State-of-the-Art Research on Chemiresistive Gas Sensors in Korea: Emphasis on the Achievements of the Research Labs of Professors Hyoun Woo Kim and Sang Sub Kim
Abstract
:1. Introduction: Overview of the Oxide-Based Gas Sensors
2. Gas Sensors Based on Morphology Engineering: Core–Shell (C-S) Sensing Materials
3. Self-Heated Gas Sensors
4. Irradiated Gas Sensors
5. Flexible Gas Sensors
6. Other Resistive-Based Gas Sensors (Glass/Silicon/MOF-Based Gas Sensors)
6.1. Glass Gas Sensors
6.2. Si-Based Gas Sensors
6.3. Metal–Organic Framework (MOF)-Based Gas Sensors
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Brugha, R.; Edmondson, C.; Davies, J.C. Outdoor air pollution and cystic fibrosis. Paed. Resp. Rev. 2018, 28, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Fuller, R.; Fisher, S.; Suk, W.A.; Sly, P.; Chiles, T.C.; O’Reilly, S. Pollution and children’s health. Sci. Total Environ. 2019, 650, 2389–2394. [Google Scholar] [CrossRef]
- Pifferi, S.; Menini, A. Odorant detection and discrimination in the olfactory system. Sens. Microsyst. 2011, 91, 3–18. [Google Scholar]
- Chiu, S.-W.; Tang, K.-T. Towards a chemiresistive sensor-integrated electronic nose: A review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef] [Green Version]
- Gruber, B.; David, F.; Sandra, P. Capillary gas chromatography-mass spectrometry: Current trends and perspectives. Trends Ana. Chem. 2020, 124, 115475. [Google Scholar] [CrossRef]
- Anyakudo, F.; Adams, E.; Schepdael, A. Thin-layer chromatography–flame ionization detection. Chromatographia 2020, 83, 149–157. [Google Scholar] [CrossRef]
- You, Y.; Moussa, S.G.; Zhang, L.; Fu, L.; Beck, J.; Staebler, R. Quantifying fugitive gas emissions from an oil sands tailings pond with open-path Fourier transform infrared measurements. Atmos. Meas. Tech. 2021, 14, 945–959. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, J.-H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef] [Green Version]
- Brattain, W.H.; Bardeen, J. Surface properties of germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Heiland, G. Zum Einfluß von Wasserstoff auf die elektrische Leitfähigkeit an der Oberfläche von Zinkoxydkristallen. Z. Für Phys. 1957, 148, 15–27. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Taguchi, N. A Metal Oxide Gas Sensor. Japanese Patent Application No. 45-38200, 1962. Available online: https://hal.archives-ouvertes.fr/tel-00509149v1/html_references (accessed on 2 November 2021).
- Shaver, P.J. Activated tungsten oxide gas detectors. Appl. Phys. Lett. 1967, 11, 255–257. [Google Scholar] [CrossRef]
- Ihokura, K.; Watson, J. The Stannic Oxide Gas Sensor: Principles and Applications; CRC Press: Roca Barton, FL, USA, 1994. [Google Scholar]
- Salehi, A. A highly sensitive self-heated SnO2 carbon monoxide sensor. Sens. Actuators B Chem. 2003, 96, 88–93. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Reduced graphene oxide (rGO)-loaded metal-oxide nanofiber gas sensors: An overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef]
- Han, D.; Zhao, M. Facile and simple synthesis of novel iron oxide foam and used as acetone gas sensor with sub-ppm level. J. Alloys Compd. 2020, 815, 152406. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Yang, Z.; Wang, Q. Facile hydrothermal synthesis of mesoporous In2O3 nanoparticles with superior formaldehyde-sensing properties. Physica E 2018, 97, 38–44. [Google Scholar] [CrossRef]
