Computational Identification and 3D Morphological Characterization of Renal Glomeruli in Optically Cleared Murine Kidneys
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Optical Clearing
2.3. Image Acquisition
2.4. Image Processing
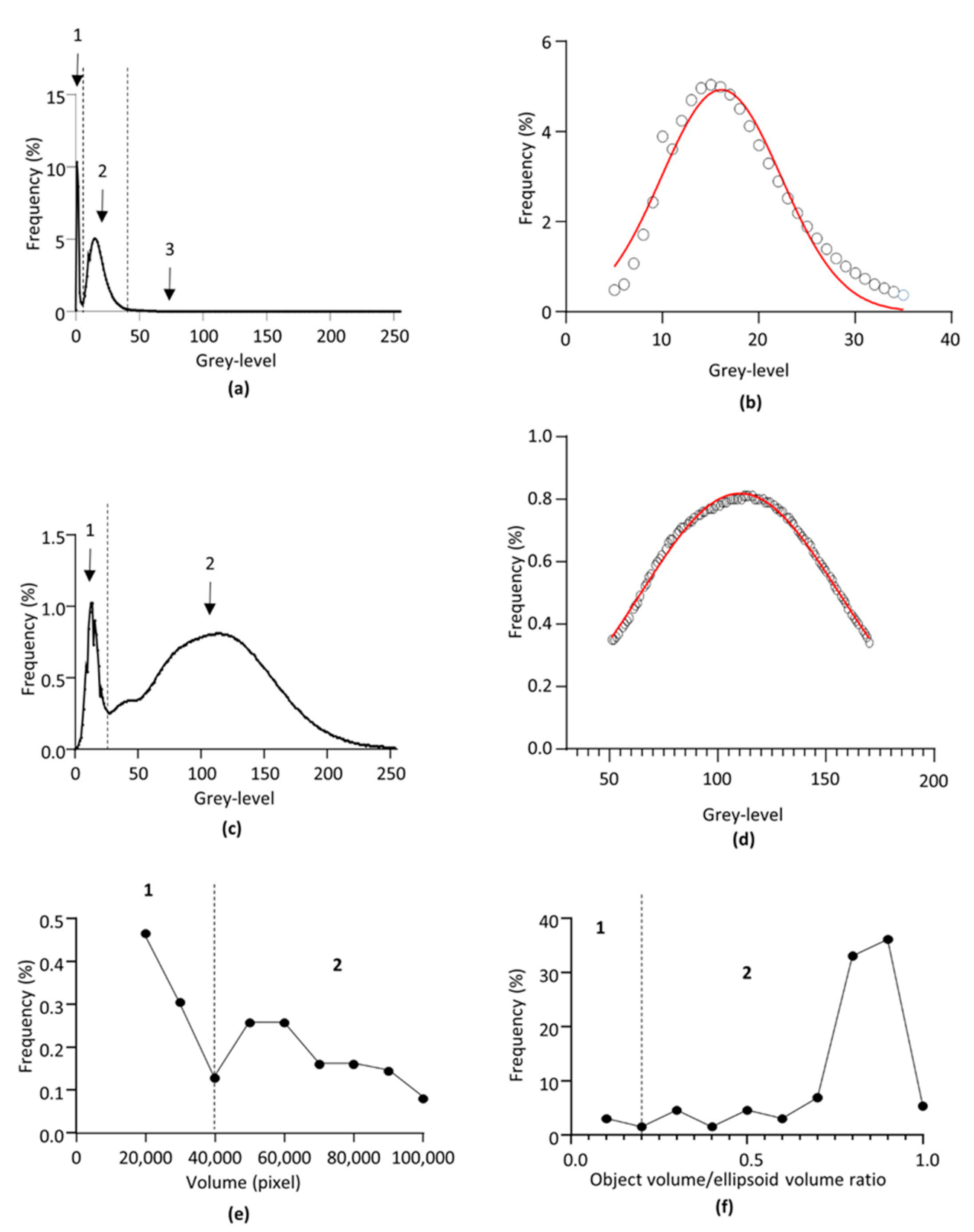
2.4.1. Grey-Level Segmentations and Glomerulus-Background Interface Determination
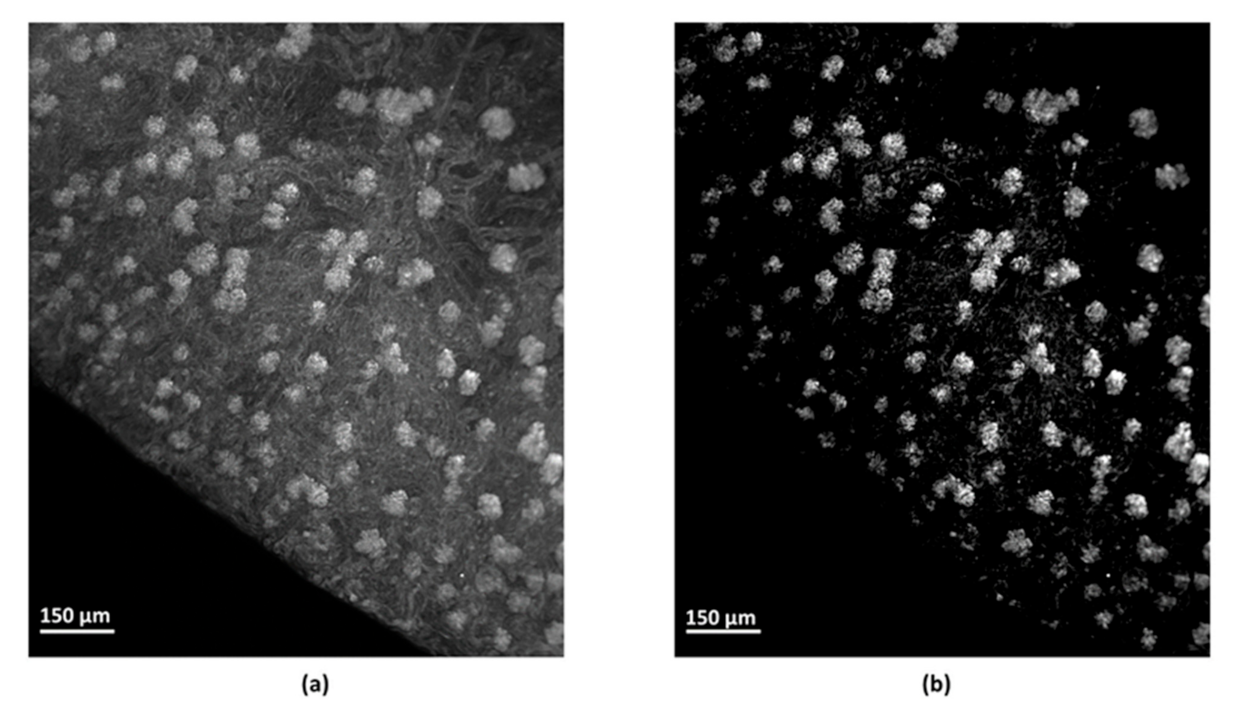
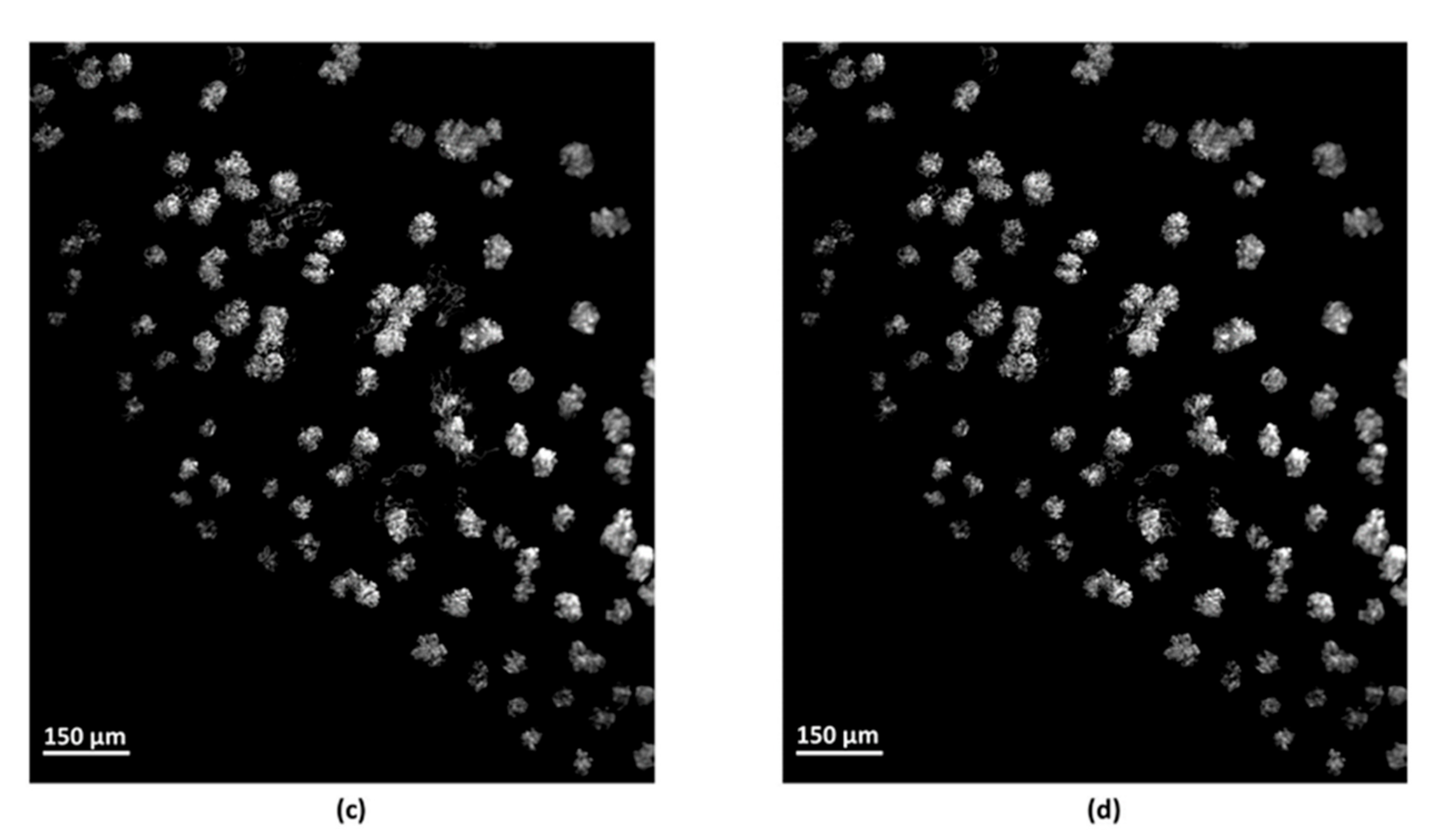
2.4.2. Filtering
2.4.3. Removal of Lectin-Labelled Non-Glomerular Objects
2.4.4. Segmentation Efficiency for the Individual Identification of the Glomeruli
2.5. Data Extraction and Calculated Parameters
2.5.1. Glomerular Volume Density
2.5.2. Glomerular Numerical Density
2.5.3. Surface Area
2.5.4. Volume
2.5.5. Compactness
2.6. Statistical Analysis
3. Results
3.1. Shrinkage
3.2. Identification of the Objects of Interest and Segmentation Efficiency
3.3. Glomerular External Surface
3.4. Glomerular Volume and Numerical Density
3.5. Surface Area, Volume and Compactness
4. Discussion
4.1. 3D Imaging
4.2. 3D Image Processing
4.3. Number, Volume and Morphology of the Glomeruli
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, M.A. Anatomy and Physiology of the Kidney. AORN J. 1998, 68, 799–820. [Google Scholar] [CrossRef]
- Pollak, M.R.; Quaggin, S.E.; Hoenig, M.P.; Dworkin, L.D. The Glomerulus: The Sphere of Influence. Clin. J. Am. Soc. Nephrol. 2014, 9, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Rush, B.M.; Small, S.A.; Stolz, D.B.; Tan, R.J. An Efficient Sieving Method to Isolate Intact Glomeruli from Adult Rat Kidney. JoVE 2018, 141, e58162. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. Chronic Kidney Disease as Cause of Cardiovascular Morbidity and Mortality. Nephrol. Dial. Transplant. 2005, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.C. Renal Relevant Radiology: Use of Ultrasound in Kidney Disease and Nephrology Procedures. Clin. J. Am. Soc. Nephrol. 2014, 9, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Caramori, M.L.; Basgen, J.M.; Mauer, M. Glomerular Structure in the Normal Human Kidney: Differences between Living and Cadaver Donors. J. Am. Soc. Nephrol. 2003, 14, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Workeneh, B.; Busque, S.; Blouch, K.; Derby, G.; Myers, B.D. Glomerular Function, Structure, and Number in Renal Allografts from Older Deceased Donors. J. Am. Soc. Nephrol. 2009, 20, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sheng, J.; He, H.; Chen, X.; Li, J.; Tan, R.; Wang, L.; Lan, H.-Y. A Simple and Highly Purified Method for Isolation of Glomeruli from the Mouse Kidney. Am. J. Physiol. Ren. Physiol. 2019, 317, F1217–F1223. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Grann, B.; Kristoffersen, I.B.; Skriver, E.; Thomsen, J.S.; Andreasen, A. Three-Dimensional Reconstruction of the Rat Nephron. Am. J. Physiol. Ren. Physiol. 2014, 306, F664–F671. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Brunson, J.C.; Sardi, J. Analysis of the Three Dimensional Structure of the Kidney Glomerulus Capillary Network. Sci Rep. 2020, 10, 20334. [Google Scholar] [CrossRef] [PubMed]
