Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ti–Me Dry Electrode Preparation
2.2. Chemical and (Micro)Structural Analysis
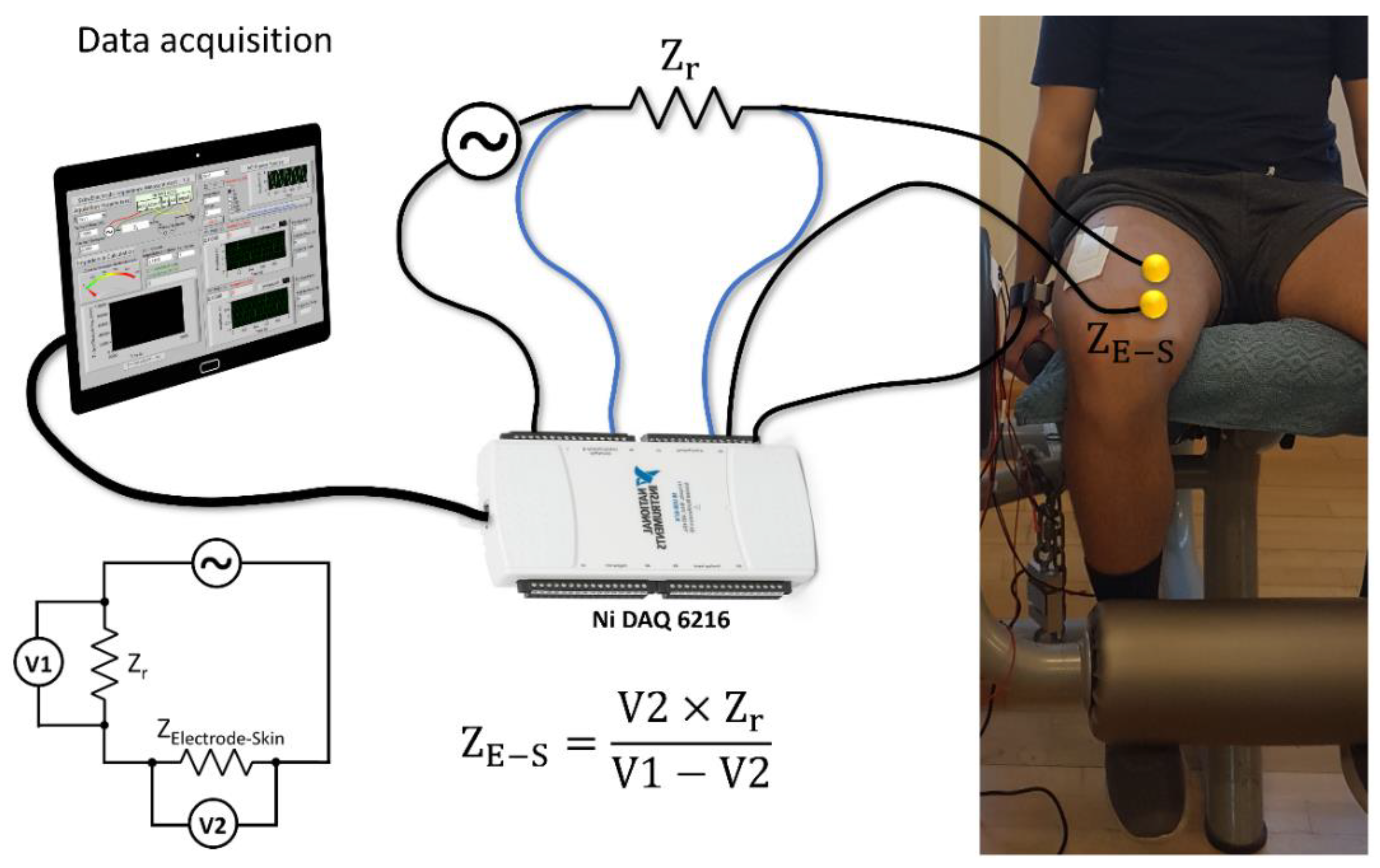
2.3. In-Vivo Electrode–Skin Interfacial Impedance Measurements
3. Results
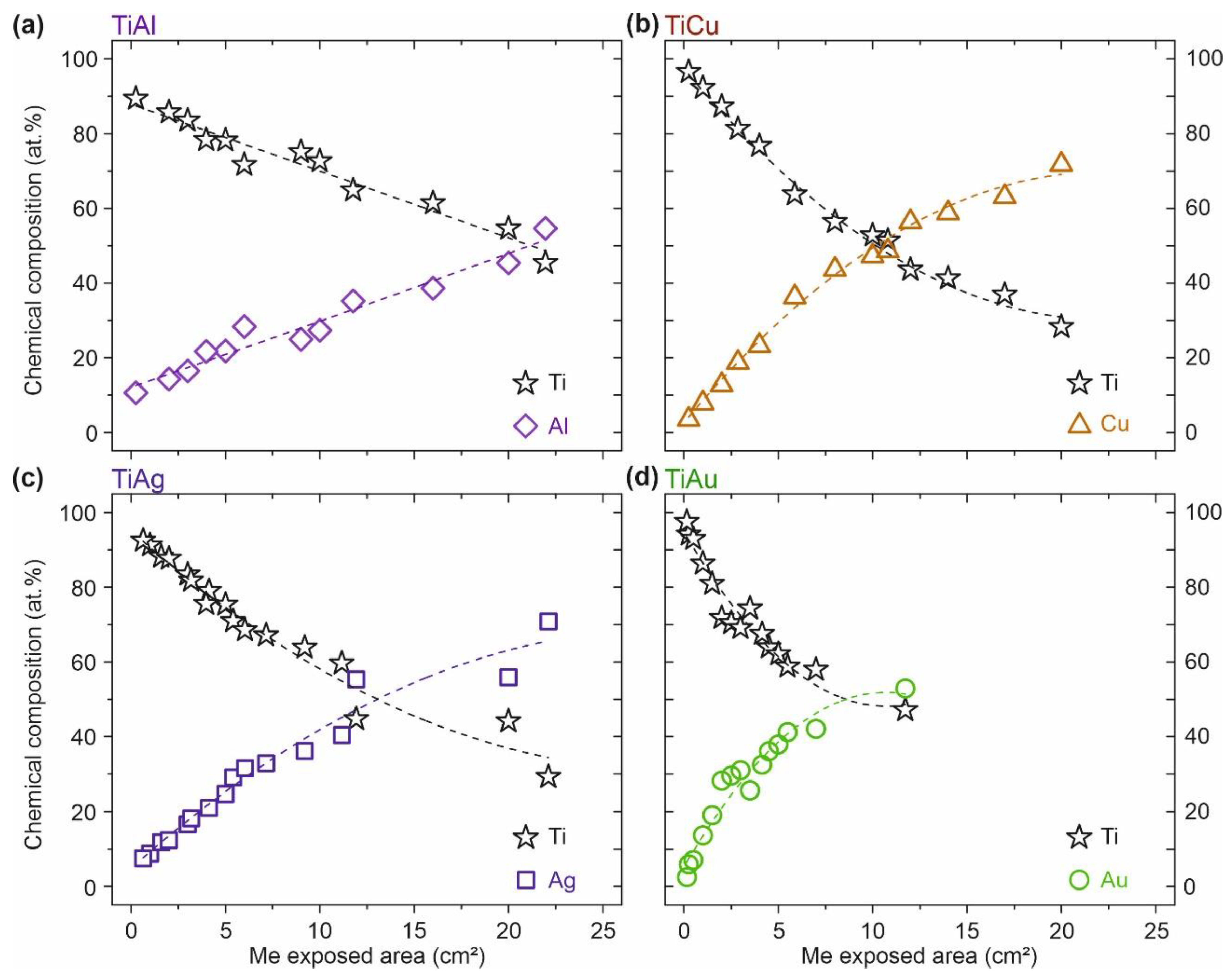
3.1. Ti–Me Systems—Chemical Composition
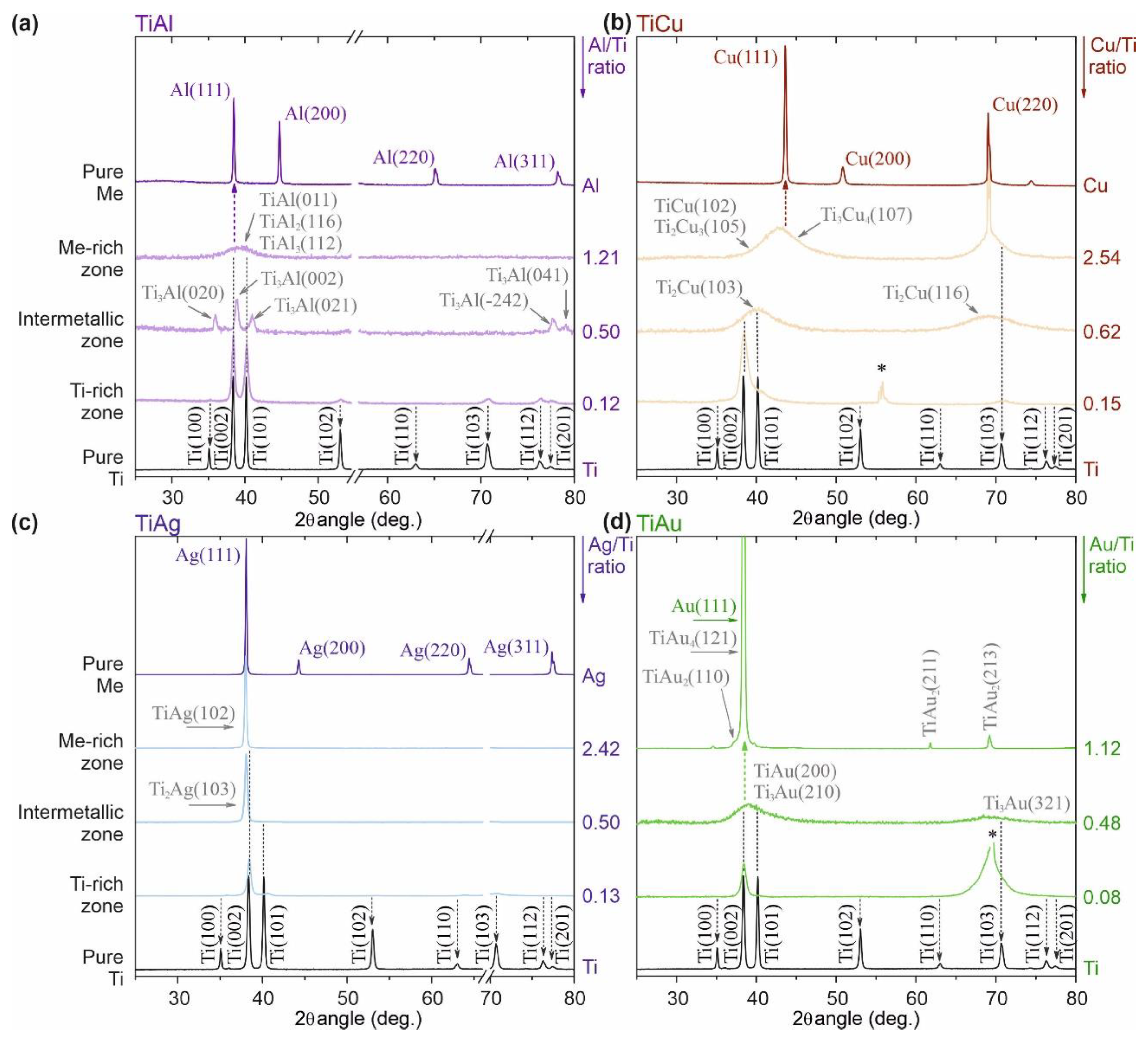
3.2. Ti–Me Systems—Structural Composition
3.3. Ti–Me Systems—Morphological Evolution
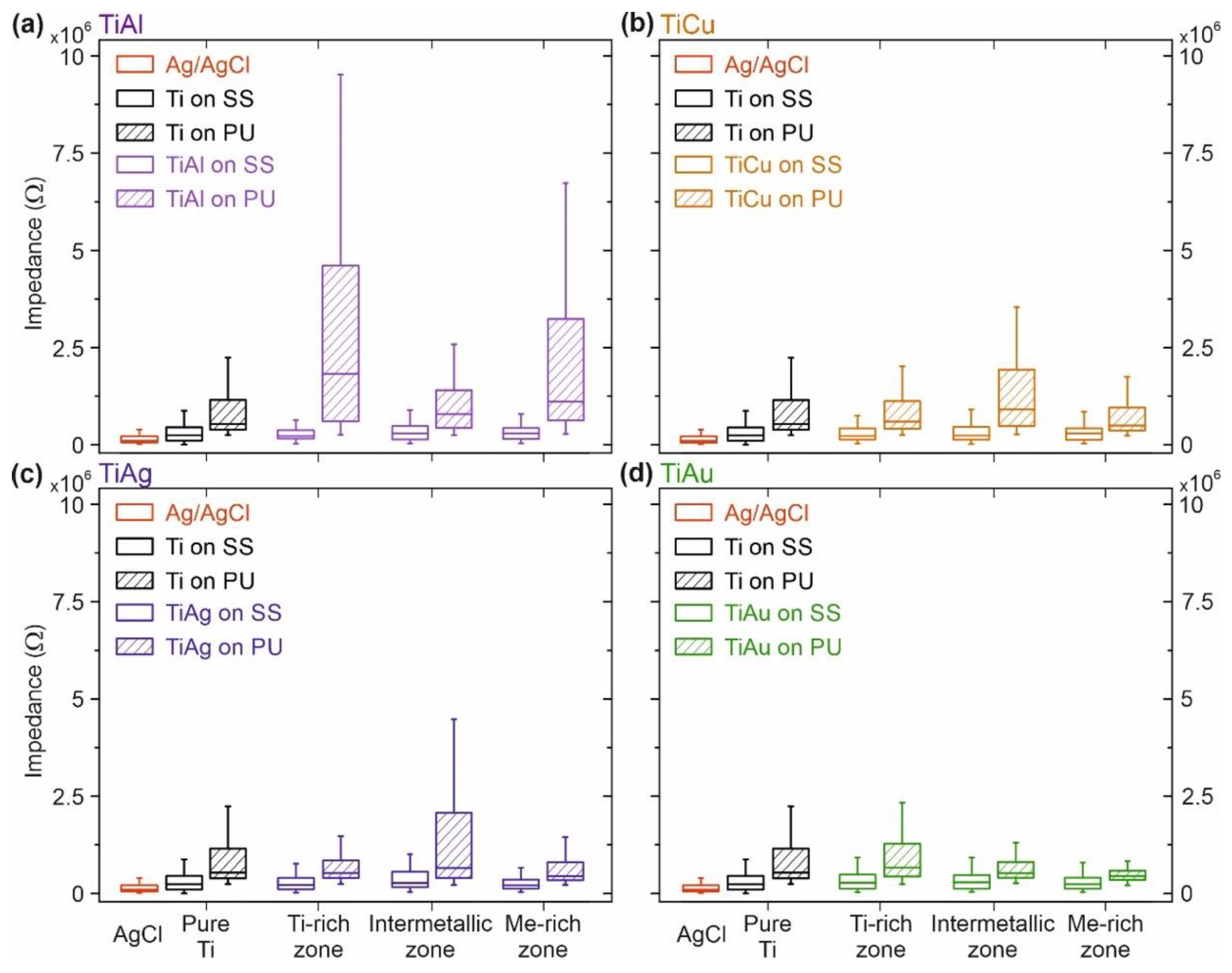
3.4. Electrode–Skin Impedance Measurements
4. Conclusions
- (i)
- Increased biocompatibility according to literature when compared to Ag/AgCl electrodes;
- (ii)
- Impedance values in the same order of magnitude as the Ag/AgCl reference electrodes;
- (iii)
- Reusability and long durability, even after repetitive applications;
- (iv)
- Reduced variability of the impedance values recorded;
- (v)
- Reduced costs of material and production, capable of being equitably used in new fields of application for reusable electrodes in mobile electrophysiological monitoring.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Avenel-audran, M.; Goossens, A.; Zimerson, E.; Bruze, M. Contact dermatitis from electrocardiograph-monitoring electrodes: Role of p-tert -butylphenol-formaldehyde resin. Contact Dermat. 2003, 48, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Gao, X.; Liu, Y.; Liu, H. Surface bioelectric dry Electrodes: A review. Measurement 2021, 183, 109774. [Google Scholar] [CrossRef]
- Fiedler, P.; Haueisen, J.; Jannek, D.; Griebel, S.; Zentner, L.; Vaz, F.; Fonseca, C. Comparison of three types of dry electrodes for electroencephalography. Acta IMEKO 2014, 3, 33–37. [Google Scholar] [CrossRef]
- Freire, F.C.M.; Becchi, M.; Ponti, S.; Miraldi, E.; Strigazzi, A. Impedance spectroscopy of conductive commercial hydrogels for electromyography and electroencephalography. Physiol. Meas. 2010, 31, S157–S167. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, P.; Fiedler, P.; Schinaia, L.; Vasconcelos, B.; Martins, A.C.; Amaral, M.H.; Comani, S.; Haueisen, J.; Fonseca, C. Alginate-based hydrogels as an alternative to electrolytic gels for rapid EEG monitoring and easy cleaning procedures. Sens. Actuators B Chem. 2017, 247, 273–283. [Google Scholar] [CrossRef]
