Metal–Organic-Framework- and MXene-Based Taste Sensors and Glucose Detection
Abstract
:1. Introduction
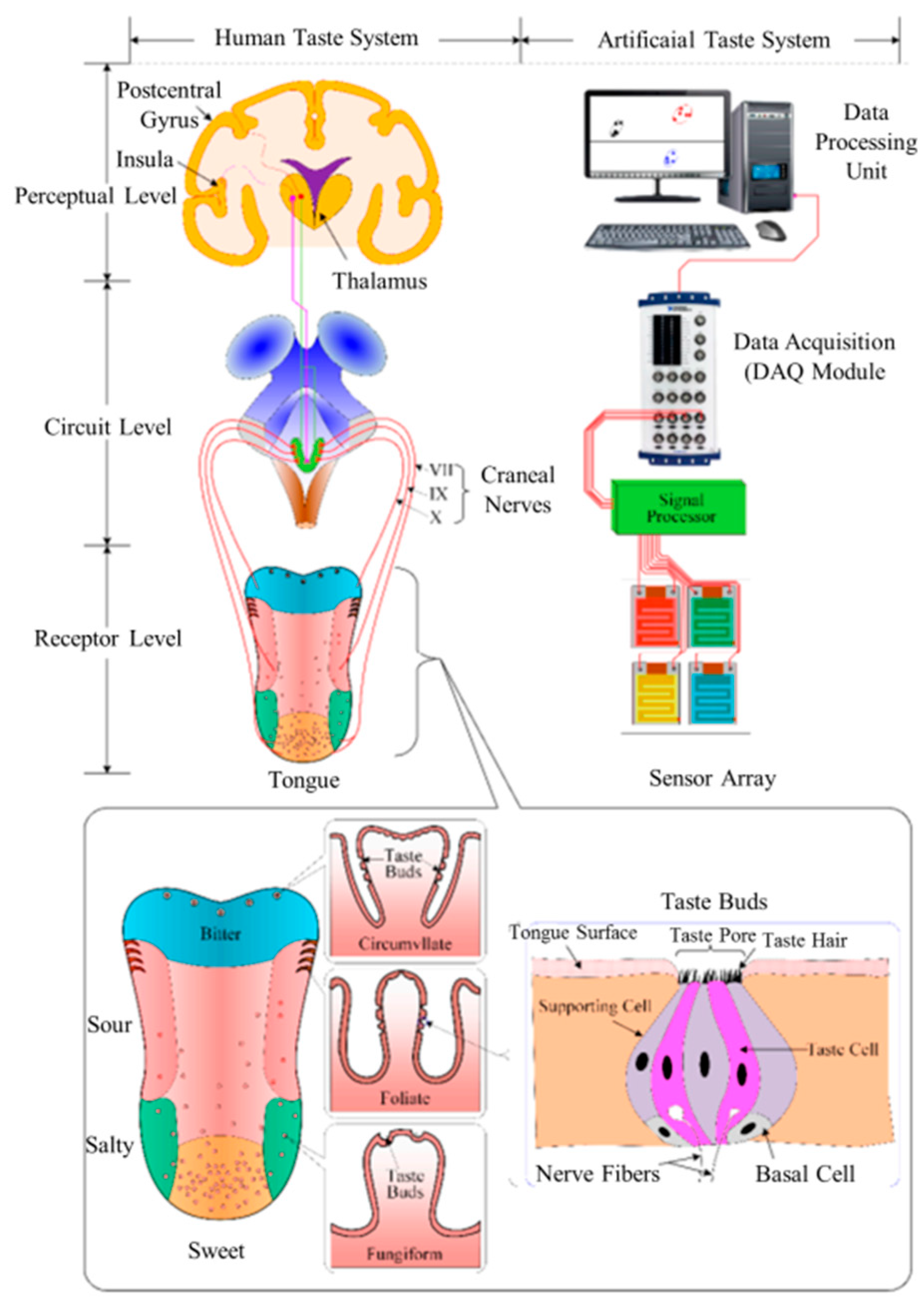
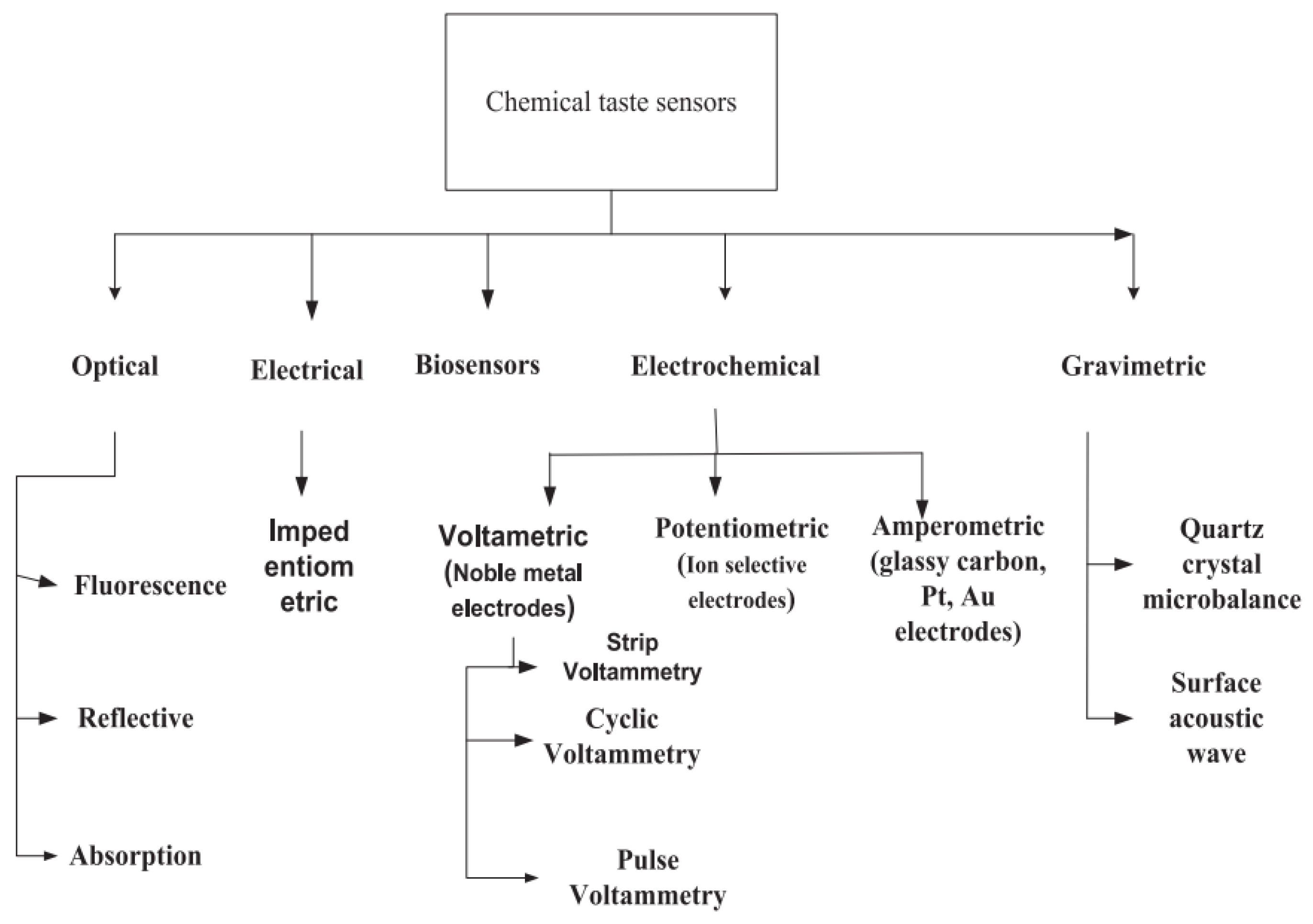
2. Fundamentals of Taste Sensor
3. MOFs for Taste Sensing
3.1. MOFs as Taste Sensors
3.2. MOF-Based Electrochemical Sensors for Glucose Detection
3.2.1. Pristine MOFs
3.2.2. MOF-Derived Metal Compounds
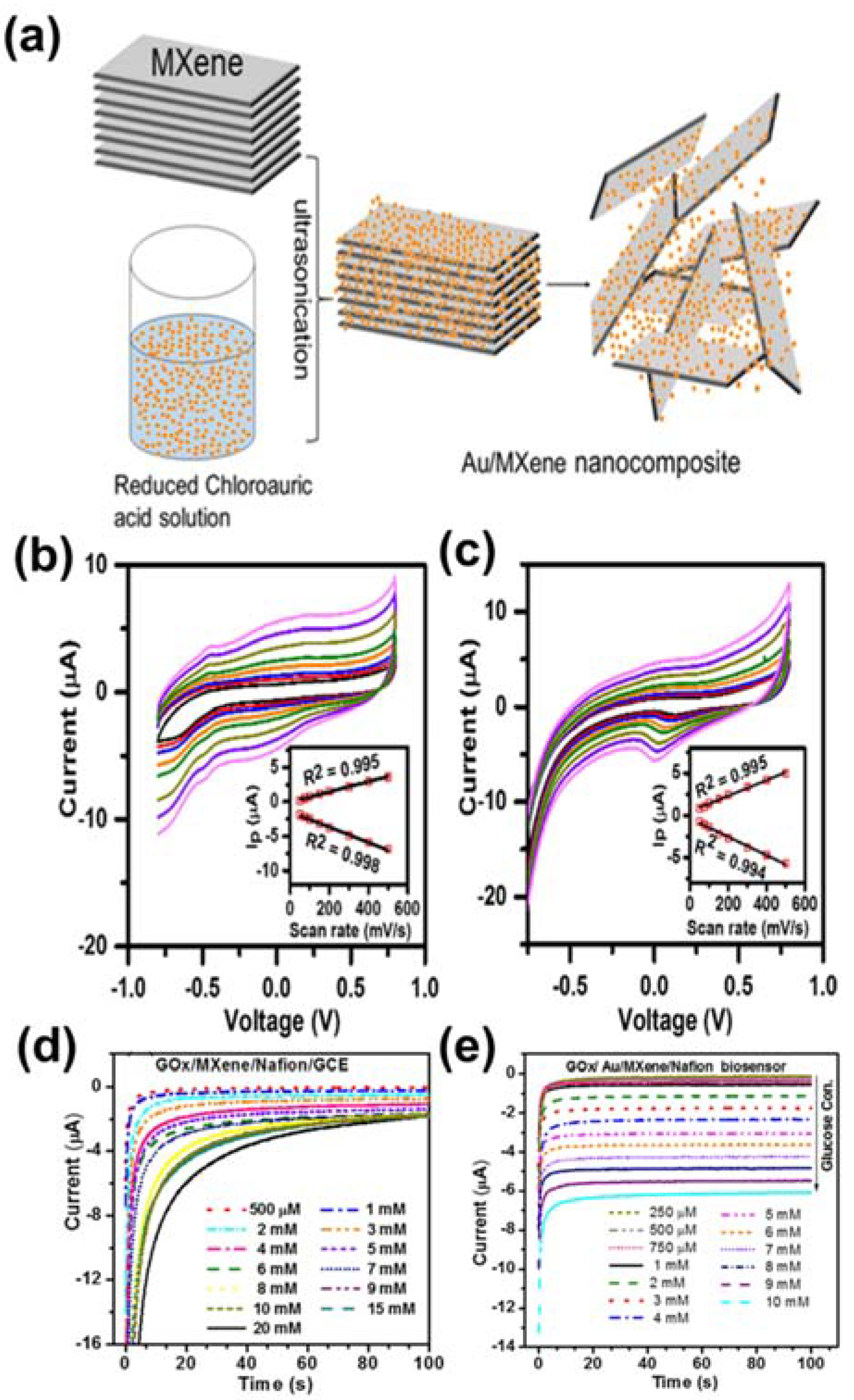
4. MXenes for Glucose Detection
4.1. MXenes
4.2. MXene-Based Composites
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toko, K. A taste sensor. Meas. Sci. Technol. 1998, 9, 1919. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste sensor: Electronic tongue with lipid membranes. Anal. Sci. 2019, 36, 19R008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhu, L.; Zhang, W.; Wei, Z. Application of the voltammetric electronic tongue based on nanocomposite modified electrodes for identifying rice wines of different geographical origins. Anal. Chim. Acta 2019, 1050, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Ye, B. An efficient electrochemical glucose sensor based on porous nickel-based metal organic framework/carbon nanotubes composite (Ni-MOF/CNTs). J. Alloys Compd. 2018, 767, 651–656. [Google Scholar] [CrossRef]
- Sinha, A.; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Banerjee, R.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. A review on combined odor and taste sensor systems. J. Food Eng. 2016, 190, 10–21. [Google Scholar] [CrossRef]
- Ahn, H.H.; Lee, M.S. Adsorption of Pd(II) onto Zr(IV) Based Metal-Organic Framework UIO-66-NH2 from Hydrochloric Acid Solution. Korean J. Met. Mater. 2019, 57, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, T.; Choi, D. Optimization of ZnO/Cu/ZnO Flexible Transparent Conductive Electrodes Fabricated by Magnetron Sputtering. Korean J. Met. Mater. 2019, 57, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Ramyashree, M.; Priya, S.S.; Freudenberg, N.C.; Sudhakar, K.; Tahir, M. Metal-organic framework-based photocatalysts for carbon dioxide reduction to methanol: A review on progress and application. J. CO2 Util. 2020, 43, 101374. [Google Scholar]
- Tekalgne, M.A.; Do, H.; Hasani, A.; Van Le, Q.; Jang, H.; Ahn, S.; Kim, S. Two-dimensional materials and metal-organic frameworks for the CO2 reduction reaction. Mater. Today Adv. 2020, 5, 100038. [Google Scholar] [CrossRef]
- Budnikova, Y.H. Recent advances in metal–organic frameworks for electrocatalytic hydrogen evolution and overall water splitting reactions. Dalton Trans. 2020, 49, 12483–12502. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, R.; Xu, Q. Metal–organic framework based catalysts for hydrogen evolution. Adv. Energy Mater. 2018, 8, 1801193. [Google Scholar] [CrossRef]
- Kukulka, W.; Cendrowski, K.; Michalkiewicz, B.; Mijowska, E. MOF-5 derived carbon as material for CO2 absorption. RSC Adv. 2019, 9, 18527–18537. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Zuluaga, S.; Fuentes, E.; Mattson, E.C.; Veyan, J.-F.; Wang, H.; Li, J.; Thonhauser, T.; Chabal, Y.J. Trapping gases in metal-organic frameworks with a selective surface molecular barrier layer. Nat. Commun. 2016, 7, 13871. [Google Scholar] [CrossRef]
