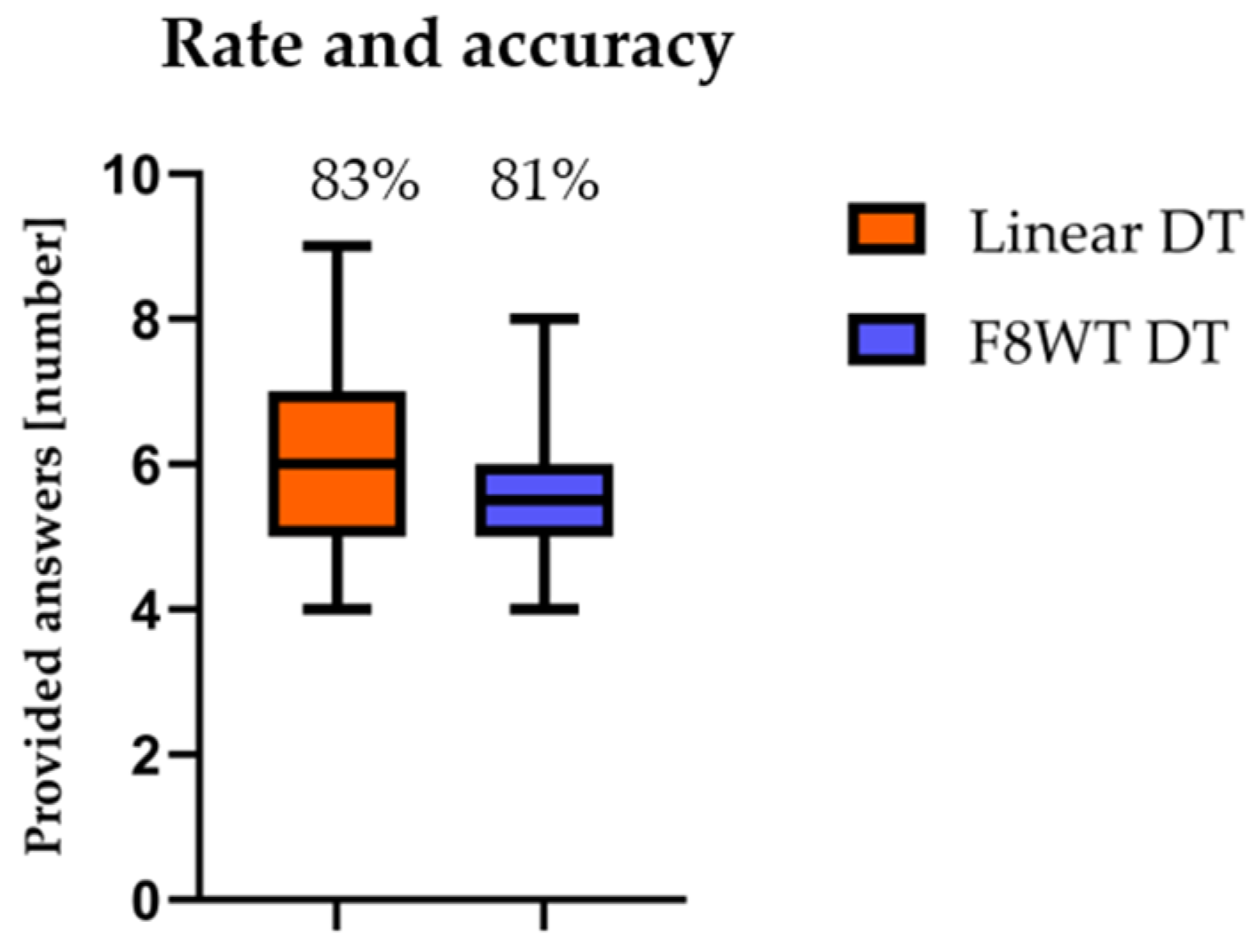
3.1. Rate and Accuracy of Counting Performance
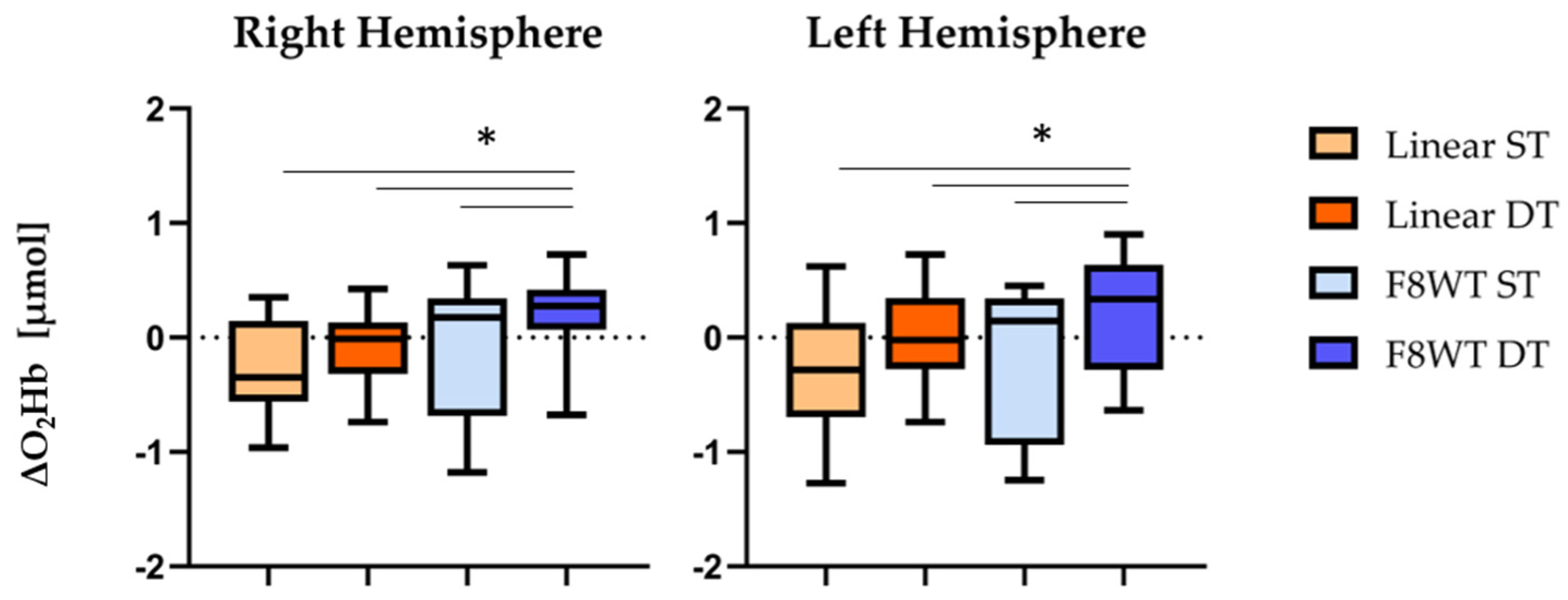
3.2. The fNIRS Results
3.3. IMUs’ Results
3.3.1. Temporal Parameters
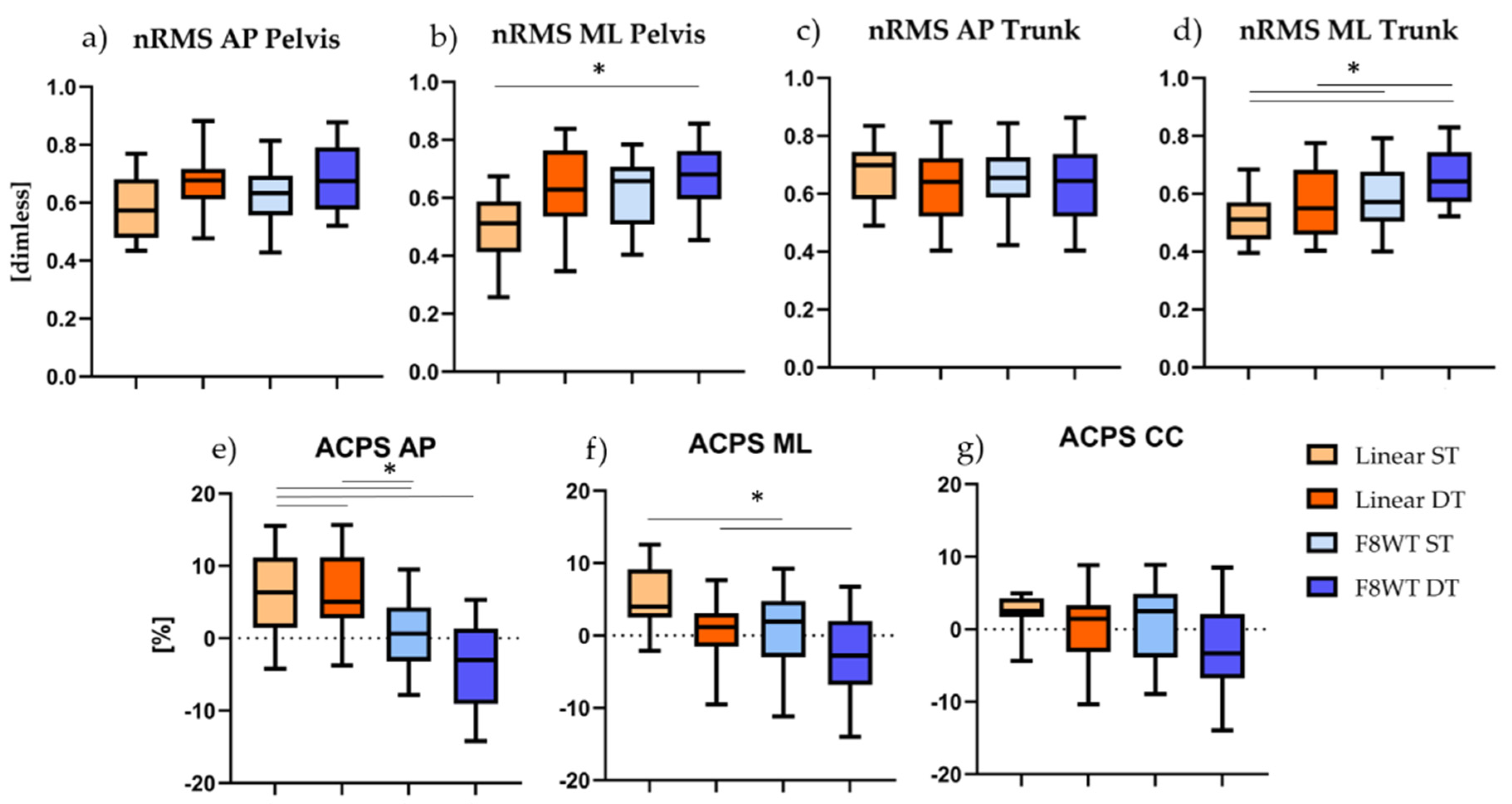
3.3.2. Gait Quality indices
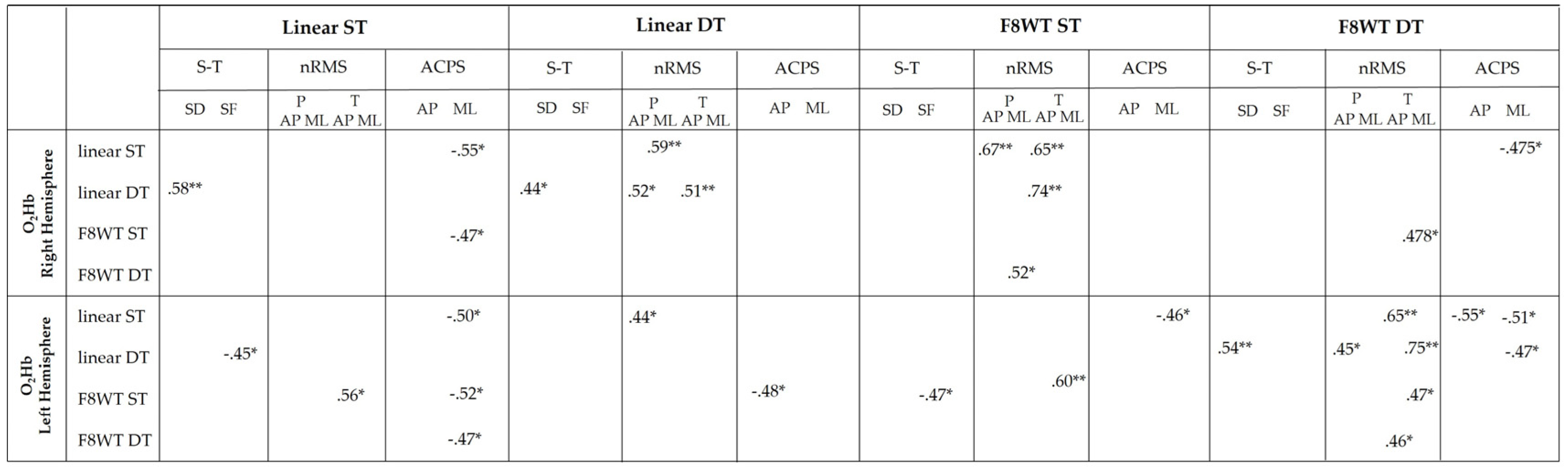
3.4. Correlation Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menant, J.C.; Maidan, I.; Alcock, L.; Al-Yahya, E.; Cerasa, A.; Clark, D.J.; de Bruin, E.D.; Fraser, S.; Gramigna, V.; Hamacher, D.; et al. A consensus guide to using functional near-infrared spectroscopy in posture and gait research. Gait Posture 2020, 82, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Ferrari, M. A Mini-Review on Functional Near-Infrared Spectroscopy (fNIRS): Where Do We Stand, and Where Should We Go? Photonics 2019, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Thiers, A.; Hamacher, D.; Schega, L. Functional near-infrared spectroscopy in movement science: A systematic review on cortical activity in postural and walking tasks. Neurophotonics 2017, 4, 041403. [Google Scholar] [CrossRef] [Green Version]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Miyai, I.; Tanabe, H.C.; Sase, I.; Eda, H.; Oda, I.; Konishi, I.; Tsunazawa, Y.; Suzuki, T.; Yanagida, T.; Kubota, K. Cortical mapping of gait in humans: A near-infrared spectroscopic topography study. Neuroimage 2001, 14, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Miyai, I.; Ono, T.; Oda, I.; Konishi, I.; Kochiyama, T.; Kubota, K. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: An optical imaging study. Neuroimage 2004, 23, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Udina, C.; Avtzi, S.; Durduran, T.; Holtzer, R.; Rosso, A.L.; Castellano-Tejedor, C.; Perez, L.