- Kleffner-Canucci, K.; Luu, P.; Naleway, J.; Tucker, D.M. A novel hydrogel electrolyte extender for rapid application of EEG sensors and extended recordings. J. Neurosci. Methods 2012, 206, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.-L.; Wu, J.-T.; Xia, Y.-H.; He, Q.-G.; Jin, H.-G. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 2020, 17, 051004. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Li, M.; Duan, Y.Y. Towards real-life EEG applications: Novel superporous hydrogel-based semi-dry EEG electrodes enabling automatically ‘charge–discharge’ electrolyte. J. Neural Eng. 2021, 18, 046016. [Google Scholar] [CrossRef]
- Mota, A.R.; Duarte, L.; Rodrigues, D.; Martins, A.C.; Machado, A.V.; Vaz, F.; Fiedler, P.; Haueisen, J.; Nóbrega, J.M.; Fonseca, C. Development of a quasi-dry electrode for EEG recording. Sens. Actuators A Phys. 2013, 199, 310–317. [Google Scholar] [CrossRef]
- Pedrosa, P.; Fiedler, P.; Pestana, V.; Vasconcelos, B.; Gaspar, H.; Amaral, M.H.; Freitas, D.; Haueisen, J.; Nóbrega, J.M.; Fonseca, C. In-Service characterization of a polymer wick-based quasi-dry electrode for rapid pasteless electroencephalography. Biomed. Eng./Biomed. Tech. 2018, 63, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, L.; Chen, L.; Zhang, Y.; He, Z.; Zhang, W.; Zhao, J.; Lu, D.; He, J.; Zhu, H.; et al. Flexible and stretchable dry active electrodes with PDMS and silver flakes for bio-potentials sensing systems. IEEE Sens. J. 2021, 21, 12255–12268. [Google Scholar] [CrossRef]
- Heijs, J.J.A.; Havelaar, R.J.; Fiedler, P.; van Wezel, R.J.A.; Heida, T. Validation of soft multipin dry EEG electrodes. Sensors 2021, 21, 6827. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.S.; Piña-Martínez, E.; Roberts, R.; Rodriguez-Leal, E. Impact of size and shape for textile surface electromyography electrodes: A study of the biceps brachii muscle. Text. Res. J. 2021, 1–149. [Google Scholar] [CrossRef]