- Navale, S.T.; Jadhav, V.V.; Tehare, K.K.; Sagar, R.U.R.; Biswas, C.S.; Galluzzi, M.; Liang, W.; Patil, V.B.; Mane, R.S.; Stadler, F.J. Solid-state synthesis strategy of ZnO nanoparticles for the rapid detection of hazardous Cl2. Sens. Actuators B Chem. 2017, 238, 1102–1110. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, N.; Kannan, J.C.; Krishnakumar, T.; Leonardi, S.G.; Neri, G. Sensing behavior to ethanol of tin oxide nanoparticles prepared by microwave synthesis with different irradiation time. Sens. Actuators B Chem. 2014, 194, 96–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, L.Z.; Yuan, C.X.; Zhang, C.L.; Liu, S.; Peng, Y.Q.; Li, H.R.; Zhang, M. A ppb-level formaldehyde gas sensor based on rose-like nickel oxide nanoparticles prepared using electrodeposition process. Nano 2016, 11, 1650009. [Google Scholar] [CrossRef]
- Mane, A.T.; Kulkarni, S.B.; Navale, S.T.; Ghanwat, A.A.; Shinde, N.M.; Kim, J.; Patil, V.B. NO2 sensing properties of nanostructured tungsten oxide thin films. Ceram. Interfaces 2014, 40, 16495–16502. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Y.; Ma, C.; He, W. In-situ growth of Co3O4 nanoparticles based on electrospray for an acetone gas sensor. J. Alloys Compd. 2021, 854, 157234. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas sensors based on CeO2 nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards H2S and NO2 gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Navale, S.T.; Yang, Z.B.; Liu, C.; Cao, P.J.; Patil, V.B.; Ramgir, N.S.; Mane, R.S.; Stadler, F.J. Enhanced acetone sensing properties of titanium dioxide nanoparticles with a sub-ppm detection limit. Sens. Actuators B Chem. 2018, 255, 1701–1710. [Google Scholar] [CrossRef]
- Peng, F.; Sun, Y.; Yu, W.; Lu, Y.; Hao, J.; Cong, R.; Ge, M.; Shi, J.; Dai, N. Studies on sensing properties and mechanism of CuO nanoparticles to H2S gas. Nanomaterials 2020, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.; Xia, Y. A Solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J Am. Chem. Soc. 2003, 125, 16176–16177. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, T.; Yuan, M.; Kang, M.; Lu, G.; Wang, R.; Fan, H.; He, Y.; Yang, H. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B Chem. 2009, 143, 93–98. [Google Scholar] [CrossRef]
- Huang, F.; Yang, W.; He, F.; Liu, S. Controlled synthesis of flower-like In2O3 microrods and their highly improved selectivity toward ethanol. Sens. Actuators B Chem. 2016, 235, 86–93. [Google Scholar] [CrossRef]
- Tan, W.; Tan, J.; Li, L.; Dun, M.; Huang, X. Nanosheets-assembled hollowed-out hierarchical Co3O4 microrods for fast response/recovery gas sensor. Sens. Actuators B Chem. 2017, 249, 66–75. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, G.; Du, W.; Tian, Q. Synthesis and excellent acetone sensing properties of porous WO3 nanofibers. Vacuum 2016, 124, 32–39. [Google Scholar] [CrossRef]
- Yuan, Z.; Yin, L.; Ding, H.; Huang, W.; Shuai, C.; Deng, J. One-step synthesis of single-crystalline ZnO nanowires for the application of gas sensor. J. Mater. Sci. Mater. Electron. 2018, 29, 11559–11565. [Google Scholar] [CrossRef]
- Tan, J.; Huang, X. Ultra-thin nanosheets-assembled hollowed-out hierarchical α-Fe2O3 nanorods: Synthesis via an interface reaction route and its superior gas sensing properties. Sens. Actuators B Chem. 2016, 237, 159–166. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Zhang, L.; Jiang, C.; Siu-Wai Kong, E.; Zhang, Y. Preparation of high aspect ratio nickel oxide nanowires and their gas sensing devices with fast response and high sensitivity. J. Mater. Chem. 2012, 22, 8327–8335. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Li, H.-Y.; Yang, X.-N.; Guo, X. NO sensing by single crystalline WO3 nanowires. Sens. Actuators B 2015, 219, 346. [Google Scholar] [CrossRef]
