Applying Noncontact Sensing Technology in the Customized Product Design of Smart Clothes Based on Anthropometry
Abstract
:1. Introduction
2. Methods
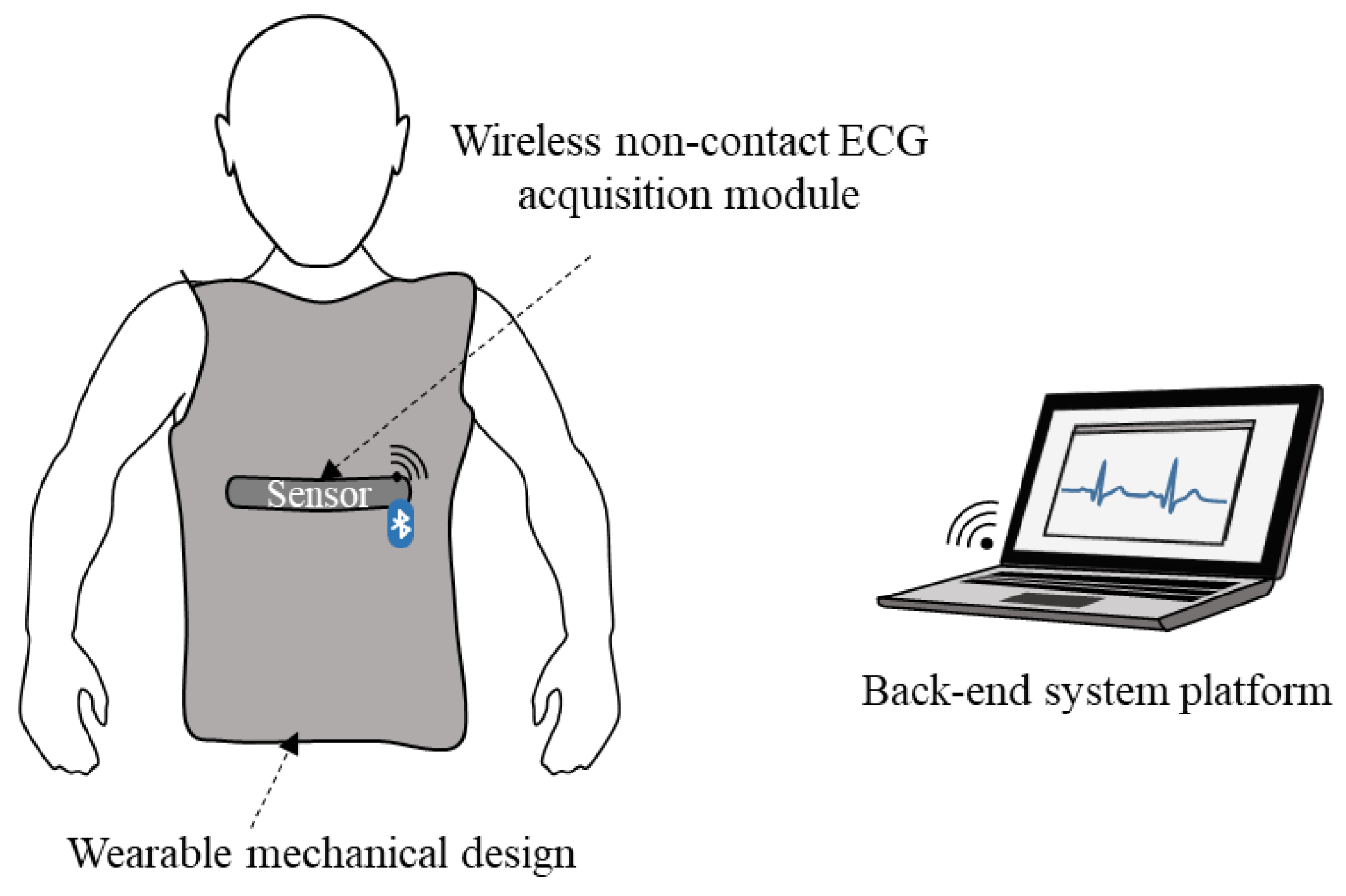
2.1. Design and Implementation of Smart Clothes
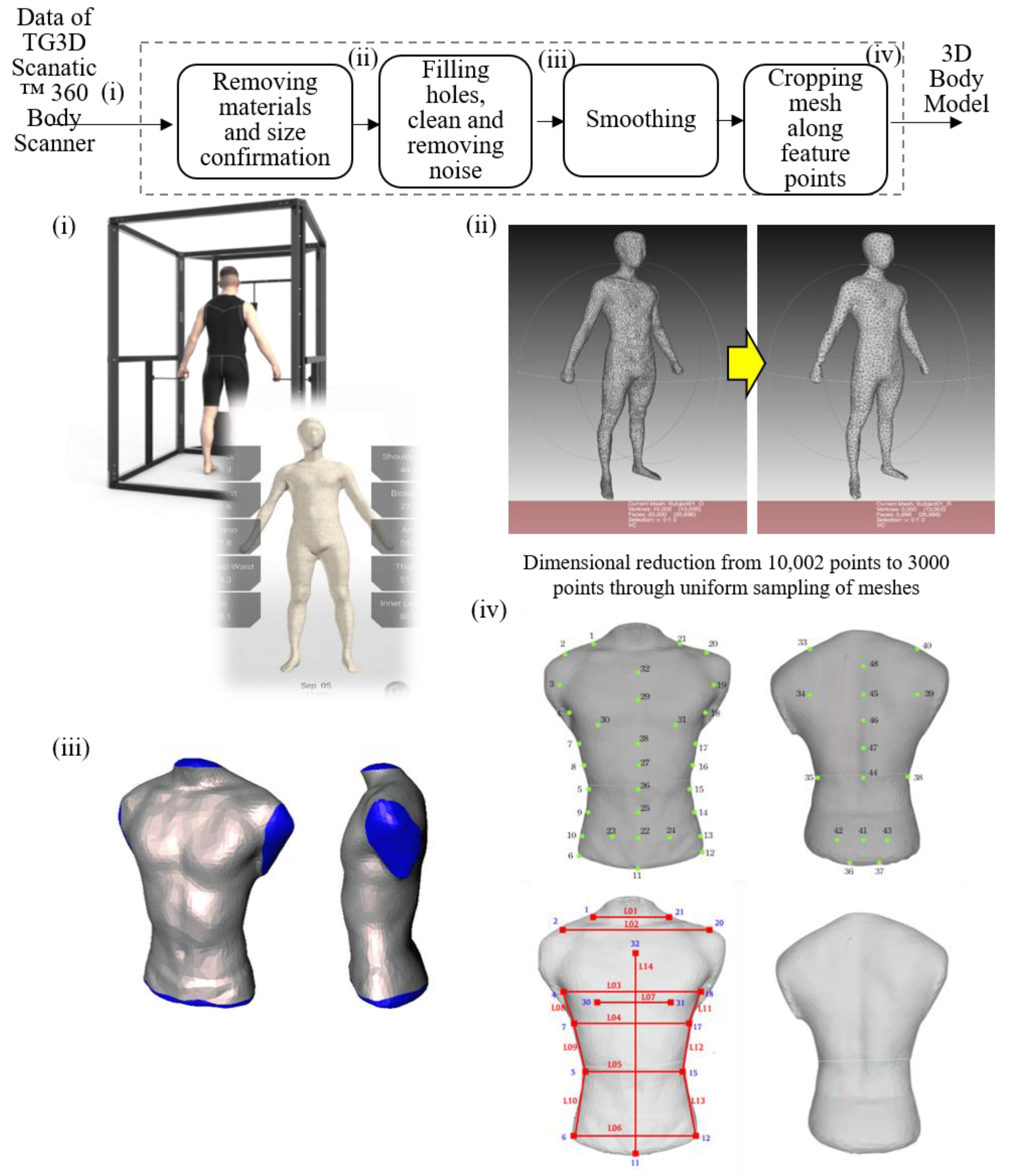
2.2. Mechanical Design of Smart Clothes
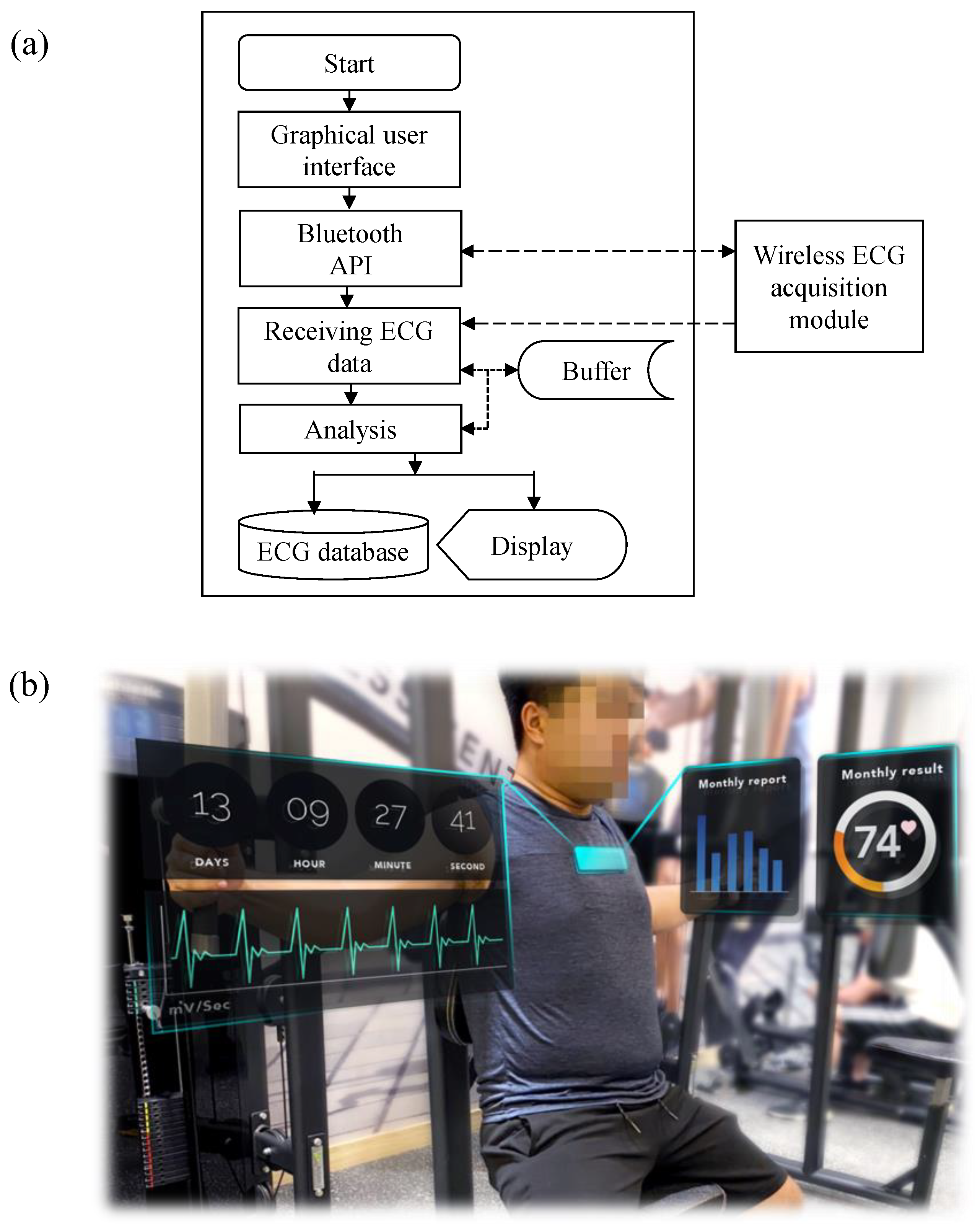
2.3. Back-End System Platform
3. Results
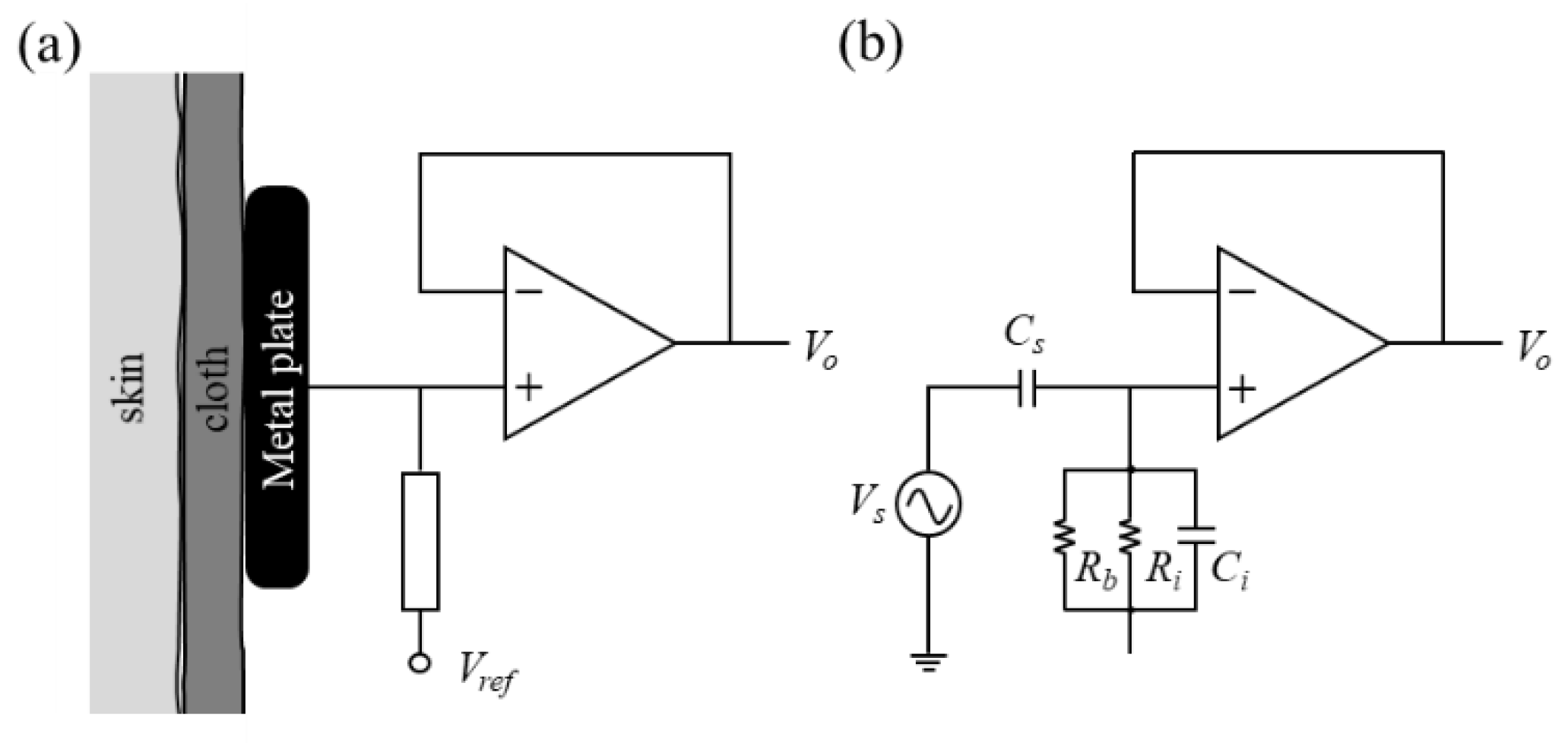
3.1. Electrical Specification and Signal Quality of Flexible Noncontact Electrode
3.2. Signal Quality of Smart Clothes under Different Motion Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef]
- Baba, A.; Burke, M. Measurement of the electrical properties of ungelled ECG electrodes. Int. J. Biol. Biomed. Eng. 2008, 2, 89–97. [Google Scholar]
- Ribeiro, D.M.D.; Fu, L.S.; Carlos, L.A.D.; Cunha, J.P.S. A Novel Dry Active Biosignal Electrode Based on an Hybrid Organic-Inorganic Interface Material. IEEE Sens. J. 2011, 11, 2241–2245. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Yang, B.; Yang, C. PDMS-Based Low Cost Flexible Dry Electrode for Long-Term EEG Measurement. IEEE Sens. J. 2012, 12, 2898–2904. [Google Scholar] [CrossRef]