- Fiedler, P.; Strohmeier, D.; Hunold, A.; Griebel, S.; Muhle, R.; Schreiber, M.; Pedrosa, P.; Vasconcelos, B.; Fonseca, C.; Vaz, F.; et al. Modular multipin electrodes for comfortable dry EEG. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5705–5708. [Google Scholar]
- Pedrosa, P.; Fiedler, P.; Lopes, C.C.; Alves, E.; Barradas, N.P.; Haueisen, J.; Machado, A.V.; Fonseca, C.; Vaz, F. Ag:TiN-Coated polyurethane for Dry biopotential electrodes: From polymer plasma interface activation to the first EEG measurements. Plasma Process. Polym. 2016, 13, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.S.; Fiedler, P.; Küchler, N.; Domingues, R.P.; Lopes, C.; Borges, J.; Haueisen, J.; Vaz, F. Dry electrodes for surface electromyography based on architectured titanium thin films. Materials 2020, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Thap, T.; Yoon, K.-H.; Lee, J. Graphite Based Electrode for ECG Monitoring: Evaluation under Freshwater and Saltwater Conditions. Sensors 2016, 16, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.-H.; Chen, S.-H.; Chang, C.-L.; Lin, C.-T.; Hairston, W.; Mrozek, R. New flexible silicone-based EEG dry sensor material compositions exhibiting improvements in lifespan, conductivity, and reliability. Sensors 2016, 16, 1826. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Samanta, K.; Lee, H.; Lee, K.; Tiwari, A.P.; Lee, J.; Yang, J.; Lee, H. Highly stretchable and conductive silver nanoparticle embedded graphene flake electrode prepared by in situ dual reduction reaction. Sci. Rep. 2015, 5, 14177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffini, G.; Dunne, S.; Fuentemilla, L.; Grau, C.; Farrés, E.; Marco-Pallarés, J.; Watts, P.C.P.; Silva, S.R.P. First human trials of a dry electrophysiology sensor using a carbon nanotube array interface. Sens. Actuators