- Cheng, K.; Li, Y.; Gao, Z.; Chen, F.; You, C.; Sun, B. Two-dimensional metal organic framework for effective gas absorption. Inorg. Chem. Commun. 2019, 101, 27–31. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Tavra, C.; Baxter, E.F.; Tian, T.; Bennett, T.D.; Slater, N.K.; Cheetham, A.K.; Fairen-Jimenez, D. Amorphous metal–organic frameworks for drug delivery. Chem. Commun. 2015, 51, 13878–13881. [Google Scholar] [CrossRef] [Green Version]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Zhang, L.T.; Zhou, Y.; Han, S.T. The Role of Metal–Organic Frameworks in Electronic Sensors. Angew. Chem. 2021, 60, 15192–15212. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.O.; Hupp, J.T. Metal–organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hönicke, I.M.; Senkovska, I.; Bon, V.; Baburin, I.A.; Bönisch, N.; Raschke, S.; Evans, J.D.; Kaskel, S. Balancing mechanical stability and ultrahigh porosity in crystalline framework materials. Angew. Chem. Int. Ed. 2018, 57, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Chung, W.-C.; Wei, Z.; Gu, Z.-Y.; Jiang, H.-L.; Chen, Y.-P.; Darensbourg, D.J.; Zhou, H.-C. Construction of ultrastable porphyrin Zr metal–organic frameworks through linker elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef]
- Banerjee, D.; Kim, S.J.; Parise, J.B. Lithium based metal− organic framework with exceptional stability. Cryst. Growth Des. 2009, 9, 2500–2503. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A Ni-based redox-active metal-organic framework for sensitive and non-enzymatic detection of glucose. J. Electroanal. Chem. 2018, 822, 43–49. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, S.; Li, X.; Zhang, G.; Guo, X.; Xue, H.; Pang, H. Facile synthesis of ultrathin Ni-MOF nanobelts for high-efficiency determination of glucose in human serum. J. Mater. Chem. 2017, 5, 5234–5239. [Google Scholar] [CrossRef]
- Wiwasuku, T.; Boonmak, J.; Siriwong, K.; Ervithayasuporn, V.; Youngme, S. Highly sensitive and selective fluorescent sensor based on a multi-responsive ultrastable amino-functionalized Zn(II)-MOF for hazardous chemicals. Sens. Actuators B Chem. 2019, 284, 403–413. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, L.; Sun, M.; Deng, D.; Su, Y.; Lv, Y. Amino-functionalized metal-organic frameworks nanoplates-based energy transfer probe for highly selective fluorescence detection of free chlorine. Anal. Chem. 2016, 88, 3413–3420. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.M.T.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Van Le, Q. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Khazaei, M.; Mishra, A.; Venkataramanan, N.S.; Singh, A.K.; Yunoki, S. Recent advances in MXenes: From fundamentals to applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Anasori, B.; Gogotsi, Û.G. 2D Metal Carbides and Nitrides (MXenes); Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2TX MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Meshkian, R.; Näslund, L.-Å.; Halim, J.; Lu, J.; Barsoum, M.W.; Rosen, J. Synthesis of two-dimensional molybdenum carbide, Mo2C, from the gallium based atomic laminate Mo2Ga2C. Scr. Mater. 2015, 108, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zha, X.; Chen, F.Y.; Ye, Q.; Eklund, P.; Du, S.; Huang, Q. A two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5. Angew. Chem. Int. Ed. 2016, 55, 5008–5013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihsanullah, I. Potential of MXenes in water desalination: Current status and perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Ding, L.; Wang, H. MXene-Based Membranes for Separation Applications. Small Science 2021, 1, 2100013. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, Y.; Xie, Z.; Wang, D.; Yuan, Y.; Kang, H.; Su, B.; Cheng, Z.; Liu, Y. Modified MXene/holey graphene films for advanced supercapacitor electrodes with superior energy storage. Adv. Sci. 2018, 5, 1800750. [Google Scholar] [CrossRef]