-M.; Soto-Bagaria, L.; Inzitari, M. Functional Near-Infrared Spectroscopy to Study Cerebral Hemodynamics in Older Adults During Cognitive and Motor Tasks: A Review. Front. Aging Neurosci. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.B.; Louie, D.R.; Peters, S.; Liu-Ambrose, T.; Boyd, L.A.; Eng, J.J. Brain activity during real-time walking and with walking interventions after stroke: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.; Moon, S.; Ranchet, M.; Vukas, R.R.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.; Devos, H. Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: A systematic review. Exp. Gerontol. 2019, 128, 1–23. [Google Scholar] [CrossRef]
- Pelicioni, P.H.S.; Tijsma, M.; Lord, S.R.; Menant, J. Prefrontal cortical activation measured by fNIRS during walking: Effects of age, disease and secondary task. PeerJ 2019, 7, e6833. [Google Scholar] [CrossRef] [Green Version]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.I.B.; Lin, K.H. Walking while performing working memory tasks changes the prefrontal cortex hemodynamic activations and gait kinematics. Front. Behav. Neurosci. 2016, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, K.; Onaral, B.; Verghese, J. fNIRS Study of Walking and Walking while Talking in Young and Old Individuals. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011, 66, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schack, J.; Pripp, A.H.; Mirtaheri, P.; Steen, H.; Güler, E.; Gjøvaag, T. Increased prefrontal cortical activation during challenging walking conditions in persons with lower limb amputation–an fNIRS observational study. Physiother. Theory Pract. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Schieppati, M. Tuning of a Basic Coordination Pattern Constructs Straight-Ahead and Curved Walking in Humans. J. Neurophysiol. 