A Phys. 2008, 144, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Martínez, J.; Seseña-Rubfiaro, A.; Godínez-Fernández, R.; Aguilar-Elguezabal, A. Electrodes made of multi-wall carbon nanotubes on PVDF-filters have low electrical resistance and are able to record electrocardiograms in humans. Microelectron. Eng. 2016, 166, 10–14. [Google Scholar] [CrossRef]
- Grozea, C.; Voinescu, C.D.; Fazli, S. Bristle-sensors—low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J. Neural Eng. 2011, 8, 025008. [Google Scholar] [CrossRef]
- Fiedler, P.; Griebel, S.; Pedrosa, P.; Fonseca, C.; Vaz, F.; Zentner, L.; Zanow, F.; Haueisen, J. Multichannel EEG with novel Ti/TiN dry electrodes. Sens. Actuators A Phys. 2015, 221, 139–147. [Google Scholar] [CrossRef]
- Leleux, P.; Badier, J.-M.; Rivnay, J.; Bénar, C.; Hervé, T.; Chauvel, P.; Malliaras, G.G. Conducting polymer electrodes for electroencephalography. Adv. Healthc. Mater. 2014, 3, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Etiemble, A.; Lopes, C.; Nkou Bouala, G.I.; Borges, J.; Malchère, A.; Langlois, C.; Vaz, F.; Steyer, P. Fracture resistance of Ti-Ag thin films deposited on polymeric substrates for biosignal acquisition applications. Surf. Coat. Technol. 2019, 358, 646–653. [Google Scholar] [CrossRef]
- Ullas Pradhan, U.; Reddy, N.; Chandrashekar, K.; Mohan, C.B. Titanium dioxide based bioelectric sensor for the acquisition of electrocardiogram signals. Microchem. J. 2021, 160, 105656. [Google Scholar] [CrossRef]
- Greulich, C.; Kittler, S.; Epple, M.; Muhr, G.; Köller, M. Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs). Langenbeck’s Arch. Surg. 2009, 394, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Fonseca, P.; Matamá, T.; Gomes, A.; Louro, C.; Paiva, S.; Vaz, F. Protective Ag:TiO2thin films for pressure sensors in orthopedic prosthesis: The importance of composition, structural and morphological features on the biological response of the coatings. J. Mater. Sci. Mater. Med. 2014, 25, 2069–2081. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Lopes, C.; Gabor, C.; Cristea, D.; Costa, R.; Domingues, R.P.; Rodrigues, M.S.; Borges, J.; Alves, E.; Barradas, N.P.; Munteanu, D.; et al. Evolution of the mechanical properties of Ti-based intermetallic thin films doped with different metals to be used as biomedical devices. Appl. Surf. Sci. 2019, 144617. [Google Scholar] [CrossRef]
- Lopes, C.; Vieira, M.; Borges, J.; Fernandes, J.; Rodrigues, M.S.S.; Alves, E.; Barradas, N.P.P.; Apreutesei, M.; Steyer, P.; Tavares, C.J.J.; et al. Multifunctional Ti–Me (Me = Al, Cu) thin film systems for biomedical sensing devices. Vacuum 2015, 122, 353–359. [Google Scholar] [CrossRef]
- Lopes, C.; Gonçalves, C.; Borges, J.; Polcar, T.; Rodrigues, M.S.; Barradas, N.P.; Alves, E.; Le Bourhis, E.; Couto, F.M.; Macedo, F.; et al. Evolution of the functional properties of titanium–silver thin films for biomedical applications: Influence of in-vacuum annealing. Surf. Coat. Technol. 2015, 261, 262–271. [Google Scholar] [CrossRef]
- Ferreira, A.; Lopes, C.; Martin, N.; Lanceros-Méndez, S.; Vaz, F. Nanostructured functional Ti-Ag electrodes for large deformation sensor applications. Sens. Actuators A Phys. 2014, 220, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, P.; Muhle, R.; Griebel, S.; Pedrosa, P.; Fonseca, C.; Vaz, F.; Zanow, F.; Haueisen, J. Contact pressure and flexibility of multipin dry EEG electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 750–757. [Google Scholar] [CrossRef]
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Supriyanto, E.; Zanow, F.; Haueisen, J. Novel multipin electrode cap system for dry electroencephalography. Brain Topogr. 2015, 28, 647–656. [Google Scholar] [CrossRef]
- Morais, D.S.; Ávila, B.; Lopes, C.; Rodrigues, M.A.; Vaz, F.; Machado, A.V.; Fernandes, M.H.; Guedes, R.M.; Lopes, M.A. Surface functionalization of polypropylene (PP) by chitosan immobilization to enhance human fibroblasts viability. Polym. Test. 2020, 86, 106507. [Google Scholar] [CrossRef]