- Navarro-Suárez, A.M.; Maleski, K.; Makaryan, T.; Yan, J.; Anasori, B.; Gogotsi, Y. 2D titanium carbide/reduced graphene oxide heterostructures for supercapacitor applications. Batter. Supercaps 2018, 1, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, C.; Ci, S.; Ma, Y.; Liu, T.; Gao, L.; Qian, P.; Ji, C.; Su, Y. Accelerating 2D MXene catalyst discovery for the hydrogen evolution reaction by computer-driven workflow and an ensemble learning strategy. J. Mater. Chem. A 2020, 8, 23488–23497. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Banjade, H.R.; Thenuwara, A.C.; Anasori, B.; Yan, Q.; Strongin, D.R. Electrocatalytic CO2 reduction on earth abundant 2D Mo2C and Ti3C2 MXenes. Chem. Commun. 2021, 57, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liao, G.; Liu, S. Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. NPJ 2D Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for Sensing Applications: Recent Progress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef]
- Mehdi Aghaei, S.; Aasi, A.; Panchapakesan, B. Experimental and Theoretical Advances in MXene-Based Gas Sensors. ACS Omega 2021, 6, 2450–2461. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, M.M.; Chowdhury, H.K. Two-dimensional mxene-based flexible nanostructures for functional nanodevices: A review. J. Mater. Chem. A 2021, 9, 3231–3269. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for supercapacitors: Status, challenges and prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rehman, M.A.; Lee, S.; Kim, M.; Hong, H.; Park, J.-Y.; Seo, Y. Supercapacitors based on Ti3C2Tx MXene extracted from supernatant and current collectors passivated by CVD-graphene. Sci. Rep. 2021, 11, 649. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.; Zhao, D.; Zhang, X.; Zhuo, J.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Ti3C2Tx MXene for electrode materials of supercapacitors. J. Mater. Chem. A 2021, 9, 11501–11529. [Google Scholar] [CrossRef]
- Khan, M.; Rahaman, R.; Khalilian, A.; Kang, S.-W. A high sensitivity IDC-electronic tongue using dielectric/sensing membranes with solvatochromic dyes. Sensors 2016, 16, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.-Y. Photoluminescent metal–organic frameworks and their application for sensing biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Wang, F.; Pu, Y.; Zhang, X.; Zhang, F.; Cheng, H.; Zhao, Y. A series of multifunctional lanthanide metal-organic frameworks for luminescent sensing and photocatalytic applications. J. Lumin. 2019, 206, 192–198. [Google Scholar] [CrossRef]
- Lee, T.; Lee, H.L.; Tsai, M.H.; Cheng, S.-L.; Lee, S.-W.; Hu, J.-C.; Chen, L.-T. A biomimetic tongue by photoluminescent metal–organic frameworks. Biosens. Bioelectron. 2013, 43, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lin, T.Y.; Lee, H.L.; Chang, Y.H.; Tsai, Y.C. Biomimetic Taste Receptors with Chiral Recognition by Photoluminescent Metal-Organic Frameworks Chelated with Polyaniline Helices. Chem. Eur. J. 2016, 22, 1406–1414. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Xu, G.; Lu, Y.; Li, S.; Hu, L.; Li, J.; Liu, Q. Biomimetic sensor for sweet taste detection based on graphene composite materials. Sens. Actuators B Chem. 2017, 251, 909–917. [Google Scholar] [CrossRef]