- Oh, T.I.; Yoon, S.; Kim, T.E.; Wi, H.; Kim, K.J.; Woo, E.J.; Sadleir, R.J. Nanofiber Web Textile Dry Electrodes for Long-Term Biopotential Recording. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 204–211. [Google Scholar]
- Abu-Saude, M.J.; Morshed, B.I. Patterned Vertical Carbon Nanotube Dry Electrodes for Impedimetric Sensing and Stimulation. IEEE Sens. J. 2015, 15, 5851–5858. [Google Scholar] [CrossRef]
- Yokus, M.A.; Jur, J.S. Fabric-Based Wearable Dry Electrodes for Body Surface Biopotential Recording. IEEE Trans. Biomed. Eng. 2016, 63, 423–430. [Google Scholar] [CrossRef]
- Jung, H.; Moon, J.-H.; Baek, D.-H.; Lee, J.-H.; Choi, Y.-Y.; Hong, J.-S.; Lee, S.-H. CNT/PDMS Composite Flexible Dry Electrodesfor Long-Term ECG Monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef]
- Galli, A.; Peri, E.; Zhang, Y.; Vullings, R.; van der Ven, M.; Giorgi, G.; Ouzounov, S.; Harpe, P.J.A.; Mischi, M. Dedicated Algorithm for Unobtrusive Fetal Heart Rate Monitoring Using Multiple Dry Electrodes. Sensors 2021, 21, 4298. [Google Scholar] [CrossRef]
