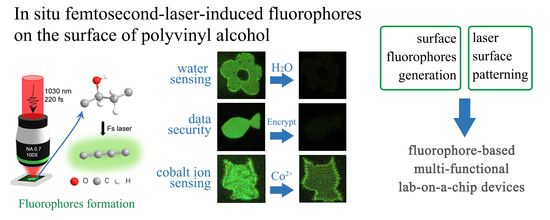
In Situ Femtosecond-Laser-Induced Fluorophores on Surface of Polyvinyl Alcohol for H2O/Co2+ Sensing and Data Security
Abstract
:1. Introduction
2. Materials and Methods
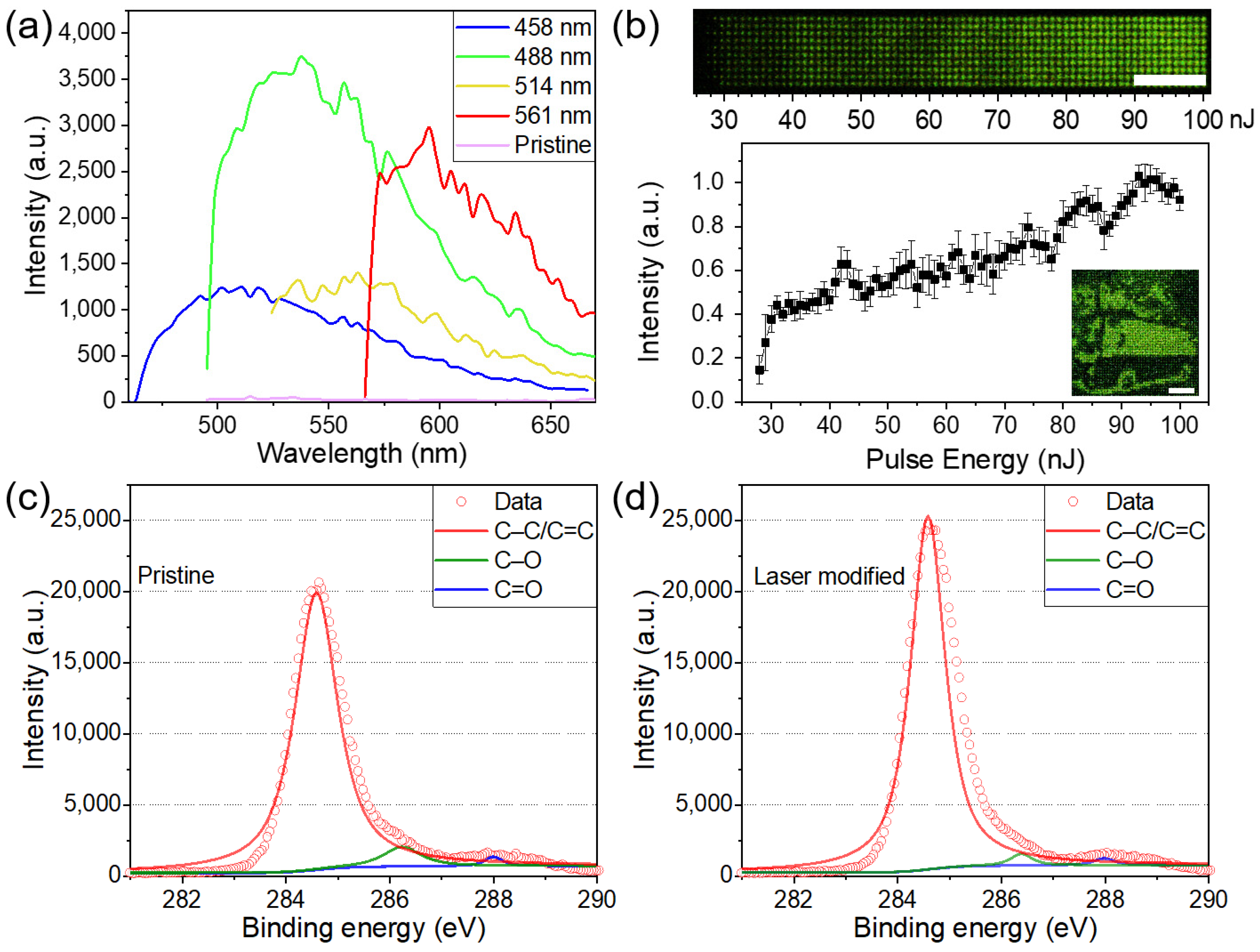
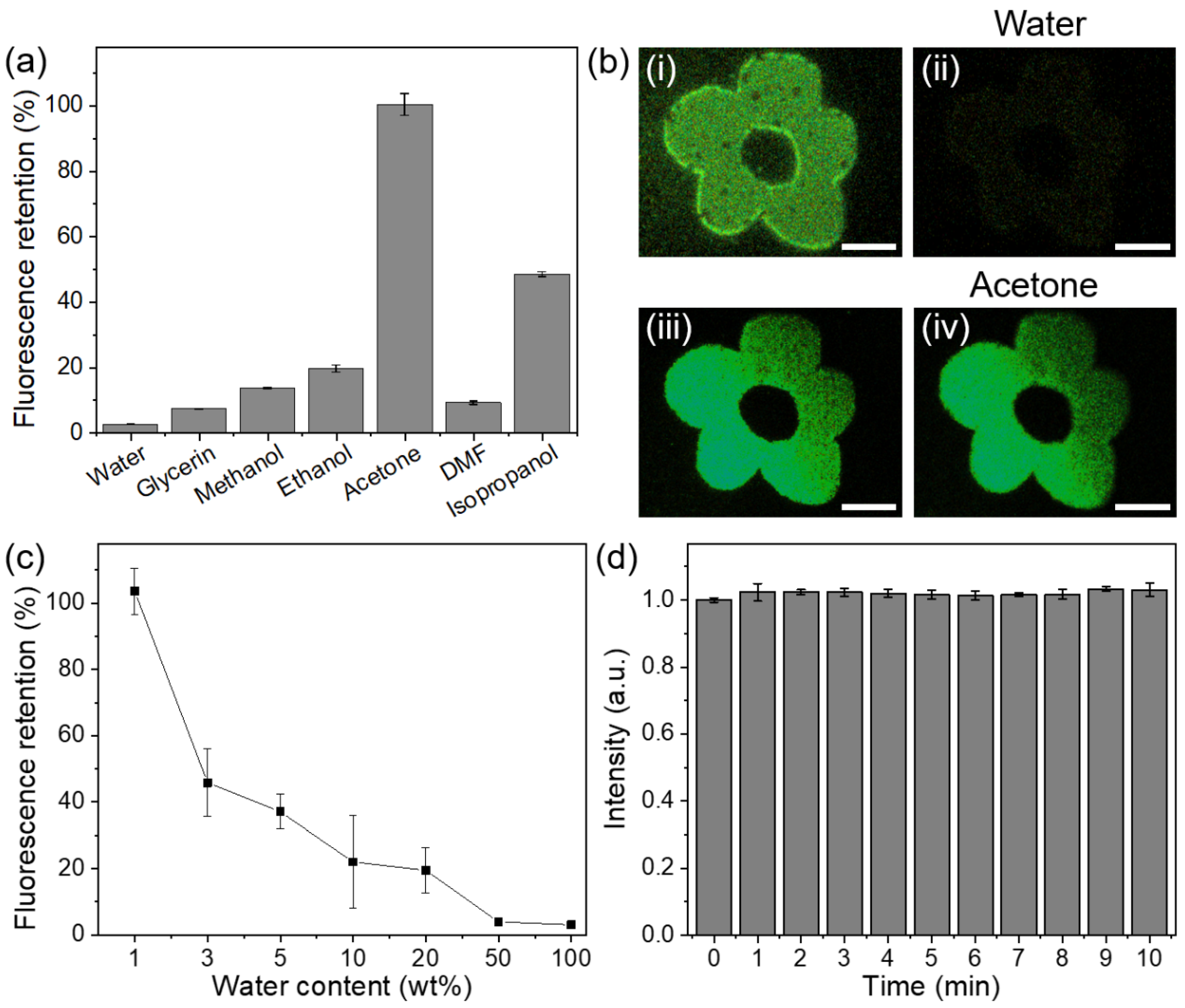
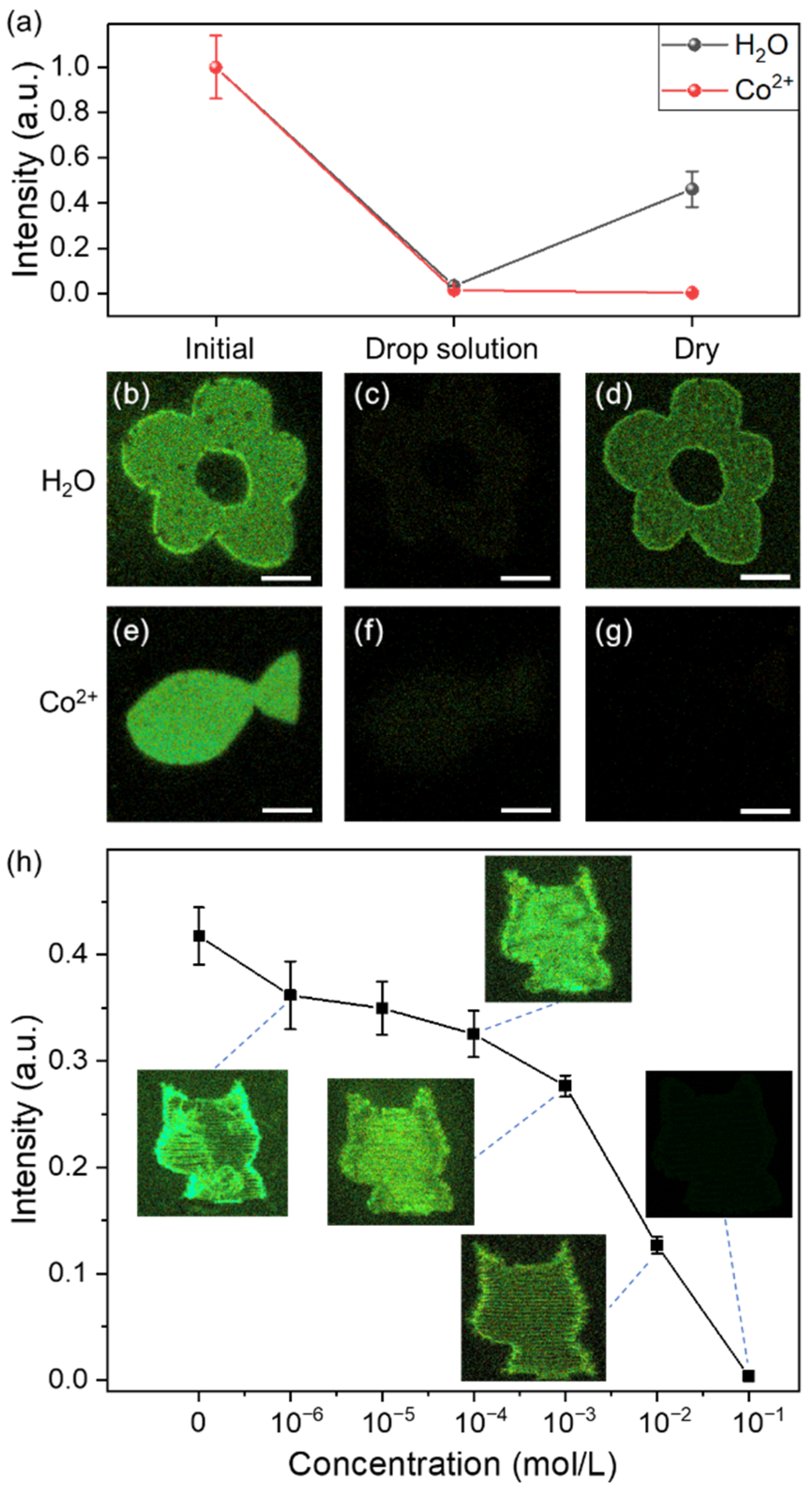
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, Q.; Liu, H.; Wang, B.; Wu, Q.; Pu, R.; Zhou, C.; Huang, B.; Peng, X.; Ågren, H.; He, S. Achieving High-Efficiency Emission Depletion Nanoscopy by Employing Cross Relaxation in Upconversion Nanoparticles. Nat. Commun. 2017, 8, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow Luminescent Carbon Dots for Drug Delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Cheng, H.; Lin, H.; Humphrey, M.G.; Zhang, C. A Hydrothermal Route to Water-Stable Luminescent Carbon Dots as Nanosensors for PH and Temperature. Carbon 2015, 82, 87–95. [Google Scholar] [CrossRef]
- Yang, M.; Tang, Q.; Meng, Y.; Liu, J.; Feng, T.; Zhao, X.; Zhu, S.; Yu, W.; Yang, B. Reversible “off-On” Fluorescence of Zn2+ Passivated Carbon Dots: Mechanism and Potential for the Detection of EDTA and Zn2+. Langmuir 2018, 34, 7767–7775. [Google Scholar] [CrossRef]
- Noun, F.; Jury, E.A.; Naccache, R. Elucidating the Quenching Mechanism in Carbon Dot-Metal Interactions–Designing Sensitive and Selective Optical Probes. Sensors 2021, 21, 1391. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liu, X.; Xie, Y.; Lv, H.; Wang, Z.; Yang, H.; Han, A.; Yang, X.; Zang, L. A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer. Sensors 2017, 17, 2517. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.H.; Gourevich, I.; Oh, J.K.; Jonkman, J.E.N.; Kumacheva, E. A Multidye Nanostructured Material for Optical Data Storage and Security Data Encryption. Adv. Mater. 2004, 16, 516–520. [Google Scholar] [CrossRef]
- Le, X.; Shang, H.; Yan, H.; Zhang, J.; Lu, W.; Liu, M.; Wang, L.; Lu, G.; Xue, Q.; Chen, T. A Urease-Containing Fluorescent Hydrogel for Transient Information Storage. Angew. Chem. Int. Ed. 2021, 60, 3640–3646. [Google Scholar] [CrossRef]
- Shi, L.; Meng, L.; Jiang, F.; Ge, Y.; Li, F.; Wu, X.G.; Zhong, H. In Situ Inkjet Printing Strategy for Fabricating Perovskite Quantum Dot Patterns. Adv. Funct. Mater. 2019, 29, 1903648. [Google Scholar] [CrossRef]
- Gansel, J.K.; Thiel, M.; Rill, M.S.; Decker, M.; Bade, K.; Saile, V.; Von Freymann, G.; Linden, S.; Wegener, M. Gold Helix Photonic Metamaterial as Broadband Circular Polarizer. Science 2009, 325, 1513–1515. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Wan, W.; Yang, H.; Gong, Q.; Li, Y. Fast Fabrication of Silver Helical Metamaterial with Single-Exposure Femtosecond Laser Photoreduction. Nanophotonics 2019, 8, 1087–1093. [Google Scholar] [CrossRef]
- Kallepalli, D.L.N.; Alshehri, A.M.; Marquez, D.T.; Andrzejewski, L.; Scaiano, J.C.; Bhardwaj, R. Ultra-High Density Optical Data Storage in Common Transparent Plastics. Sci. Rep. 2016, 6, 26163. [Google Scholar] [CrossRef]