- Mao, Y.; Tian, S.; Gong, S.; Qin, Y.; Han, J.; Deng, S. A Broad-Spectrum Sweet Taste Sensor Based on Ni(OH)2/Ni Electrode. Sensors 2018, 18, 2758. [Google Scholar] [CrossRef] [Green Version]
- Toyota, K.; Cui, H.; Abe, K.; Habara, M.; Toko, K.; Ikezaki, H. Sweetness sensor with lipid/polymer membranes: Response to various sugars. Sens. Mater. 2011, 23, 475–482. [Google Scholar]
- Hasani, A.; Do, H.H.; Tekalgne, M.; Hong, S.H.; Jang, H.W.; Kim, S.Y. Recent progress of two-dimensional materials and metal–organic framework-based taste sensors. J. Korean Ceram. Soc. 2020, 57, 353–367. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Lu, W.; Sun, X. High-performance non-enzymatic glucose detection: Using a conductive Ni-MOF as an electrocatalyst. J. Mater. Chem. B 2020, 8, 5411–5415. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wan, Y.; Wang, Y.; Liu, T.; Zou, P.; Wang, X.; Zhao, Q.; Ding, F.; Rao, H. Flower-like Ni(II)-based Metal-organic Framework-decorated Ag Nanoparticles: Fabrication, Characterization and Electrochemical Detection of Glucose. Electroanalysis 2019, 31, 2179–2186. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Gong, J.; Ji, Z.; Nie, Q. Efficient nonenzymatic sensors based on Ni-MOF microspheres decorated with Au nanoparticles for glucose detection. J. Electron. Mater. 2020, 49, 4754–4763. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, D.; Yan, L.; Lai, H.; Chu, X.-Q.; Ge, D.; Chen, X. Enhanced non-enzymatic glucose sensing based on porous ZIF-67 hollow nanoprisms. New J. Chem. 2021, 45, 10031–10039. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Yuniasari, R.; Amri, F.; Abrori, S.; Septiani, N.; Rezki, M.; Fahmi, M.; Yuliarto, B. A graphene-modified Co-BDC metal-organic frameworks (Co-MOF) for electrochemical non-enzymatic glucose sensing. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1045, 012010. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Lian, H.; Sun, X.; Liu, B. Cobalt metal-organic framework modified carbon cloth/paper hybrid electrochemical button-sensor for nonenzymatic glucose diagnostics. Sens. Actuators B Chem. 2021, 329, 129205. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhangsun, H.; Zhao, S.; Zhao, Y.; Wang, L. Carbon cloth-supported nanorod-like conductive Ni/Co bimetal MOF: A stable and high-performance enzyme-free electrochemical sensor for determination of glucose in serum and beverage. Food Chem. 2021, 349, 129202. [Google Scholar] [CrossRef]
- Li, S.; Zhan, X.; Bai, W.; Zheng, J. Controllable synthesis of Ni/Co-TCPP MOFs with different morphologies and their application in electrochemical detection of glucose. J. Electrochem. Soc. 2020, 167, 127506. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
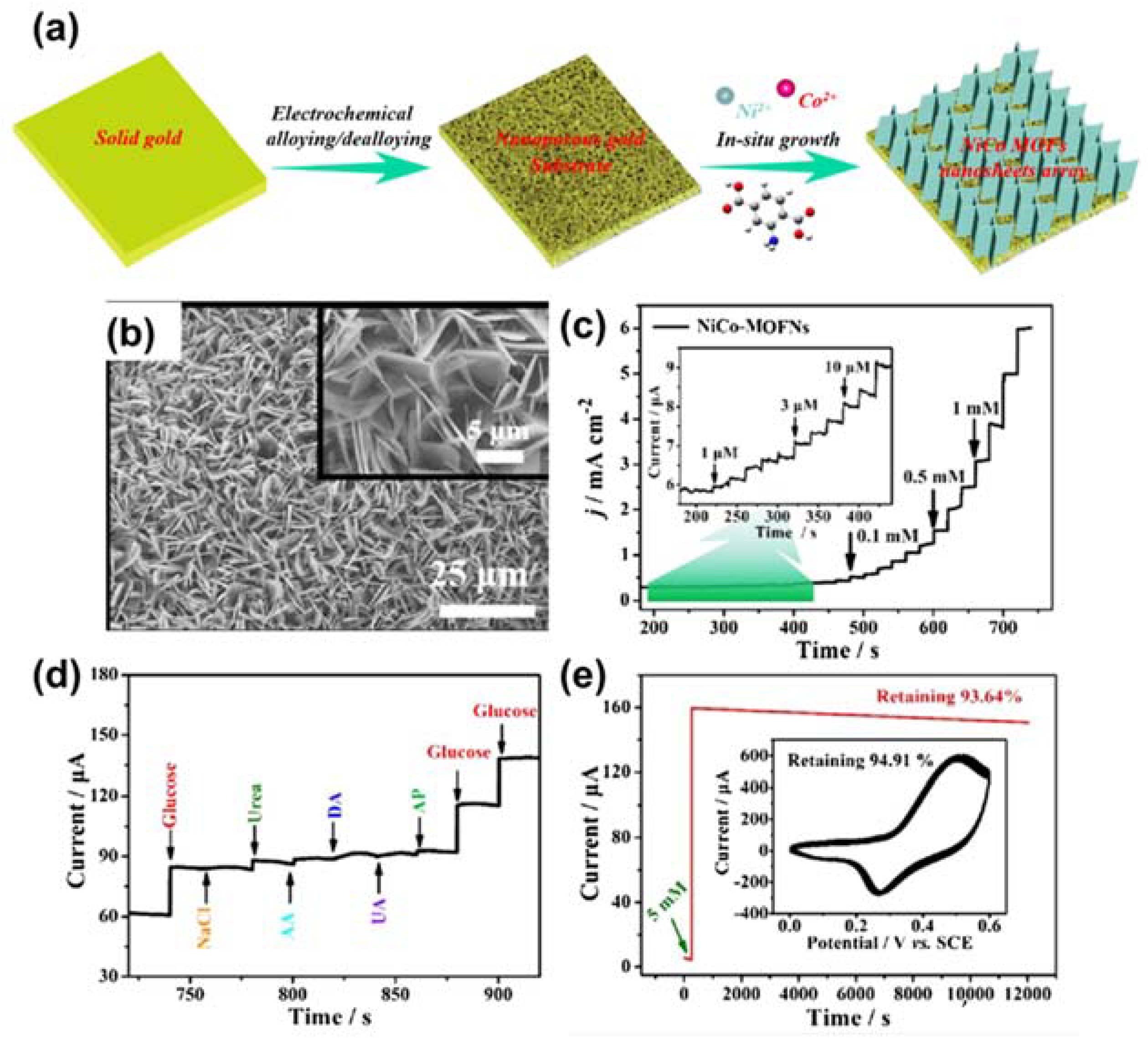
- Li, W.; Lv, S.; Wang, Y.; Zhang, L.; Cui, X. Nanoporous gold induced vertically standing 2D NiCo bimetal-organic framework nanosheets for non-enzymatic glucose biosensing. Sens. Actuators B Chem. 2019, 281, 652–658. [Google Scholar] [CrossRef]