- Carlos Quagliano Amado, J. Thermal resistance properties of polyurethanes and its composites. In Thermosoftening Plastics; IntechOpen: London, UK, 2020. [Google Scholar]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar]
- Grace, J.M.; Gerenser, L.J. Plasma treatment of polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Barradas, N.P.; Jeynes, C. Advanced physics and algorithms in the IBA DataFurnace. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 1875–1879. [Google Scholar] [CrossRef] [Green Version]
- Barradas, N.P.; Alves, E.; Jeynes, C.; Tosaki, M. Accurate simulation of backscattering spectra in the presence of sharp resonances. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 247, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Seniam.org. Available online: http://www.seniam.org/ (accessed on 29 October 2021).
- Fish, R.M.; Geddes, L.A. Conduction of electrical current to and through the human body: A review. Eplasty 2009, 9, e44. [Google Scholar]
- Wang, Y.; Tian, Y.; Zhu, J.; She, H.; Yokoi, H.; Jiang, Y.; Huang, Q. A study on the classification effect of sEMG signals in different vibration environments based on the lda algorithm. Sensors 2021, 21, 6234. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Josephson, M.E. Frequency content and characteristics of ventricular conduction. J. Electrocardiol. 2015, 48, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Polachan, K.; Chatterjee, B.; Weigand, S.; Sen, S. Human body–electrode interfaces for wide-frequency sensing and communication: A review. Nanomaterials 2021, 11, 2152. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Chang, K.; Music, D.; to Baben, M.; Lange, D.; Bolvardi, H.; Schneider, J.M. Modeling of metastable phase formation diagrams for sputtered thin films. Sci. Technol. Adv. Mater. 2016, 17, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Mitterer, C.; Mayrhofer, P.H.; Musil, J. Thermal stability of PVD hard coatings. Vacuum 2003, 71, 279–284. [Google Scholar] [CrossRef]