2004, 91, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Godi, M.; Giardini, M.; Schieppati, M. Walking along curved trajectories. Changes with age and Parkinson’s disease. Hints to rehabilitation. Front. Neurol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Formisano, R.; Buzzi, M.G.; Vannozzi, G. Does Curved Walking Sharpen the Assessment of Gait Disorders? An Instrumented Approach Based on Wearable Inertial Sensors. Sensors 2020, 20, 5244. [Google Scholar] [CrossRef]
- Hess, R.J.; Brach, J.S.; Piva, S.R.; VanSwearingen, J.M. Walking Skill Can Be Assessed in Older Adults: Validity of the Figure-of-8 Walk Test. Phys. Ther. 2010, 90, 89–99. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Johansen-Berg, H.; Kischka, U.; Zarei, M.; Cockburn, J.; Dawes, H. Prefrontal cortex activation while walking under dual-task conditions in stroke: A multimodal imaging study. Neurorehabil. Neural Repair 2016, 30, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Reelick, M.F.; Bloem, B.R.; Giladi, N.; Deutsch, J.E.; Hausdorff, J.M.; Claassen, J.A.H.; Mirelman, A. The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: An fNIRS study. Neurorehabil. Neural Repair 2016, 30, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Nieuwhof, F.; Reelick, M.; Giladi, N.; Hausdorff, J.M. Increased frontal brain activation during walking while dual tasking: An fNIRS study in healthy young adults. J. Neuroeng. Rehabil. 2014, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Shustack, S.; Giladi, N.; Hausdorff, J.M. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017, 115, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.A.; Lühmann, A.v.; Scholkmann, F.; Gervain, J.; Dan, I.; Ayaz, H.; Boas, D.; Cooper, R.J.; Culver, J.; Elwell, C.E.; et al. Best practices for fNIRS publications. Neurophotonics 2021, 8, 1–34. [Google Scholar]