- Zou, H.; Tian, D.; Lv, C.; Wu, S.; Lu, G.; Guo, Y.; Liu, Y.; Yu, Y.; Ding, K. The synergistic effect of Co/Ni in ultrathin metal–organic framework nanosheets for the prominent optimization of non-enzymatic electrochemical glucose detection. J. Mater. Chem. B 2020, 8, 1008–1016. [Google Scholar] [CrossRef]
- Lan, W.; Zhi-Wei, L.; Ying, M.; ZHANG, J.-J.; Guang-Quan, M.; Hai-Jun, D.; Jian-Shan, Y. Cu (II) Metal-Organic Framework Encapsulated in Carbon Paste Electrode for High-Performance Non-Enzymatic Glucose Sensing. Chin. J. Anal. Chem. 2020, 48, e20038–e20046. [Google Scholar]
- Sun, Y.; Li, Y.; Wang, N.; Xu, Q.Q.; Xu, L.; Lin, M. Copper-based Metal-organic Framework for Non-enzymatic Electrochemical Detection of Glucose. Electroanalysis 2018, 30, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, X.; Liu, H.; Zhai, Y.; Li, L.; Wen, H. 2D MOF with electrochemical exfoliated graphene for nonenzymatic glucose sensing: Central metal sites and oxidation potentials. Anal. Chim. Acta 2020, 1122, 9–19. [Google Scholar] [CrossRef]
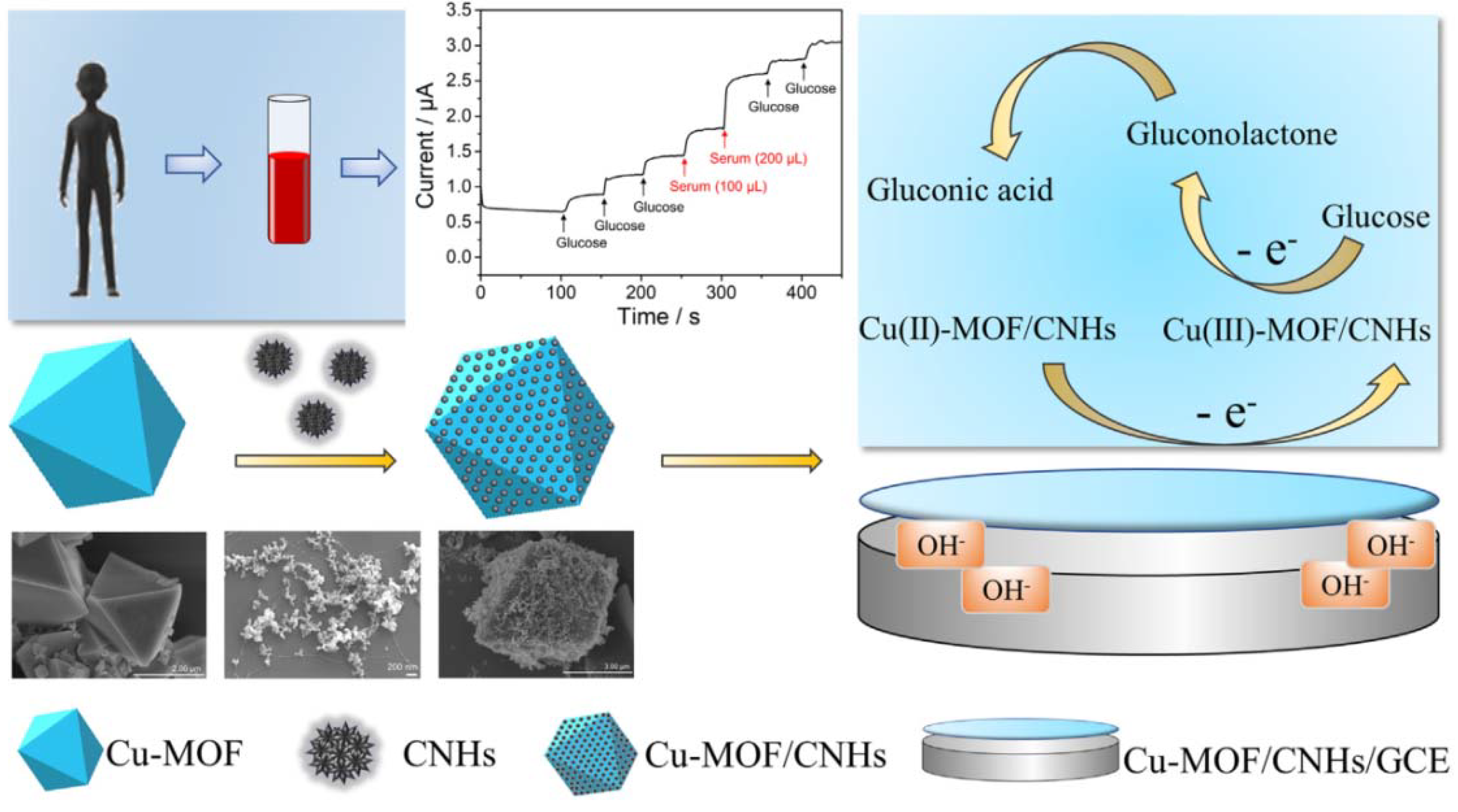
- Zheng, W.; Liu, Y.; Yang, P.; Chen, Y.; Tao, J.; Hu, J.; Zhao, P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanal. Chem. 2020, 862, 114018. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Enzyme-free glucose sensor based on layer-by-layer electrodeposition of multilayer films of multi-walled carbon nanotubes and Cu-based metal framework modified glassy carbon electrode. Biosens. Bioelectron. 2019, 135, 45–49. [Google Scholar] [CrossRef]
- Yuan, A.; Lu, Y.; Zhang, X.; Chen, Q.; Huang, Y. Two-dimensional iron MOF nanosheet as a highly efficient nanozyme for glucose biosensing. J. Mater. Chem. B 2020, 8, 9295–9303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A nanosized metal–organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 2013, 138, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, X.; Shi, W.; Huang, Y. Metal–organic framework MIL-53(Fe): Facile microwave-assisted synthesis and use as a highly active peroxidase mimetic for glucose biosensing. RSC Adv. 2015, 5, 17451–17457. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Li, R.; Ye, C.; Zhao, G.; Wang, Y. Ni-Based metal–organic framework derived Ni@C nanosheets on a Ni foam substrate as a supersensitive non-enzymatic glucose sensor. J. Mater. Chem. B 2017, 5, 5549–5555. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Li, J.; Xu, F.; Sun, L.; Bu, Y.; Zou, Y.; Kraatz, H.-B.; Rosei, F. Tunable hierarchical surfaces of CuO derived from metal–organic frameworks for non-enzymatic glucose sensing. Inorg. Chem. Front. 2020, 7, 1512–1525. [Google Scholar] [CrossRef]
- Vilian, A.E.; Dinesh, B.; Rethinasabapathy, M.; Hwang, S.-K.; Jin, C.-S.; Huh, Y.S.; Han, Y.-K. Hexagonal Co3O4 anchored reduced graphene oxide sheets for high-performance supercapacitors and non-enzymatic glucose sensing. J. Mater. Chem. A 2018, 6, 14367–14379. [Google Scholar] [CrossRef]
- Shu, Y.; Yan, Y.; Chen, J.; Xu, Q.; Pang, H.; Hu, X. Ni and NiO nanoparticles decorated metal–organic framework nanosheets: Facile synthesis and high-performance nonenzymatic glucose detection in human serum. ACS Appl. Mater. Interfaces 2017, 9, 22342–22349. [Google Scholar] [CrossRef] [PubMed]
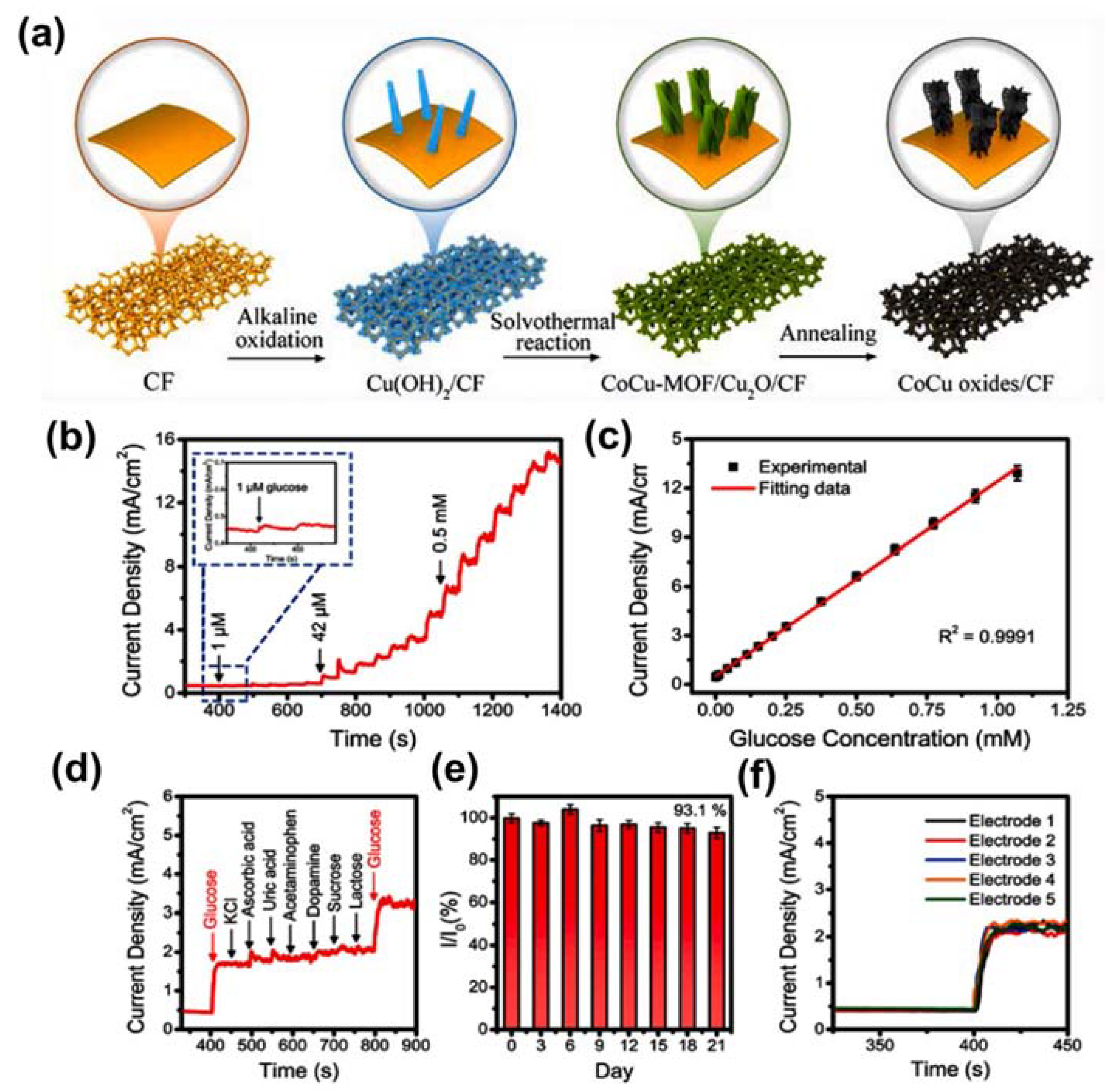