- Virtanen, J.; Somppi, S.; Törnqvist, H.; Jeyhani, V.; Fiedler, P.; Gizatdinova, Y.; Majaranta, P.; Väätäjä, H.; Cardó, A.V.; Lekkala, J.; et al. Evaluation of Dry Electrodes in Canine Heart Rate Monitoring. Sensors 2018, 18, 1757. [Google Scholar] [CrossRef] [Green Version]
- Pani, D.; Dessì, A.; Saenz-Cogollo, J.F.; Barabino, G.; Fraboni, B.; Bonfiglio, A. Fully Textile, PEDOT:PSS Based Electrodes for Wearable ECG Monitoring Systems. IEEE Trans. Biomed. Eng. 2016, 63, 540–549. [Google Scholar] [CrossRef]
- Pei, W.; Zhang, H.; Wang, Y.; Guo, X.; Xing, X.; Huang, Y.; Xie, Y.; Yang, X.; Chen, H. Skin-Potential Variation Insensitive Dry Electrodes for ECG Recording. IEEE Trans. Biomed. Eng. 2017, 64, 463–470. [Google Scholar] [CrossRef]
- Lou, C.; Li, R.; Li, Z.; Liang, T.; Wei, Z.; Run, M.; Yan, X.; Liu, X. Flexible Graphene Electrodes for Prolonged Dynamic ECG Monitoring. Sensors 2016, 16, 1833. [Google Scholar] [CrossRef] [Green Version]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuator A-Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Chen, Y.-H.; de Beeck, M.O.