- Baldelomar, E.J.; Charlton, J.R.; de Ronde, K.A.; Bennett, K.M. In Vivo Measurements of Kidney Glomerular Number and Size in Healthy and Os /+ Mice Using MRI. Am. J. Physiol. Ren. Physiol. 2019, 317, F865–F873. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Silva, D.N.; Moreira, A.F.; Correia, I.J. Optical Clearing Methods: An Overview of the Techniques Used for the Imaging of 3D Spheroids. Biotechnol. Bioeng. 2019, 116, 2742–2763. [Google Scholar] [CrossRef] [PubMed]
- Renier, N.; Adams, E.L.; Kirst, C.; Wu, Z.; Azevedo, R.; Kohl, J.; Autry, A.E.; Kadiri, L.; Umadevi Venkataraju, K.; Zhou, Y.; et al. Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell 2016, 165, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.T.; Levine, S.T.; Haynes, S.M.; Gutierrez, P.; Baratta, J.L.; Tan, Z.; Longmuir, K.J. Use of Labeled Tomato Lectin for Imaging Vasculature Structures. Histochem. Cell Biol. 2015, 143, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ollion, J.; Cochennec, J.; Loll, F.; Escudé, C.; Boudier, T. TANGO: A Generic Tool for High-Throughput 3D Image Analysis for Studying Nuclear Organization. Bioinformatics 2013, 29, 1840–1841. [Google Scholar] [CrossRef] [PubMed]
- Bribiesca, E. A Measure of Compactness for 3D Shapes. Comput. Math. Appl. 2000, 40, 1275–1284. [Google Scholar] [CrossRef]
- Nicolas, N.; Roux, E. 3D Imaging and Quantitative Characterization of Mouse Capillary Coronary Network Architecture. Biology 2021, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Bonvalet, J.P.; Champion, M.; Courtalon, A.; Farman, N.; Vandewalle, A.; Wanstok, F. Number of Glomeruli in Normal and Hypertrophied Kidneys of Mice and Guinea-Pigs. J. Physiol. 