Electrochemical Characterization of Modified Glassy Carbon Electrodes for Non-Enzymatic Glucose Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation and Characterization of Copper Sulfides
2.3. Preparation of Modified Electrodes
2.4. Electrochemical Characterization of Modified Electrodes
2.5. Glucose Detection
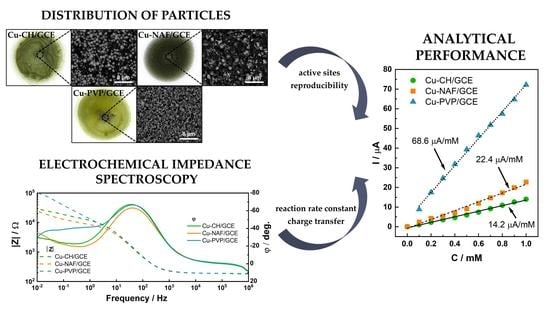
3. Results
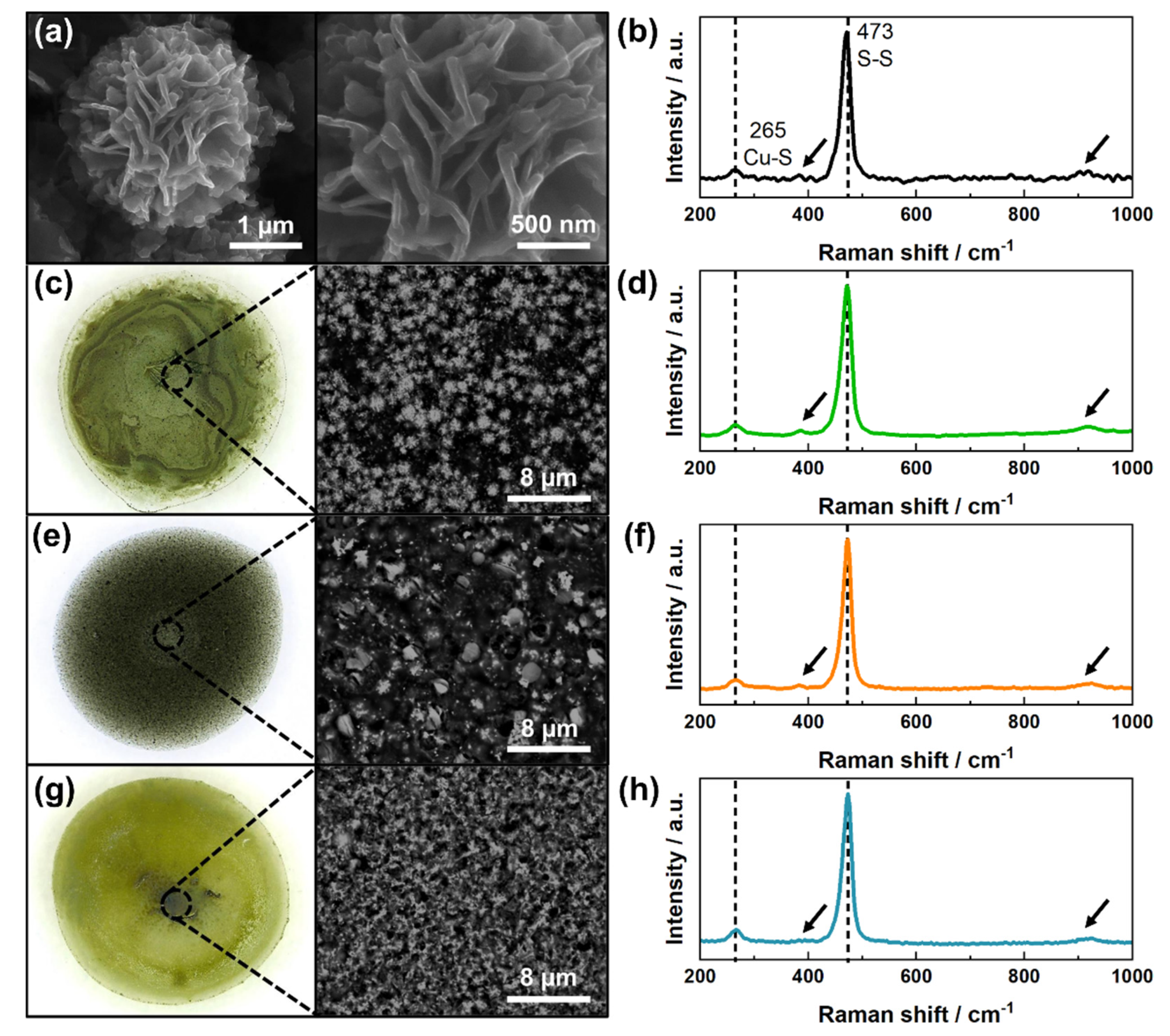
3.1. Characterization of Copper Sulfides and Drop-Casted Layers
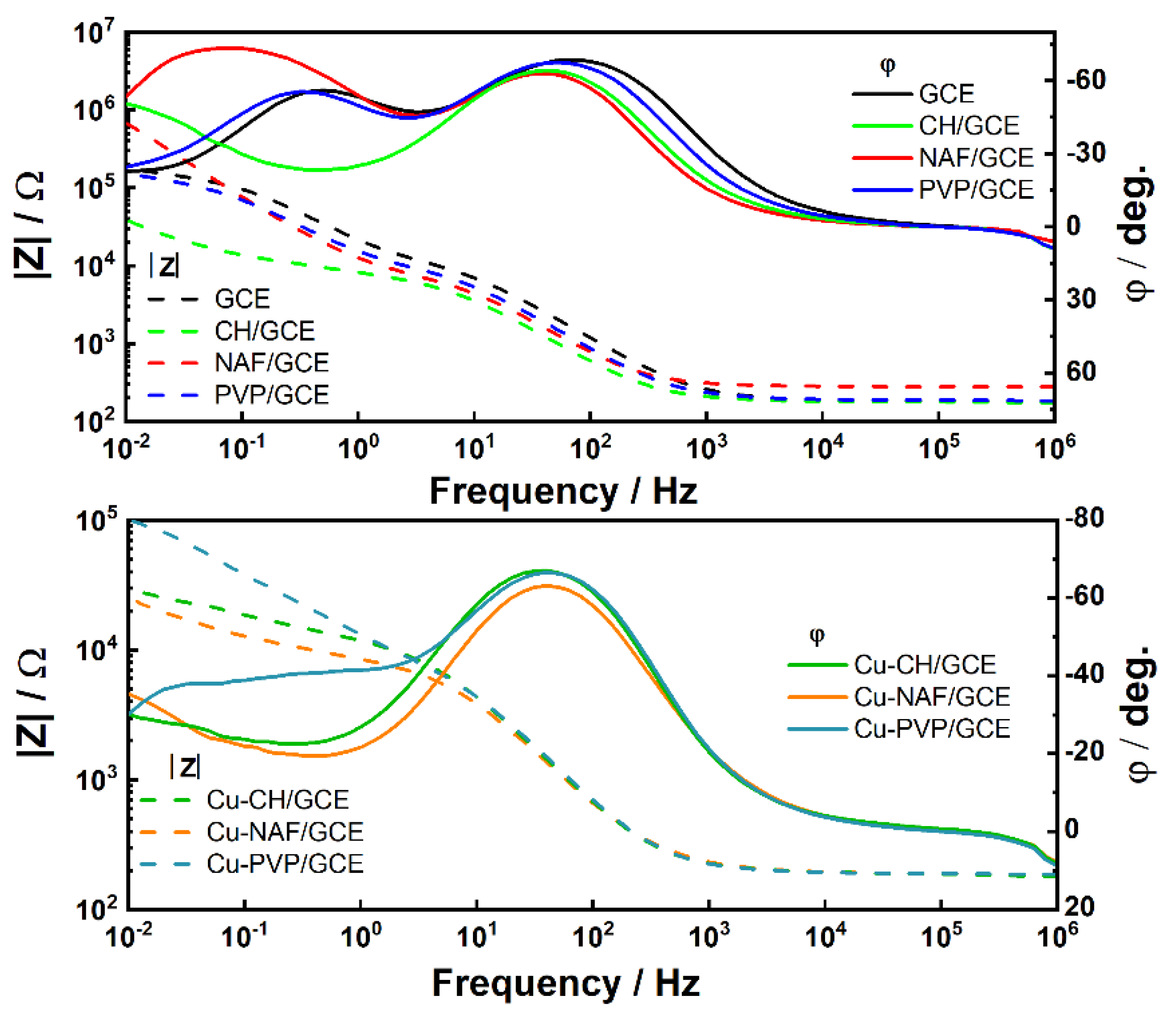
3.2. Electrochemical Characterization of Modified Electrodes
3.3. Glucose Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Dhara, K.; Mahapatra, D.R. Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials. Microchim. Acta 2018, 185, 1–32. [Google Scholar] [CrossRef]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent advances in enzymatic and non-enzymatic electrochemical glucose sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Takamura, K. Catalytic oxidation of biological components on platinum electrodes modified by adsorbed metals. Anodic oxidation of catecholamines. J. Electroanal. Chem. 1982, 141, 571–582. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, D. Electrocatalysis: Present and future. J. Appl. Electrochem. 1984, 14, 403–415. [Google Scholar] [CrossRef]
- Burke, L.D. Premonolayer oxidation and its role in electrocatalysis. Electrochim. Acta 1994, 39, 1841–1848. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187–1197. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Wang, Z.; Haidry, A.A.; Yao, Z.; Haque, E.; Wang, Y.; Li, G.; Daeneke, T.; McConville, C.F.; et al. Low dimensional materials for glucose sensing. Nanoscale 2021, 13, 11017–11040. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Z.; Hermerschmidt, F.; List-Kratochvil, E.J.W.; Pinna, N. Impact of different intermediate layers on the morphology and crystallinity of TiO2 grown on carbon nanotubes by atomic layer deposition. Adv. Mater. Interfaces 2021, 8, 2100759. [Google Scholar] [CrossRef]
- Yeh, J.; Zhu, B.; Wang, K.I.K.; Salcic, Z.; Kannappan, K.; Partridge, A. Modelling diffusion impedance in the sensing of micron-sized particles. Sens. Actuators B Chem. 2016, 237, 329–340. [Google Scholar] [CrossRef]
- Madej, M.; Fendrych, K.; Porada, R.; Flacha, M.; Kochana, J.; Baś, B. Application of Fe(III)-exchanged clinoptilolite/graphite nanocomposite for electrochemical sensing of amitriptyline. Microchem. J. 2021, 160, 105648. [Google Scholar] [CrossRef]