- Cooper, R.J.; Selb, J.; Gagnon, L.; Phillip, D.; Schytz, H.W.; Iversen, H.K.; Ashina, M.; Boas, D.A. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 2012, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brigadoi, S.; Ceccherini, L.; Cutini, S.; Scarpa, F.; Scatturin, P.; Selb, J.; Gagnon, L.; Boas, D.A.; Cooper, R.J. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage 2014, 85, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamini, E.; Ligorio, G.; Summa, A.; Vannozzi, G.; Cappozzo, A.; Sabatini, A.M. Estimating Orientation Using Magnetic and Inertial Sensors and Different Sensor Fusion Approaches: Accuracy Assessment in Manual and Locomotion Tasks. Sensors 2014, 14, 18625–18649. [Google Scholar] [CrossRef] [Green Version]
- Iosa, M.; Bini, F.; Marinozzi, F.; Fusco, A.; Morone, G.; Koch, G.; Cinnera, A.M.; Bonnì, S.; Paolucci, S. Stability and Harmony of Gait in Patients with Subacute Stroke. J. Med. Biol. Eng. 2016, 36, 635–643. [Google Scholar] [CrossRef]
- Mazzà, C.; Iosa, M.; Pecoraro, F.; Cappozzo, A. Control of the upper body accelerations in young and elderly women during level walking. J. Neuroeng. Rehabil. 2008, 5, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Ohsugi, H.; Ohgi, S.; Shigemori, K.; Schneider, E.B. Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neurosci. 2013, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Makizako, H.; Shimada, H.; Park, H.; Tsutsumimoto, K.; Uemura, K.; Suzuki, T. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: A fNIRS study. Aging Clin. Exp. Res. 2013, 25, 539–544. [Google Scholar] [CrossRef]
- Salzman, T.; Vallejo, D.T.; Polskaia, N.; Michaud, L.; St-Amant, G.; Lajoie, Y.; Fraser, S. Hemodynamic and behavioral changes in older adults during cognitively demanding dual tasks. Brain Behav. 2021, 11, e02021. [Google Scholar] [CrossRef] [PubMed]