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; van Hoof, C. Soft, Comfortable Polymer Dry Electrodes for High Quality ECG and EEG Recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, A.; Akabane, Y.; Kato, T.; Hoshino, H.; Kataoka, S.; Ishiyama, Y. Capacitive sensing of electrocardiographic potential through cloth from the dorsal surface of the body in a supine position: A preliminary study. IEEE Trans. Biomed. Eng. 2007, 54, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yu, C.; Dong, Y. Capacitively Coupled Electrocardiogram Measuring System and Noise Reduction by Singular Spectrum Analysis. IEEE Sens. J. 2016, 16, 3802–3810. [Google Scholar] [CrossRef]
- Lin, B.; Chou, W.; Wang, H.; Huang, Y.; Pan, J. Development of Novel Non-Contact Electrodes for Mobile Electrocardiogram Monitoring System. IEEE J. Transl. Eng. Health Med. 2013, 1, 1–8. [Google Scholar]
- Fong, E.-M.; Chung, W.-Y. A Hygroscopic Sensor Electrode for Fast Stabilized Non-Contact ECG Signal Acquisition. Sensors 2015, 15, 19237–19250. [Google Scholar]
- Leicht, L.; Eilebrecht, B.; Weyer, S.; Leonhardt, S.; Teichmann, D. Closed-Loop Control of Humidification for Artifact Reduction in Capacitive ECG Measurements. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 300–313. [Google Scholar] [CrossRef]