- Svanidze, E.; Besara, T.; Ozaydin, M.F.; Tiwary, C.S.; Wang, J.K.; Radhakrishnan, S.; Mani, S.; Xin, Y.; Han, K.; Liang, H.; et al. High hardness in the biocompatible intermetallic compound-Ti3Au. Sci. Adv. 2016, 2, e1600319. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.L. The Cu-Ti (Copper-Titanium) system. Bull. Alloy Phase Diagr. 1983, 4, 81–95. [Google Scholar] [CrossRef]
- Batalu, D.; Coşmeleaţǎ, G.; Aloman, A. Critical analysis of the Ti-Al phase diagrams. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2006, 68, 77–90. [Google Scholar]
- Murray, J.L.; Bhansali, K.J. The Ag-Ti (Silver-Titanium) system. Bull. Alloy Phase Diagr. 1983, 4, 178–183. [Google Scholar] [CrossRef]
- Murray, J.L. The Au-Ti (Gold-Titanium) system. Bull. Alloy Phase Diagr. 1983, 4, 278–283. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [Green Version]
- Ohring, M. Substrate surfaces and thin-film nucleation. In Materials Science of Thin Films; Elsevier: Amsterdam, The Netherlands, 2002; pp. 357–415. ISBN 0125249756. [Google Scholar]
- Lopes, C.; Gonçalves, C.; Pedrosa, P.; Macedo, F.; Alves, E.; Barradas, N.P.; Martin, N.; Fonseca, C.; Vaz, F. TiAgx thin films for lower limb prosthesis pressure sensors: Effect of composition and structural changes on the electrical and thermal response of the films. Appl. Surf. Sci. 2013, 285, 10–18. [Google Scholar] [CrossRef]
- Pelliccione, M.; Lu, T.M. Evolution of Thin Film Morphology; Materials Science; Springer: New York, NY, USA, 2008; Volume 108. [Google Scholar]
- Chu, J.P.; Jang, J.S.C.; Huang, J.C.; Chou, H.S.; Yang, Y.; Ye, J.C.; Wang, Y.C.; Lee, J.W.; Liu, F.X.; Liaw, P.K.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Films 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Turnow, H.; Wendrock, H.; Menzel, S.; Gemming, T.; Eckert, J. Structure and properties of sputter deposited crystalline and amorphous Cu-Ti films. Thin Solid Films 2016, 598, 184–188. [Google Scholar] [CrossRef]