- Chen, W.; Yan, Z.; Tian, J.; Liu, S.; Gao, J.; Zhang, J. Flexible Four-Dimensional Optical Data Storage Enabled by Single-Pulse Femtosecond Laser Irradiation in Thermoplastic Polyurethane. Opt. Lett. 2021, 46, 3211–3214. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zheng, H.Y.; Xia, H.M. Femtosecond Laser-Induced Modification of Surface Wettability of PMMA for Fluid Separation in Microchannels. Microfluid. Nanofluid. 2011, 10, 225–229. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Gan, Z.; Su, J.; Gao, Y. In Situ Localized Formation of Cesium Lead Bromide Nanocomposites for Fluorescence Micro-Patterning Technology Achieved by Organic Solvent Polymerization. J. Mater. Chem. C 2020, 8, 3409–3417. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. 2021, 60, 8608–8624. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, H.; Li, B.; Xie, Y.; Gong, X.; Liu, X.; Li, H.; Zhao, Y. Photoresponsive Luminescent Polymeric Hydrogels for Reversible Information Encryption and Decryption. Adv. Sci. 2019, 6, 1901529. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.; Si, J.; Yan, L.; Hou, X. Electron-Hole Recombination Dynamics in Carbon Nanodots. Carbon 2015, 95, 659–663. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.Y.; Zhang, Z. ying Synthesis and Surface Photochemistry of Graphitized Carbon Quantum Dots. J. Colloid Interface Sci. 2011, 356, 416–421. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Li, J.; Xie, Z.; Sun, Z. Tailoring Color Emissions from N-Doped Graphene Quantum Dots for Bioimaging Applications. Light Sci. Appl. 2015, 4, e364. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Size-Dependent Structural and Optical Characteristics of Glucose-Derived Graphene Quantum Dots. Part. Part. Syst. Charact. 2013, 30, 523–531. [Google Scholar] [CrossRef]