- De Faria, R.A.D.; Iden, H.; Dias Heneine, L.G.; Matencio, T.; Messaddeq, Y. Non-enzymatic impedimetric sensor based on 3-aminophenylboronic acid functionalized screen-printed carbon electrode for highly sensitive glucose detection. Sensors 2019, 19, 1686. [Google Scholar] [CrossRef] [Green Version]
- Siuzdak, K.; Niedzia, P.; Sobaszek, M.; Łęga, T.; Sawczak, M.; Czaczyk, E.; Dziąbowska, K.; Ossowski, T.; Nidzworski, D.; Bogdanowicz, R. Biomolecular influenza virus detection based on the electrochemical impedance spectroscopy using the nanocrystalline boron-doped diamond electrodes with covalently bound antibodies. Sens. Actuators B Chem. 2019, 280, 263–271. [Google Scholar] [CrossRef]
- Chelaghmia, M.L.; Fisli, H.; Nacef, M.; Brownson, D.A.C.; Affoune, A.M.; Satha, H.; Banks, C.E. Disposable non-enzymatic electrochemical glucose sensors based on screen-printed graphite macroelectrodes modified via a facile methodology with Ni, Cu, and Ni/Cu hydroxides are shown to accurately determine glucose in real human serum blood samples. Anal. Methods 2021, 13, 2812–2822. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Abroa-Nemeir, I.; Halima, H.B.; Gallardo-Gonzalez, J.; El Ami El Hassani, N.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; et al. Electrochemical impedance spectroscopy determination of glyphosate using a molecularly imprinted chitosan. Sens. Actuators B Chem. 2020, 309, 127753. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Fatibello-filho, O.; Brett, C.M.A. Electrochemical impedance studies of chitosan-modified electrodes for application in electrochemical sensors and biosensors. Electrochim. Acta 2010, 55, 6239–6247. [Google Scholar] [CrossRef]
- Madej, M.; Matoga, D.; Skaźnik, K.; Porada, R.; Baś, B.; Kochana, J. A voltammetric sensor based on mixed proton-electron conducting composite including metal-organic framework JUK-2 for determination of citalopram. Microchim. Acta 2021, 188, 184. [Google Scholar] [CrossRef]
- Raquel, G.-G.; Fernández Abedul, M.T. Electrochemical impedance spectroscopy for characterization of electrode surfaces: Carbon nanotubes on gold electrodes. In Laboratory Methods in Dynamic Electroanalysis; Fernandez Abedu, M.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–126. [Google Scholar]