- Spinelli, E.; Haberman, M.; García, P.; Guerrero, F. A capacitive electrode with fast recovery feature. Physiol. Meas. 2012, 33, 1277–1288. [Google Scholar] [CrossRef]
- Serteyn, A.; Vullings, R.; Meftah, M.; Bergman, J.W.M. S Motion Artifacts in Capacitive ECG Measurements: Reducing the Combined Effect of DC Voltages and Capacitance Changes Using an Injection Signal. IEEE Trans. Biomed. Eng. 2015, 62, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.M.; Jung, T.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef]
- Drefuss, H.; Dreyfuss, H.H. The Measure of Man: Human Factors in Design; Whitney Library of Design: New York, NY, USA, 1967. [Google Scholar]
- Chu, C.-H.; Wang, I.-J. Mass Customized Design of Cosmetic Masks Using Three-Dimensional Parametric Human Face Models Constructed from Anthropometric Data. J. Comput. Inf. Sci. Eng. 2018, 18, 3. [Google Scholar] [CrossRef]
- Berntson, G.; Thomas Bigger, J., Jr.; Eckberg, D.; Grossman, P.; Kaufmann, P.; Malik, M.; Nagaraja, H.; Porges, S.; Saul, J.; Stone, P.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Majumder, S.; Chen, L.; Marinov, O.; Chen, C.; Mondal, T.; Deen, M.J. Noncontact Wearable Wireless ECG Systems for Long-Term Monitoring. IEEE Rev. Biomed. Eng. 2018, 11, 306–321. [Google Scholar] [CrossRef]
- Trindade, I.G.; da Silva, J.M.; Miguel, R.; Pereira, M.; Lucas, J.; Oliveira, L.; Valentim, B.; Barreto, J.; Silva, M.S. Design and Evaluation of Novel Textile Wearable Systems for the Surveillance of Vital Signals. Sensors 2016, 16, 1573. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Amano, Y.; Sato, T.; Saito, S.; Inoue, M. A Smart Shirt Made with Conductive Ink and Conductive Foam for the Measurement of Electrocardiogram Signals with Unipolar Precordial Leads. Fibers 2015, 3, 463–477. [Google Scholar] [CrossRef]
- Liu, B.; Tang, H.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Wearable carbon nanotubes-based polymer electrodes for ambulatory electrocardiographic measurements. Sens. Actuators A Phys. 2017, 265, 79–85. [Google Scholar] [CrossRef]


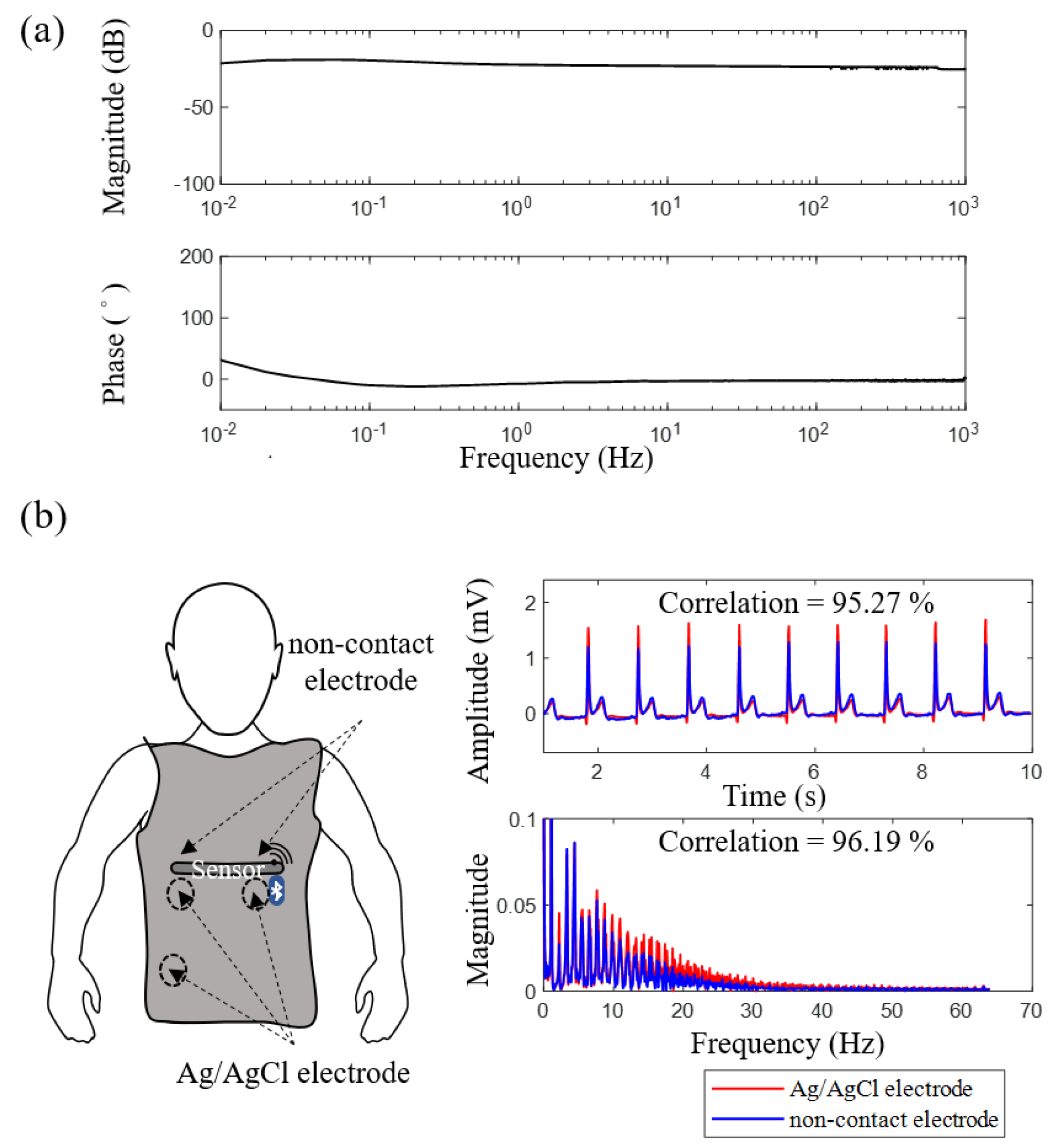



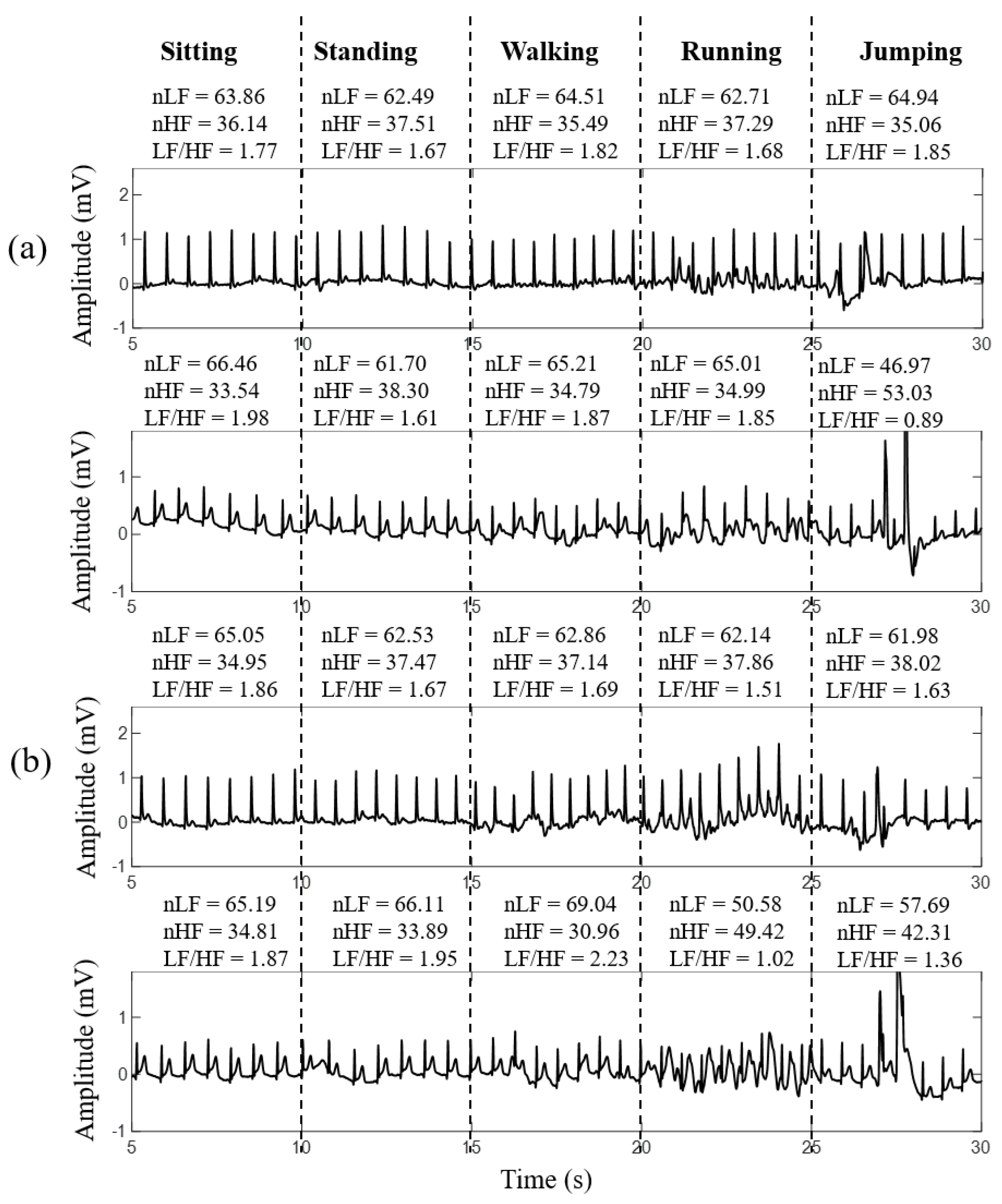
| Non-Contact Electrodes | Ag/AgCl Electrodes | |