- Wei, C.; Li, X.; Xiang, W.; Yu, Z.; Liu, Q. MOF derived seaweed-like CoCu oxides nanorod arrays for electrochemical non-enzymatic glucose sensing with ultrahigh sensitivity. Sens. Actuators B Chem. 2020, 324, 128773. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, L.; Wang, X.; Chai, L.; Wang, Y.; Jiang, M.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. General approach to MOF-derived core-shell bimetallic oxide nanowires for fast response to glucose oxidation. Sens. Actuators B Chem. 2020, 306, 127551. [Google Scholar] [CrossRef]
- Archana, V.; Xia, Y.; Fang, R.; Gnana Kumar, G. Hierarchical CuO/NiO-carbon nanocomposite derived from metal organic framework on cello tape for the flexible and high performance nonenzymatic electrochemical glucose sensors. ACS Sustain. Chem. Eng. 2019, 7, 6707–6719. [Google Scholar] [CrossRef]
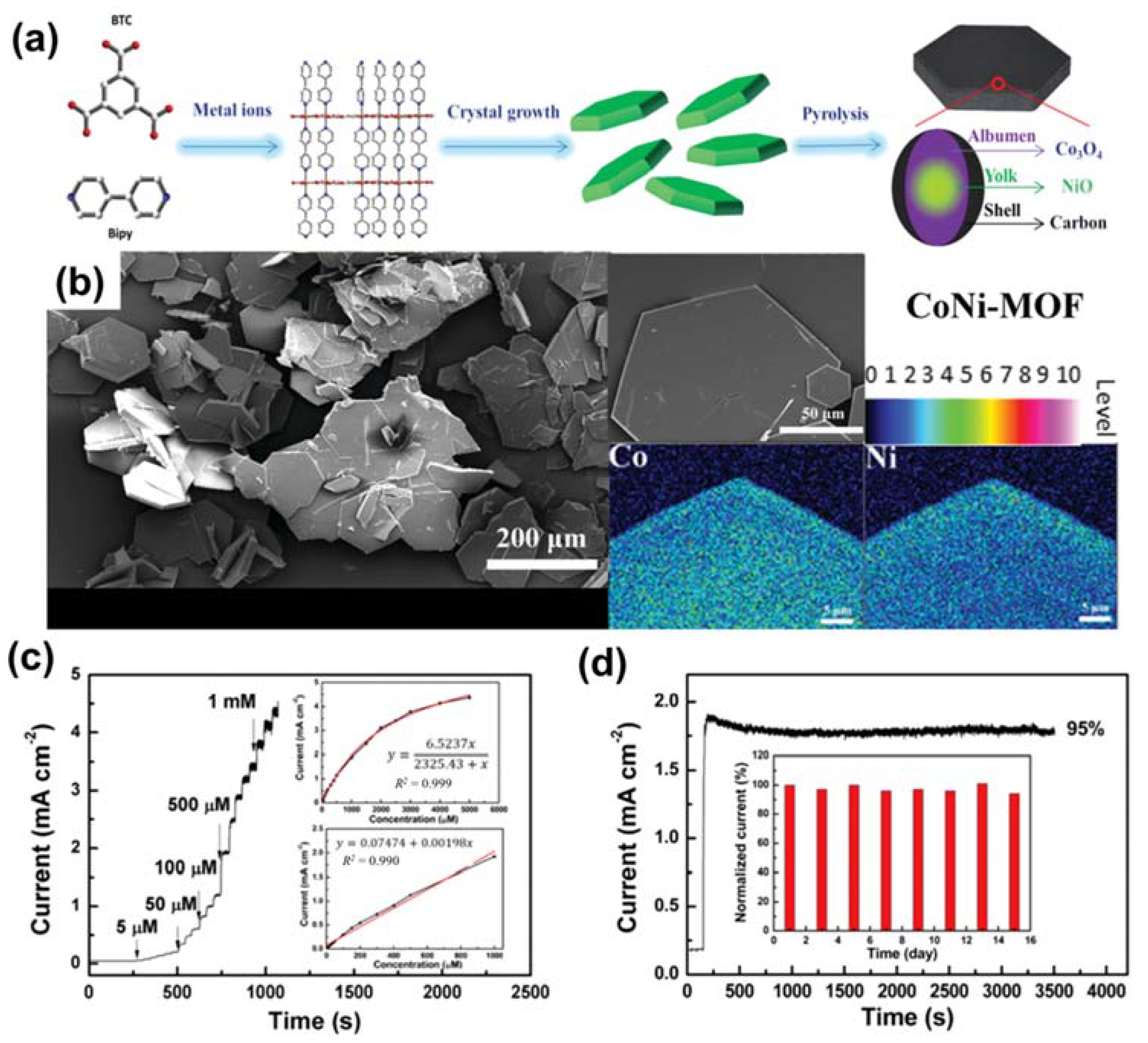
- Zhang, X.; Zhang, Y.; Guo, W.; Wan, K.; Zhang, T.; Arbiol, J.; Zhao, Y.-Q.; Xu, C.-L.; Xu, M.; Fransaer, J. A yolk–albumen–shell structure of mixed Ni–Co oxide with an ultrathin carbon shell for high-sensitivity glucose sensors. Mater. Adv. 2020, 1, 908–917. [Google Scholar] [CrossRef]
- Abrori, S.A.; Septiani, N.L.W.; Anshori, I.; Suendo, V.; Yuliarto, B. Metal-Organic-Framework FeBDC-Derived Fe3O4 for Non-Enzymatic Electrochemical Detection of Glucose. Sensors 2020, 20, 4891. [Google Scholar] [CrossRef]
- Yu, C.; Cui, J.; Wang, Y.; Zheng, H.; Zhang, J.; Shu, X.; Liu, J.; Zhang, Y.; Wu, Y. Porous HKUST-1 derived CuO/Cu2O shell wrapped Cu(OH)2 derived CuO/Cu2O core nanowire arrays for electrochemical nonenzymatic glucose sensors with ultrahigh sensitivity. Appl. Surf. Sci. 2018, 439, 11–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xia, J.; Zhang, F.; Wang, Z. MOF-derived porous Ni2P/graphene composites with enhanced electrochemical properties for sensitive nonenzymatic glucose sensing. ACS Appl. Mater. Interfaces 2018, 10, 39151–39160. [Google Scholar] [CrossRef]
- Jee, H.-W.; Paeng, K.-J.; Myung, N.; Rajeshwar, K. Electrochemical Deposition of a Metal–Organic Framework and Subsequent Conversion to Cobalt Selenide. ACS Appl. Electron. Mater. 2020, 2, 1358–1364. [Google Scholar] [CrossRef]
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable synthesis of Ti3C2Tx mxene. Adv. Eng. Mater. 2020, 22, 1901241. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Srivastava, P.; Mishra, A.; Mizuseki, H.; Lee, K.-R.; Singh, A.K. Mechanistic insight into the chemical exfoliation and functionalization of Ti3C2 MXene. ACS Appl. Mater. Interfaces 2016, 8, 24256–24264. [Google Scholar] [CrossRef] [PubMed]
- Berdiyorov, G. Effect of surface functionalization on the electronic transport properties of Ti3C2 MXene. EPL 2015, 111, 67002. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.; Jin, J.; Li, Y.; Dong, Z.; Li, R. A novel glucose sensor based on MoS2 nanosheet functionalized with Ni nanoparticles. Electrochim. Acta 2014, 136, 41–46. [Google Scholar] [CrossRef]
- Zhang, J.; Han, D.; Wang, S.; Zhang, X.; Yang, R.; Ji, Y.; Yu, X. Electrochemical detection of adenine and guanine using a three-dimensional WS2 nanosheet/graphite microfiber hybrid electrode. Electrochem. Commun. 2019, 99, 75–80. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhang, Y.; Liu, M.; Liu, Y. 2D titanium carbide MXenes as emerging optical biosensing platforms. Biosens. Bioelectron. 2020, 171, 112730. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Zaidi, S.A.; Naqvi, R.A. 2D transition metal carbides (MXene) for electrochemical sensing: A review. Crit. Rev. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