- Ahmad, R.; Tripathy, N.; Ahn, M.; Bhat, K.S.; Wang, Y.; Yoo, J.; Kwon, D.; Yang, H. Highly efficient non-enzymatic glucose sensor based on CuO modified vertically-grown ZnO nanorods on electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Murphy, A.; O’riordan, A.; O’connell, I. Equivalent impedance models for electrochemical nanosensor-based integrated system design. Sensors 2021, 21, 3259. [Google Scholar] [CrossRef] [PubMed]
- Mazurków, J.; Kusior, A.; Radecka, M. Nonenzymatic glucose sensors based on copper sulfides: Effect of binder-particles interactions in drop-casted suspensions on electrodes electrochemical performance. Sensors 2021, 21, 802. [Google Scholar] [CrossRef] [PubMed]
- Kusior, A.; Jelen, P.; Mazurkow, J.; Nieroda, P.; Radecka, M. Synthesis of anisotropic Cu2−xS-based nanostructures by thermal oxidation. J. Therm. Anal. Calorim. 2019, 138, 4321–4329. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Lin, Y.; He, Y.; Tang, D. Enzyme-free amperometric glucose sensor using a glassy carbon electrode modified with poly(vinyl butyral) incorporating a hybrid nanostructure composed of molybdenum disulfide and copper sulfide. Microchim. Acta 2017, 184, 807–814. [Google Scholar] [CrossRef]
- Taylor, L.S.; Langkilde, F.W.; Zografi, G. Fourier transform Raman spectroscopic study of the interaction of water vapor with amorphous polymers. J. Pharm. Sci. 2001, 90, 888–901. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kaliyaraj Selva Kumar, A.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Barik, P.; Pal, S.; Pradhan, M. On-demand nanoparticle-on-mirror (NPoM) structure for cost-effective surface-enhanced Raman scattering substrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120193. [Google Scholar] [CrossRef]
- Cruz, J.; Kawasaki, M.; Gorski, W. Electrode coatings based on chitosan scaffolds. Anal. Chem. 2000, 72, 680–686. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Jara, C.C.; Cerro-Lopez, M.; Quiroz, M.A. Chitosan-modified glassy carbon electrodes: Electrochemical behaviour as a function of the preparation method and pH. Can. J. Anal. Sci. Spectrosc. 2009, 54, 53–62. [Google Scholar]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Zhang, Z.L.; Bao, L.; Pang, D.W. Surface structure-related electrochemical behaviors of glassy carbon electrodes. Electrochem. Commun. 2008, 10, 181–185. [Google Scholar] [CrossRef]
- Bystron, T.; Sramkova, E.; Dvorak, F.; Bouzek, K. Glassy carbon electrode activation—A way towards highly active, reproducible and stable electrode surface. Electrochim. Acta 2019, 299, 963–970. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Jedlińska, K.; Porada, R.; Lipińska, J.; Baś, B. The bi-band silver microelectrode: Fabrication, characterization and analytical study. Sens. Actuators B Chem. 2020, 302, 127152. [Google Scholar] [CrossRef]