- Sciortino, A.; Marino, E.; Van Dam, B.; Schall, P.; Cannas, M.; Messina, F. Solvatochromism Unravels the Emission Mechanism of Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-Assembly and Embedding of Nanoparticles by In Situ Reduced Graphene for Preparation of a 3D Graphene/Nanoparticle Aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An Improved Hummers Method for Eco-Friendly Synthesis of Graphene Oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, H.; Li, J.; Xiang, J.; Panmai, M.; Dai, Q.; Xu, Y.; Tie, S.; Lan, S. Controllable Formation of Luminescent Carbon Quantum Dots Mediated by the Fano Resonances Formed in Oligomers of Gold Nanoparticles. Adv. Mater. 2019, 31, 1901371. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hao, Q.; Zhang, Y.; Xu, Y.; Lei, W. Fluorescence Quenchometric Method for Determination of Ferric Ion Using Boron-Doped Carbon Dots. Microchim. Acta 2016, 183, 273–279. [Google Scholar] [CrossRef]
- Wu, J.X.; Yan, B. A Dual-Emission Probe to Detect Moisture and Water in Organic Solvents Based on Green-Tb3+ Post-Coordinated Metal-Organic Frameworks with Red Carbon Dots. Dalt. Trans. 2017, 46, 7098–7105. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Wu, P.; Ma, C.; Wu, X.; Xu, M.; Luo, S.; Xu, Z.; Liu, S. Hydrothermal Synthesis of Nitrogen and Boron Co-Doped Carbon Quantum Dots for Application in Acetone and Dopamine Sensors and Multicolor Cellular Imaging. Sens. Actuators B Chem. 2019, 281, 34–43. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Fragouli, D.; Balusamy, B.; Patil, B.; Palei, M.; Sabella, S.; Uyar, T.; Athanassiou, A. Hydrochromic Carbon Dots as Smart Sensors for Water Sensing in Organic Solvents. Nanoscale Adv. 2019, 1, 4258–4267. [Google Scholar] [CrossRef] [Green Version]
- Chao, D.; Lyu, W.; Liu, Y.; Zhou, L.; Zhang, Q.; Deng, R.; Zhang, H. Solvent-Dependent Carbon Dots and Their Applications in the Detection of Water in Organic Solvents. J. Mater. Chem. C 2018, 6, 7527–7532. [Google Scholar] [CrossRef]
- Maillard, J.; Klehs, K.; Rumble, C.; Vauthey, E.; Heilemann, M.; Fürstenberg, A. Universal Quenching of Common Fluorescent Probes by Water and Alcohols. Chem. Sci. 2021, 12, 1352–1362. [Google Scholar] [CrossRef]
- Wen, H.; Zeng, X.; Xu, X.; Li, W.; Xie, F.; Xiong, Z.; Song, S.; Wang, B.; Li, X.; Cao, Y. Reversible Data Encryption-Decryption Using a PH Stimuli-Responsive Hydrogel. J. Mater. Chem. C 2021, 9, 2455–2463. [Google Scholar] [CrossRef]
- Nguyen, V.; Yan, L.; Si, J.; Hou, X. Femtosecond Laser-Assisted Synthesis of Highly Photoluminescent Carbon Nanodots for Fe3+ Detection with High Sensitivity and Selectivity. Opt. Mater. Express 2016, 6, 312–320. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Shah, M.T.; Balouch, A.; Alveroglu, E. Sensitive Fluorescence Detection of Ni2+ Ions Using Fluorescein Functionalized Fe3O4 Nanoparticles. J. Mater. Chem. C 2018, 6, 1105–1115. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of Graphene Quantum Dots-Based Optical Sensor for Toxic Metal Ion Detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; Chen, F.; Yang, Q.; Zhang, D.; Farooq, U.; Du, G.; Hou, X. Bioinspired Underwater Superoleophobic Surface with Ultralow Oil-Adhesion Achieved by Femtosecond Laser Microfabrication. J. Mater. Chem. A 2014, 2, 8790–8795. [Google Scholar] [CrossRef]
- Wang, L.; Cao, X.W.; Lv, C.; Xia, H.; Tian, W.J.; Chen, Q.D.; Juodkazis, S.; Sun, H.B. Formation of Deep-Subwavelength Structures on Organic Materials by Femtosecond Laser Ablation. IEEE J. Quantum Electron. 2018, 54, 1–7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Gao, J.; Tian, J.; Zhang, J. In Situ Femtosecond-Laser-Induced Fluorophores on Surface of Polyvinyl Alcohol for H2O/Co2+ Sensing and Data Security. Sensors 2021, 21, 7755. https://doi.org/10.3390/s21227755
Chen W, Gao J, Tian J, Zhang J. In Situ Femtosecond-Laser-Induced Fluorophores on Surface of Polyvinyl Alcohol for H2O/Co2+ Sensing and Data Security. Sensors. 2021; 21(22):7755. https://doi.org/10.3390/s21227755
Chicago/Turabian StyleChen, Weiliang, Jichao Gao, Jie Tian, and Jingyu Zhang. 2021. "In Situ Femtosecond-Laser-Induced Fluorophores on Surface of Polyvinyl Alcohol for H2O/Co2+ Sensing and Data Security" Sensors 21, no. 22: 7755. https://doi.org/10.3390/s21227755
APA StyleChen, W., Gao, J., Tian, J., & Zhang, J. (2021). In Situ Femtosecond-Laser-Induced Fluorophores on Surface of Polyvinyl Alcohol for H2O/Co2+ Sensing and Data Security. Sensors, 21(22), 7755. https://doi.org/10.3390/s21227755