- Apreutesei, M.; Steyer, P.; Billard, A.; Joly-Pottuz, L.; Esnouf, C. Zr-Cu thin film metallic glasses: An assessment of the thermal stability and phases’ transformation mechanisms. J. Alloys Compd. 2015, 619, 284–292. [Google Scholar] [CrossRef]
- Apreutesei, M.; Steyer, P.; Joly-Pottuz, L.; Billard, A.; Qiao, J.; Cardinal, S.; Sanchette, F.; Pelletier, J.M.; Esnouf, C. Microstructural, thermal and mechanical behavior of co-sputtered binary Zr-Cu thin film metallic glasses. Thin Solid Films 2014, 561, 53–59. [Google Scholar] [CrossRef]
- Clancy, E.A.; Morin, E.L.; Merletti, R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J. Electromyogr. Kinesiol. 2002, 12, 1–16. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Gerdle, B.; Karlsson, S.; Day, S.; Djupsjöbacka, M. Acquisition, processing and analysis of the surface electromyogram. In Modern Techniques in Neuroscience Research; Springer: Berlin/Heidelberg, Germany, 1999; pp. 705–755. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, C.; Fiedler, P.; Rodrigues, M.S.; Borges, J.; Bertollo, M.; Alves, E.; Barradas, N.P.; Comani, S.; Haueisen, J.; Vaz, F. Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study. Sensors 2021, 21, 8143. https://doi.org/10.3390/s21238143
Lopes C, Fiedler P, Rodrigues MS, Borges J, Bertollo M, Alves E, Barradas NP, Comani S, Haueisen J, Vaz F. Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study. Sensors. 2021; 21(23):8143. https://doi.org/10.3390/s21238143
Chicago/Turabian StyleLopes, Cláudia, Patrique Fiedler, Marco Sampaio Rodrigues, Joel Borges, Maurizio Bertollo, Eduardo Alves, Nuno Pessoa Barradas, Silvia Comani, Jens Haueisen, and Filipe Vaz. 2021. "Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study" Sensors 21, no. 23: 8143. https://doi.org/10.3390/s21238143
APA StyleLopes, C., Fiedler, P., Rodrigues, M. S., Borges, J., Bertollo, M., Alves, E., Barradas, N. P., Comani, S., Haueisen, J., & Vaz, F. (2021). Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study. Sensors, 21(23), 8143. https://doi.org/10.3390/s21238143