- Guo, M.M.; Xia, Y.; Huang, W.; Li, Z. Electrochemical fabrication of stalactite-like copper micropillar arrays via surface rebuilding for ultrasensitive nonenzymatic sensing of glucose. Electrochim. Acta 2015, 151, 340–346. [Google Scholar] [CrossRef]
- Wei, C.; Zou, X.; Liu, Q.; Li, S.; Kang, C.; Xiang, W. A highly sensitive non-enzymatic glucose sensor based on CuS nanosheets modified Cu2O/CuO nanowire arrays. Electrochim. Acta 2020, 334, 135630. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Su, L.; Bellagamba, M.; Zhang, H.; Lei, Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosens. Bioelectron. 2010, 26, 542–548. [Google Scholar] [CrossRef]
- Keerthi, M.; Mutharani, B.; Chen, S.M.; Ranganathan, P. Carbon fibers coated with urchin-like copper sulfide for nonenzymatic voltammetric sensing of glucose. Microchim. Acta 2019, 186, 807. [Google Scholar] [CrossRef]
- Fu, Y.; Jin, W. Facile synthesis of core-shell CuS-Cu2S based nanocomposite for the high-performance glucose detection. Mater. Sci. Eng. C 2019, 105, 110120. [Google Scholar] [CrossRef]
- Huang, W.; Liu, F.; Huang, Y.; Yang, W.; Zhong, H.; Peng, J. Facile one-pot synthesis of hollow-structured CuS/Cu2S hybrid for enhanced electrochemical determination of glucose. Electrochemistry 2021, 89, 340–347. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, H.Y.; Kim, B.S. A novel CuS microflower superstructure based sensitive and selective nonenzymatic glucose detection. Sens. Actuators B Chem. 2016, 233, 93–99. [Google Scholar] [CrossRef]
- Qiana, L.; Maoa, J.; Tiana, X.; Yuanb, H.; Xiaoa, D. In situ synthesis of CuS nanotubes on Cu electrode for sensitive nonenzymatic glucose sensor. Sens. Actuators B Chem. 2013, 176, 952–959. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Tao, F.; Wang, L.; Chen, L.; Ying, Y.; Zhang, L.; Liu, H.; Xia, M. Solvothermal synthesis of sphere-like CuS microcrystals and improvement as nonenzymatic glucose sensor. J. Mater. Sci. 2013, 48, 5509–5516. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gu, A.; Wei, Y.; Fang, B. CuS nanotubes for ultrasensitive nonenzymatic glucose sensors. Chem. Commun. 2008, 5945–5947. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; Jang, D.M.; Cho, Y.J.; Kim, H.S.; Park, J.; Kim, J.U.; Choi, Y.; Lee, C.J. Nonenzymatic amperometric glucose sensing of platinum, copper sulfide, and tin oxide nanoparticle-carbon nanotube hybrid nanostructures. J. Phys. Chem. C 2009, 113, 1251–1259. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zi, J.; Li, W. Enzyme-free sensing of hydrogen peroxide and glucose at a CuS nanoflowers modified glassy carbon electrode. Electrochim. Acta 2014, 115, 126–130. [Google Scholar] [CrossRef]