- Vieilledent, S.; Kerlirzin, Y.; Dalbera, S.; Berthoz, A. Relationship between velocity and curvature of a human locomotor trajectory. Neurosci. Lett. 2001, 305, 65–69. [Google Scholar] [CrossRef]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Bustos, A.O.; Allevi, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Gait Quality Assessment in Survivors from Severe Traumatic Brain Injury: An Instrumented Approach Based on Inertial Sensors. Sensors 2019, 19, 5315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamini, E.; Iosa, M.; Belluscio, V.; Morone, G.; Tramontano, M.; Vannozzi, G. Multi-sensor assessment of dynamic balance during gait in patients with subacute stroke. J. Biomech. 2017, 61, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Menz, H. Accelerometry: A technique for quantifying movement patterns during walking. Gait Posture 2008, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Turcato, A.M.; Godi, M.; Giordano, A.; Schieppati, M.; Nardone, A. The generation of centripetal force when walking in a circle: Insight from the distribution of ground reaction forces recorded by plantar insoles. J. Neuroeng. Rehabil. 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navon, D.; Miller, J. Queuing or Sharing? A Critical Evaluation of the Single-Bottleneck Notion. Cogn. Psychol. 2002, 44, 193–251. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-Hall: Englewood Cliffs, NJ, USA, 1975; Volume 88, p. 339. [Google Scholar]
- Mori, T.; Takeuchi, N.; Izumi, S.-I. Prefrontal cortex activation during a dual task in patients with stroke. Gait Posture 2018, 59, 193–198. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Calabrese, E.; Mazzoleni, P.; Nemni, R.; Ferrarin, M. The association between impaired turning and normal straight walking in Parkinson’s disease. Gait Posture 2007, 26, 172–178. [Google Scholar] [CrossRef]