|---|---|---|
| nLF (nu) | 64.60 ± 4.82 | 65.10 ± 4.70 |
| nHF (nu) | 35.40 ± 4.82 | 34.90 ± 4.70 |
| LF/HF | 1.87 ± 0.38 | 1.91 ± 0.40 |
| Peak-to-peak amplitude | 1.63 ± 0.51 | 1.71 ± 0.45 |
| Signal correlation | 94.96 ± 2.46 | |
| Smart Clothes with Textile Electrode [30] | Smart Clothes with Conductive Ink [31] | Smart Clothes with Polymer Electrode [32] | Proposed System | |
|---|---|---|---|---|
| Area of electrodes (cm2) | 2 | 4/6 | 7.07 | 14.96 |
| Size of acquisition module | 4 × 12 × 29.2 mm3 | 90 × 28 mm2 | 100 mm long | 26 × 65 mm2 |
| Frequency band (Hz) | 0.05–100 | - | - | 0.1–100 |
| Wireless transmission | Bluetooth | XBee | - | Bluetooth |
| Electrode material | Textile | Conductive ink | CNT-PDMS polymer | Copper |
| Non-contact electrode | No | No | No | Yes |
| Influence of motion | Larger | Larger | Larger | Smaller |
| Advantages | Lower risk of skin irritation, and good washing durability. | Lower risk of skin irritation, and good washing durability. | Good biocompatibility. | Non-contact measurement. |