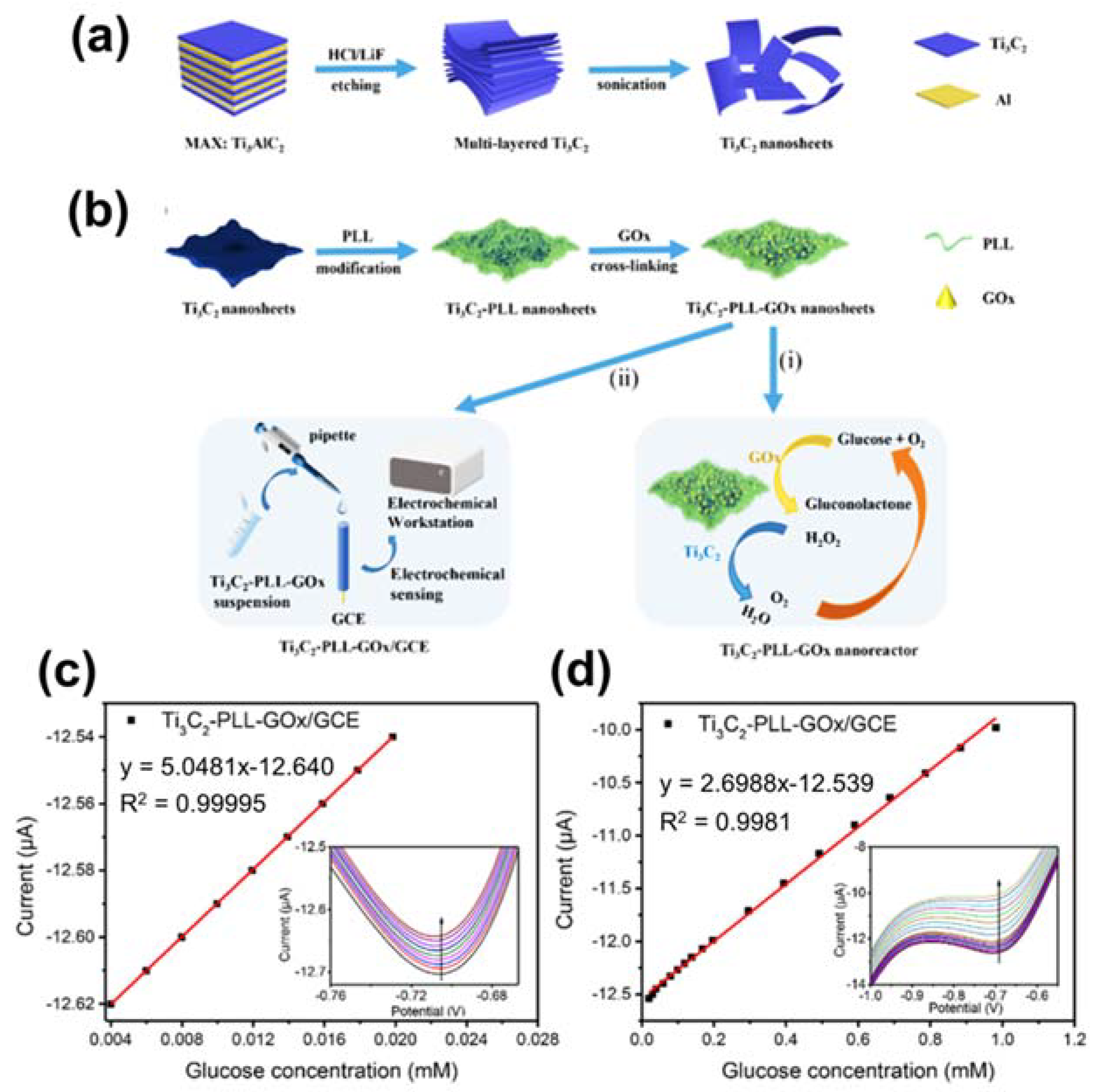
- Wu, M.; Zhang, Q.; Fang, Y.; Deng, C.; Zhou, F.; Zhang, Y.; Wang, X.; Tang, Y.; Wang, Y. Polylysine-modified MXene nanosheets with highly loaded glucose oxidase as cascade nanoreactor for glucose decomposition and electrochemical sensing. J. Colloid Interface Sci. 2021, 586, 20–29. [Google Scholar] [CrossRef]
- McNichols, R.J.; Cote, G.L. Optical glucose sensing in biological fluids: An overview. J. Biomed. Opt. 2000, 5, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, W.-D.; Ye, J.-S. Nonenzymatic electrochemical glucose sensor based on MnO2/MWNTs nanocomposite. Electrochem. Commun. 2008, 10, 1268–1271. [Google Scholar] [CrossRef]
- Feng, D.; Wang, F.; Chen, Z. Electrochemical glucose sensor based on one-step construction of gold nanoparticle–chitosan composite film. Sens. Actuators B Chem. 2009, 138, 539–544. [Google Scholar] [CrossRef]
- Chia, H.L.; Mayorga-Martinez, C.C.; Antonatos, N.; Sofer, Z.k.; Gonzalez-Julian, J.J.; Webster, R.D.; Pumera, M. MXene titanium carbide-based biosensor: Strong dependence of exfoliation method on performance. Anal. Chem. 2020, 92, 2452–2459. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and freestanding silicon/MXene composite papers for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Rakhi, R.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Xing, Y.; Xiong, P.; Tang, H.; Li, C.; Chen, S.; Zeng, R.; Han, K.; Shi, G. Three-Dimensional Porous Ti3C2Tx MXene–Graphene Hybrid Films for Glucose Biosensing. ACS Appl. Nano Mater. 2019, 2, 6537–6545. [Google Scholar] [CrossRef]
- Zhu, X.; Pang, X.; Zhang, Y.; Yao, S. Titanium carbide MXenes combined with red-emitting carbon dots as a unique turn-on fluorescent nanosensor for label-free determination of glucose. J. Mater. Chem. B 2019, 7, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
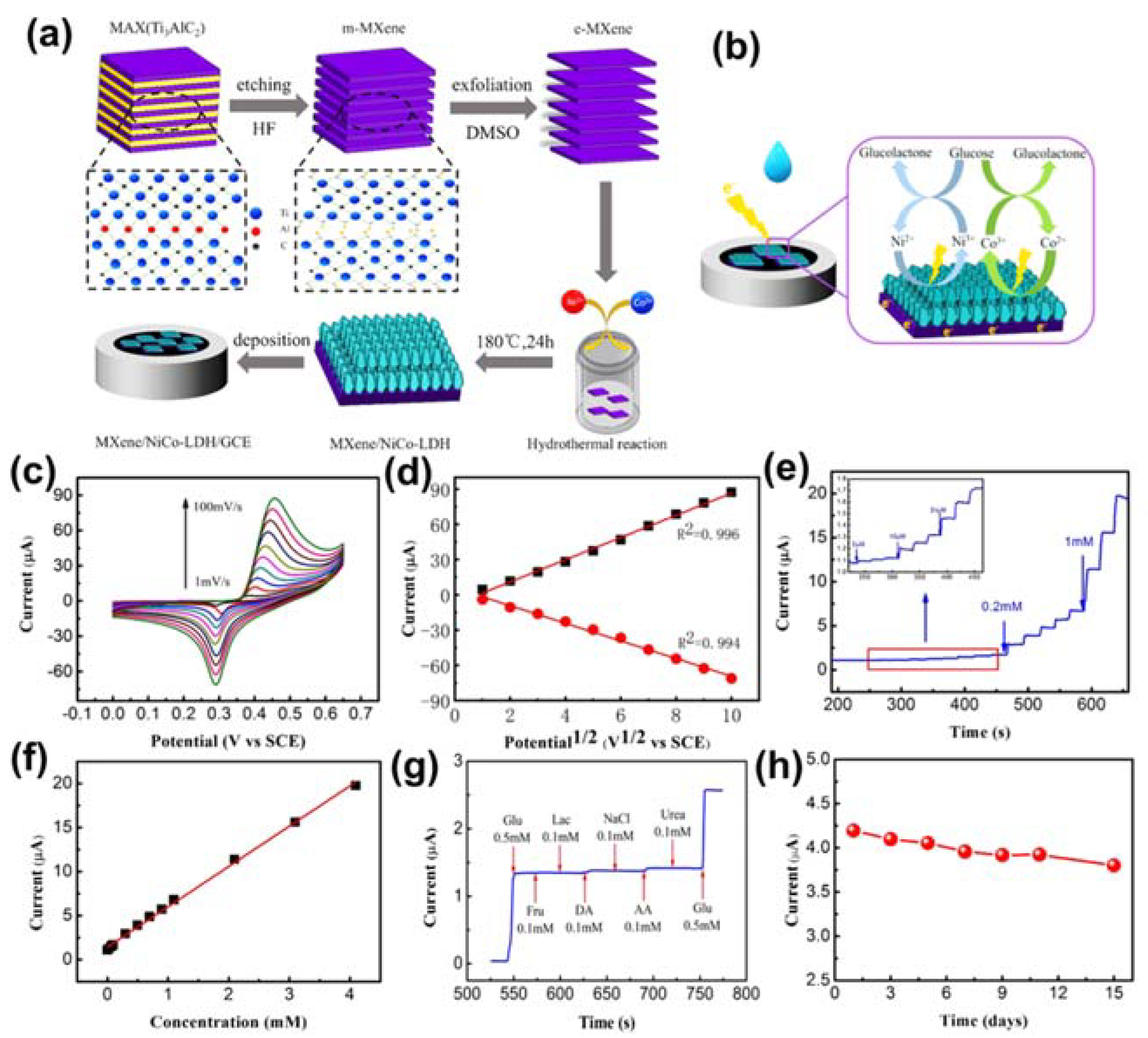
- Li, M.; Fang, L.; Zhou, H.; Wu, F.; Lu, Y.; Luo, H.; Zhang, Y.; Hu, B. Three-dimensional porous MXene/NiCo-LDH composite for high performance non-enzymatic glucose sensor. Appl. Surf. Sci. 2019, 495, 143554. [Google Scholar] [CrossRef]
- Bi, C.; Song, S.-X.; Li, H.-S.; Peng, H.-L.; Li, Q.-F. Non-enzymatic Glucose Sensor Based on Porous Foam Au/Mxene Nanocomposites. Chin. J. Chem. Phys. 2021. [Google Scholar] [CrossRef]






| Taste | Analytes | Properties |
|---|---|---|
| Saltiness | KCl, NaCl, metal ions | Providing of mineral |
| Sweetness | Glucose, sucrose, saccharin | Source of energy |
| Sourness | Acids: HCl, CH3CHOOH, etc. | Activation of metabolism |
| Bitterness | Caffein, quinine, etc. | Notice of toxicity |
| Umami | Monosodium glutamate | Providing of indispensable amino acids |
| Samples | Analytes | Method | LOD | Ref |
|---|---|---|---|---|
| MOF-76 [In(OH)(bdc)n | Sucrose, caffeine, citric acid, NaCl, monosodium glutamate | PL emission spectra | Not given | [55] |
| (+)-PAN-chelated [In(OH)(bdc)] | Sucrose, caffeine, citric acid, NaCl, monosodium glutamate | PL emission spectra | Not given | [56] |
| CPO-27(Ni) | Glucose | Electrochemical techniques | 1.46 µM | [26] |
| Ni-MIL-77 | Glucose | Electrochemical techniques | 0.25 µM | [27] |
| Conductive Ni-MOF | Glucose | Electrochemical techniques | 0.66 µM | [61] |