- Liu, X.; Ai, L.; Jiang, J. Interconnected porous hollow CuS microspheres derived from metal-organic frameworks for efficient adsorption and electrochemical biosensing. Powder Technol. 2015, 283, 539–548. [Google Scholar] [CrossRef]
- Lu, W.; Sun, Y.; Dai, H.; Ni, P.; Jiang, S.; Wang, Y.; Li, Z.; Li, Z. Fabrication of cuprous sulfide nanorods supported on copper foam for nonenzymatic amperometric determination of glucose and hydrogen peroxide. RSC Adv. 2016, 6, 90732–90738. [Google Scholar] [CrossRef]
- Xu, X.; Jin, H.; Ren, Q.; Liu, A.; Li, J.; Yin, D.; Feng, X.; Dong, X.; Wang, J.; Wang, S. Electrochemical synthesis of CuxO/Cu2S nanocomposites as nonenzymatic glucose sensor. Int. J. Electrochem. Sci. 2016, 14, 5637–5645. [Google Scholar] [CrossRef]
- Kim, W. Bin; Lee, S.H.; Cho, M.; Lee, Y. Facile and cost-effective CuS dendrite electrode for non-enzymatic glucose sensor. Sensors Actuators, B Chem. 2017, 249, 161–167. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Karuppiah, C.; Elangovan, A. Sonochemical Synthesis of Sulfur Doped Reduced Graphene Oxide Supported CuS Nanoparticles for the Non-Enzymatic Glucose Sensor Applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. A non-enzymatic glucose sensor based on the CuS nanoflakes-reduced graphene oxide nanocomposite. Anal. Methods 2018, 10, 381–388. [Google Scholar] [CrossRef]
- Cao, M.; Wang, H.; Ji, S.; Zhao, Q.; Pollet, B.G.; Wang, R. Hollow core-shell structured Cu2O@Cu1.8S spheres as novel electrode for enzyme free glucose sensing. Mater. Sci. Eng. C 2019, 95, 174–182. [Google Scholar] [CrossRef]
- Cao, M.; Wang, H.; Kannan, P.; Ji, S.; Wang, X.; Zhao, Q.; Linkov, V.; Wang, R. Highly efficient non-enzymatic glucose sensor based on CuxS hollow nanospheres. Appl. Surf. Sci. 2019, 492, 407–416. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, X.; Nie, W.; Wang, Y.; Gao, J.; Wen, W.; Selvaraj, J.N.; Zhang, X.; Wang, S. Hollow copper sulfide nanocubes as multifunctional nanozymes for colorimetric detection of dopamine and electrochemical detection of glucose. Biosens. Bioelectron. 2019, 141, 111450. [Google Scholar] [CrossRef]