1977, 269, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Fleck, D.; Ortz, L.; Papadouri, S.; Strieder, T.; Böhner, A.M.C.; van der Wolde, J.W.; Vogt, M.; Saritas, T.; Kuppe, C.; et al. Novel 3D Analysis Using Optical Tissue Clearing Documents the Evolution of Murine Rapidly Progressive Glomerulonephritis. Kidney Int. 2019, 96, 505–516. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Values |
|---|---|
| Surface | |
| R² | 0.69 |
| μ | 17,249 ± 870 µm² |
| σ | 5014 ± 930 µm² |
| Volume | |
| R² | 0.51 |
| μ | 53,579 ± 4200 µm3 |
| σ | 22,057 ± 4500 µm3 |
| Compactness | |
| R² | 0.41 |
| μ | 0.055 ± 0.01 |
| σ | 0.036 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas, N.; Nicolas, N.; Roux, E. Computational Identification and 3D Morphological Characterization of Renal Glomeruli in Optically Cleared Murine Kidneys. Sensors 2021, 21, 7440. https://doi.org/10.3390/s21227440
Nicolas N, Nicolas N, Roux E. Computational Identification and 3D Morphological Characterization of Renal Glomeruli in Optically Cleared Murine Kidneys. Sensors. 2021; 21(22):7440. https://doi.org/10.3390/s21227440
Chicago/Turabian StyleNicolas, Nabil, Nour Nicolas, and Etienne Roux. 2021. "Computational Identification and 3D Morphological Characterization of Renal Glomeruli in Optically Cleared Murine Kidneys" Sensors 21, no. 22: 7440. https://doi.org/10.3390/s21227440
APA StyleNicolas, N., Nicolas, N., & Roux, E. (2021). Computational Identification and 3D Morphological Characterization of Renal Glomeruli in Optically Cleared Murine Kidneys. Sensors, 21(22), 7440. https://doi.org/10.3390/s21227440