| Limitations | Influence of hairs, motion artifact or fabric stretch. | Influence of fabric stretch and short circuit problem caused by sweat. | Influence of hairs and motion artifact. | Influence of cloth material and thickness. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, I.-J.; Chang, W.-T.; Wu, W.-H.; Lin, B.-S. Applying Noncontact Sensing Technology in the Customized Product Design of Smart Clothes Based on Anthropometry. Sensors 2021, 21, 7978. https://doi.org/10.3390/s21237978
Wang I-J, Chang W-T, Wu W-H, Lin B-S. Applying Noncontact Sensing Technology in the Customized Product Design of Smart Clothes Based on Anthropometry. Sensors. 2021; 21(23):7978. https://doi.org/10.3390/s21237978
Chicago/Turabian StyleWang, I-Jan, Wei-Ting Chang, Wen-Hao Wu, and Bor-Shyh Lin. 2021. "Applying Noncontact Sensing Technology in the Customized Product Design of Smart Clothes Based on Anthropometry" Sensors 21, no. 23: 7978. https://doi.org/10.3390/s21237978
APA StyleWang, I.-J., Chang, W.-T., Wu, W.-H., & Lin, B.-S. (2021). Applying Noncontact Sensing Technology in the Customized Product Design of Smart Clothes Based on Anthropometry. Sensors, 21(23), 7978. https://doi.org/10.3390/s21237978