| Participant’s Characteristics | |
|---|---|
| Sex [female-male %] | 50-50 |
| Age [years] | 28.4 (5.1) |
| Body Mass [kg] | 73.5 (12.1) |
| Height [cm] | 174.9 (9.1) |
| Physical Activity [days/week] | 2.9 (2.4) |
| Hand Dominant Side [right %] | 87 |
| Linear ST | Linear DT | F8WT ST | F8WT DT | |
|---|---|---|---|---|
| WS [m/s] | 1.31 ± 0.02 *§ | 1.27 ± 0.31 | 1.19 ± 0.28 § | 1.17 ± 0.08 * |
| SF [stride/s] | 0.73 ± 0.09 | 0.71 ± 0.09 | 0.70 ± 0.13 | 0.70 ± 0.08 |
| SD [s] | 1.32 ± 0.15 | 1.31 ± 0.11 | 1.31 ± 0.11 | 1.36 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belluscio, V.; Casti, G.; Ferrari, M.; Quaresima, V.; Sappia, M.S.; Horschig, J.M.; Vannozzi, G. Modifications in Prefrontal Cortex Oxygenation in Linear and Curvilinear Dual Task Walking: A Combined fNIRS and IMUs Study. Sensors 2021, 21, 6159. https://doi.org/10.3390/s21186159
Belluscio V, Casti G, Ferrari M, Quaresima V, Sappia MS, Horschig JM, Vannozzi G. Modifications in Prefrontal Cortex Oxygenation in Linear and Curvilinear Dual Task Walking: A Combined fNIRS and IMUs Study. Sensors. 2021; 21(18):6159. https://doi.org/10.3390/s21186159
Chicago/Turabian StyleBelluscio, Valeria, Gabriele Casti, Marco Ferrari, Valentina Quaresima, Maria Sofia Sappia, Jörn M. Horschig, and Giuseppe Vannozzi. 2021. "Modifications in Prefrontal Cortex Oxygenation in Linear and Curvilinear Dual Task Walking: A Combined fNIRS and IMUs Study" Sensors 21, no. 18: 6159. https://doi.org/10.3390/s21186159
APA StyleBelluscio, V., Casti, G., Ferrari, M., Quaresima, V., Sappia, M. S., Horschig, J. M., & Vannozzi, G. (2021). Modifications in Prefrontal Cortex Oxygenation in Linear and Curvilinear Dual Task Walking: A Combined fNIRS and IMUs Study. Sensors, 21(18), 6159. https://doi.org/10.3390/s21186159