- Seo, M.H.; Yuasa, M.; Kida, T.; Huh, J.S.; Yamazoe, N.; Shimanoe, K. Microstructure control of TiO2 nanotubular films for improved VOC sensing. Sens. Actuators B Chem. 2011, 154, 251–256. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, W.; Jin, L.; Cui, F. Hierarchically porous WO3 microstructures with networks for acetylene sensing application. Mater. Lett. 2018, 214, 198–201. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.; Yan, X. Facile fabrication and enhanced formaldehyde gas sensing properties of nanoparticles-assembled chain-like NiO architectures. Mater. Lett. 2016, 185, 40–42. [Google Scholar] [CrossRef]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Li, X.; Hu, Y.; Huang, G.; Tan, W.; Huang, X. Self-template derived CuO nanowires assembled microspheres and its gas sensing properties. Sens. Actuators B Chem. 2017, 252, 1–8. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, C.; Li, L.; Du, C.; Li, X.; Kang, X.F.; Chen, W. CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: Morphology and surface structure effects on the sensing performance. Talanta 2018, 188, 41–49. [Google Scholar] [CrossRef]
- Jin, W.; Yan, S.; An, L.; Chen, W.; Yang, S.; Zhao, C.; Dai, Y. Enhancement of ethanol gas sensing response based on ordered V2O5 nanowire microyarns. Sens. Actuators B Chem. 2015, 206, 284. [Google Scholar] [CrossRef]
- Qiao, P.Y.; Zhang, L.X.; Zhu, M.Y.; Yin, Y.Y.; Zhao, Z.W.; Sun, H.N.; Dong, J.Y.; Bie, L.J. Acetylene sensing enhancement of mesoporous ZnO nanosheets with morphology and defect induced structural sensitization. Sens. Actuators B Chem. 2017, 250, 189–197. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Wen, Z.; Ye, Z. Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens. Actuators B Chem. 2017, 238, 1052–1059. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.H.; Ma, S.Y.; Jin, W.X.; Yan, S.H.; Xu, X.L.; Chen, Q. Curly porous NiO nanosheets with enhanced gas-sensing properties. Mater. Lett. 2017, 190, 252–255. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, J.; Yuan, Y.; Zhao, H.; Shi, Q.; Zhang, F.; Pei, C.; Liu, B.; Yang, H. Enhanced gas sensitivity and sensing mechanism of network structures assembled from α-Fe2O3 nanosheets with exposed {104} facets. Langmuir 2017, 33, 8671–8678. [Google Scholar] [CrossRef]
- Fu, H.T.; Zhang, Z.K.; Yang, X.H.; An, X.Z. Two-step synthesis of V2O5 nanosheets with high sensing properties toward acetone. Adv. Eng. Res. 2017, 110, 372–379. [Google Scholar]
- Bhowmik, B.; Bhattacharyya, P. Efficient gas sensor devices based on surface engineered oxygen vacancy controlled TiO2 nanosheets. IEEE Trans. Electron Dev. 2017, 64, 2357–2363. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, X.; Ge, C.; Hussain, S.; Liu, G.; Qiao, G. WO3 porous nanosheet arrays with enhanced low temperature NO2 gas sensing performance. Sens. Actuators B Chem. 2020, 316, 128050. [Google Scholar] [CrossRef]
- Kim, K.; Choi, P.; Itoh, T.; Masuda, Y. Catalyst-free highly sensitive SnO2 nanosheet gas sensors for parts per billion-level detection of acetone. ACS Appl. Mater. Interfaces 2020, 12, 51637–51644. [Google Scholar] [CrossRef]
- Li, S.; Fang, W.; Yang, Y.; Yu, H.; Wang, T.; Dong, X.; Liu, G.; Wang, J.; Yu, W.; Sh, K. Highly active and porous single-crystal In2O3 nanosheet for NOx gas sensor with excellent response at room temperature. RSC Adv. 2017, 7, 33419–33425. [Google Scholar]
- Zhou, T.; Zhang, T.; Deng, J.; Zhang, R.; Lou, Z.; Wang, L. P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance. Sens. Actuators B Chem. 2017, 242, 369–377. [Google Scholar] [CrossRef]