| AgNPs/MOF-74(Ni) | Glucose | Electrochemical techniques | 4.7 µM | [62] |
| Au/BTC | Glucose | Electrochemical techniques | 1.5 µM | [63] |
| Ni-MOF/CNTs | Glucose | Electrochemical techniques | 0.82 µM | [4] |
| ZIF-67 HNPs | Glucose | Electrochemical techniques | 0.96 µM | [64] |
| Ag@ZIF-67 | Glucose | Electrochemical techniques | 0.66 µM | [65] |
| Co-BDC-3Gr | Glucose | Electrochemical techniques | 5.39 µM | [66] |
| Co-MOF/CC | Glucose | Electrochemical techniques | 150 µM | [67] |
| NiCo-MOF/CC | Glucose | Electrochemical techniques | 100 nM | [68] |
| Ni/Co-TCPP | Glucose | Electrochemical techniques | 0.3 µM | [69] |
| Co-MOF/NF | Glucose | Electrochemical techniques | 1.3 nM | [70] |
| NiCo-MOF nanoflake | Glucose | Electrochemical techniques | 0.29 nM | [71] |
| Co/Ni-MOF | Glucose | Electrochemical techniques | 0.047 µM | [72] |
| Cu-MOF/CPE | Glucose | Electrochemical techniques | 0.11 µM | [73] |
| Cu-MOF | Glucose | Electrochemical techniques | 0.01 µM | [74] |
| Cu-MOF/EG | Glucose | Electrochemical techniques | 0.58 µM | [75] |
| Cu-MOF/CNHs | Glucose | Electrochemical techniques | 0.078 µM | [76] |
| Cu-MOF/CNT | Glucose | Electrochemical techniques | 0.4 µM | [77] |
| 2D-Fe-BTC | Glucose | Electrochemical techniques | 0.039 µM | [78] |
| Fe-MIL-88NH2 | Glucose | Electrochemical techniques | 0.48 M | [79] |
| MIL-53(Fe) | Glucose | Electrochemical techniques | 0.039 µM | [80] |
| Ni@C | Glucose | Electrochemical techniques | 50 nM | [81] |
| CuO | Glucose | Electrochemical techniques | 0.15 µM | [82] |
| Co3O4/rGO | Glucose | Electrochemical techniques | 0.4 µM | [83] |
| Ni/NiO | Glucose | Electrochemical techniques | 0.8 µM | [84] |
| CoCu oxide nanorods | Glucose | Electrochemical techniques | 0.72 μM | [85] |
| CuOx@Co3O4 | Glucose | Electrochemical techniques | 0.036 µM | [86] |
| CuO/NiO-C | Glucose | Electrochemical techniques | 37 nM | [87] |
| YASNiCo@C | Glucose | Electrochemical techniques | 0.75 M | [88] |
| Fe3O4 | Glucose | Electrochemical techniques | 15.57 M | [89] |
| Ni2P/G | Glucose | Electrochemical techniques | 0.44 μM | [90] |
| CoSe/ZIF-67 | Glucose | Electrochemical techniques | 1.53 µM | [91] |
| CuO/Cu2O@CuO/Cu2O | Glucose | Electrochemical techniques | 0.48 M | [92] |
| Samples | Analytes | Methods | LOD | Ref |
|---|---|---|---|---|
| Ti3C2-HF/TBA/GOx/GTA | Glucose | Electrochemical technique | 23.0 µM | [110] |
| Ti3C2-PLL-GOx | Glucose | Electrochemical technique | 2.6 µM | [106] |
| MXene/NiCo-LDH | Glucose | Electrochemical technique | 0.53 µM | [115] |
| Au/MXene/Nafion/GCE | Glucose | Electrochemical technique | 0.2 mM | [116] |
| GOx/Au/MXene/Nafion/GCE | Glucose | Electrochemical technique | 5.9 µM | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, H.H.; Cho, J.H.; Han, S.M.; Ahn, S.H.; Kim, S.Y. Metal–Organic-Framework- and MXene-Based Taste Sensors and Glucose Detection. Sensors 2021, 21, 7423. https://doi.org/10.3390/s21217423
Do HH, Cho JH, Han SM, Ahn SH, Kim SY. Metal–Organic-Framework- and MXene-Based Taste Sensors and Glucose Detection. Sensors. 2021; 21(21):7423. https://doi.org/10.3390/s21217423
Chicago/Turabian StyleDo, Ha Huu, Jin Hyuk Cho, Sang Mok Han, Sang Hyun Ahn, and Soo Young Kim. 2021. "Metal–Organic-Framework- and MXene-Based Taste Sensors and Glucose Detection" Sensors 21, no. 21: 7423. https://doi.org/10.3390/s21217423