- Myeni, N.; Perla, V.K.; Ghosh, S.K.; Mallick, K. Organic matrix stabilized copper sulfide nanoparticles: Synthesis, characterization and application in glucose recognition. Mater. Today Commun. 2020, 25, 101291. [Google Scholar] [CrossRef]
- Zhang, S.; Mou, X.; Cui, Z.; Hou, C.; Yang, W.; Gao, H.; Luo, X. Partial sulfidation for constructing Cu2O-CuS heterostructures realizing enhanced electrochemical glucose sensing. New J. Chem. 2021, 45, 7204–7209. [Google Scholar] [CrossRef]




| Drop-Casted Suspension | Binder | Binder wt.% | Solvent | Cu1.8S/CuS wt.% |
|---|---|---|---|---|
| CH | chitosan | 0.5 | 25% CH3COOH | - |
| NAF | Nafion | 0.5 | water/1-propanol | - |
| PVP | PVP | 1.0 | 25% CH3COOH | - |
| Cu-CH | chitosan | 0.5 | 25% CH3COOH | 0.25 |
| Cu-NAF | Nafion | 0.5 | water/1-propanol | 0.25 |
| Cu-PVP | PVP | 1.0 | 25% CH3COOH | 0.25 |
| Modified Electrode | RCT [kΩ] | WCT [kΩ/s1/2] | CPECT [μF] | k × 10−5 [m/s] |
|---|---|---|---|---|
| GCE | 111.15 | 236.68 | 4.055 | 4.61 |
| CH/GCE | 9.43 | 269.71 | 9.628 | 54.36 |
| Cu-CH/GCE | 4.67 | 49.69 | 45.262 | 20.21 |
| NAF/GCE | 5.75 | 108.14 | 2.997 | 35.75 |
| Cu-NAF/GCE | 8.03 | 50.19 | 113.810 | 11.88 |
| PVP/GCE | 119.65 | 106.49 | 17.710 | 1.69 |
| Cu-PVP/GCE | 2.81 | 35.29 | 2.438 | 23.87 |
| Electrode | Slope [µA/mM] | LOD [mM] | LOQ [mM] | Sensitivity [µA/mM·cm2] | K | Ka | R2 |
|---|---|---|---|---|---|---|---|
| Cu-CH/GCE | 14.2 ± 0.4 | 0.016 | 0.054 | 793.3 | 11.128 | 0.186 | 0.999 |
| Cu-NAFGCE | 22.4 ± 0.8 | 0.017 | 0.055 | 794.3 | 14.057 | 0.096 | 0.997 |
| Cu-PVP/GCE | 68.6 ± 1.0 | 0.006 | 0.019 | 794.7 | 65.819 | 0.227 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurków, J.M.; Kusior, A.; Radecka, M. Electrochemical Characterization of Modified Glassy Carbon Electrodes for Non-Enzymatic Glucose Sensors. Sensors 2021, 21, 7928. https://doi.org/10.3390/s21237928
Mazurków JM, Kusior A, Radecka M. Electrochemical Characterization of Modified Glassy Carbon Electrodes for Non-Enzymatic Glucose Sensors. Sensors. 2021; 21(23):7928. https://doi.org/10.3390/s21237928
Chicago/Turabian StyleMazurków, Julia Maria, Anna Kusior, and Marta Radecka. 2021. "Electrochemical Characterization of Modified Glassy Carbon Electrodes for Non-Enzymatic Glucose Sensors" Sensors 21, no. 23: 7928. https://doi.org/10.3390/s21237928
APA StyleMazurków, J. M., Kusior, A., & Radecka, M. (2021). Electrochemical Characterization of Modified Glassy Carbon Electrodes for Non-Enzymatic Glucose Sensors. Sensors, 21(23), 7928. https://doi.org/10.3390/s21237928