- Wang, H.; Yan, L.; Li, S.; Li, Y.; Liu, L.; Du, L.; Duan, H.; Cheng, Y. Acetone sensors based on microsheet assembled hierarchical Fe2O3 with different Fe3+ concentrations. Appl. Phys. A 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Chen, W.G.; Gao, T.Y.; Li, Q.Z.; Gan, H.I. Enhanced gas sensing properties of flower-like ZnO nanostructure to acetylene. Mater. Technol. 2014, 30, 96–100. [Google Scholar] [CrossRef]
- Bing, Y.; Zeng, Y.; Liu, C.; Qiao, L.; Zheng, W. Synthesis of double-shelled SnO2 nano-polyhedra and their improved gas sensing properties. Nanoscale 2015, 7, 3276–3284. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, Y.; Li, G.; Wang, X.; Xu, D.; Chen, Z.; Zhang, T.; Wang, J.; Zhang, L. Hierarchically rosette-like In2O3 microspheres for volatile organic compounds gas sensors. Sens. Actuators B Chem. 2013, 178, 302–309. [Google Scholar] [CrossRef]
- Fu, Q.; Ai, M.; Duan, Y.; Lu, L.; Tian, X.; Sun, D.; Xu, Y.; Sun, Y. Synthesis of uniform porous NiO nanotetrahedra and their excellent gas-sensing performance toward formaldehyde. RSC Adv. 2017, 7, 52312–52320. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.; Liu, D.; Wang, G.; San, X.; Shen, Y.; Jin, Q.; Meng, F. CuO hollow microspheres self-assembled with nanobars: Synthesis and their sensing properties to formaldehyde. Vacuum 2017, 144, 272–280. [Google Scholar] [CrossRef]
- Cao, S.; Chen, H. Nanorods assembled hierarchical urchin-like WO3 nanostructures: Hydrothermal synthesis, characterization, and their gas sensing properties. J. Alloys Compd. 2017, 702, 644–648. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Whang, W.-T.; Chen, C.-H. Hollow V2O5 nanoassemblies for high-performance room-temperature hydrogen sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Wang, Y.; Feng, C.; Zhou, J.; Liu, C.; Ruan, S. Xylene gas sensor based on Ni doped TiO2 bowl-like submicron particles with enhanced sensing performance. RSC Adv. 2015, 5, 28105–28110. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Metal-core@ metal oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanopart. Res. 2015, 17, 371. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Kim, S.S.; Bechelany, M. Atomic layer deposition (ALD) on inorganic or polymeric membranes. J. Appl. Phys. 2019, 126, 041101. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun metal oxide composite nanofibers gas sensors: A review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Choi, S.W.; Lee, J.W.; Lee, C.; Kim, S.S. Synthesis and gas sensing properties of TiO2–ZnO core-shell nanofibers. J. Am. Ceram. Soc. 2009, 92, 2551–2554. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.Y.; Kim, S.S. Synthesis of SnO2–ZnO core–shell nanofibers via a novel two-step process and their gas sensing properties. Nanotechnology 2009, 20, 465603. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sens. Actuators B Chem. 2020, 302, 127150. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, J.-H.; Kim, S.-H.; Kim, S.S. Dual functional sensing mechanism in SnO2–ZnO core–shell nanowires. ACS Appl. Mater. Interfaces 2014, 6, 8281–8287. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Sun, G.-J.; Kim, S.S. An approach to detecting a reducing gas by radial modulation of electron-depleted shells in core–shell nanofibers. J. Mater. Chem. A 2013, 1, 13588–13596. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, S.S. Optimum shell thickness and underlying sensing mechanism in p–n CuO–ZnO core–shell nanowires. Sens. Actuators B Chem. 2016, 222, 249–256. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Enhancement of CO and NO2 sensing in n-SnO2-p-Cu2O core-shell nanofibers by shell optimization. J. Hazard. Mater. 2019, 376, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.S. Realization of ppb-Scale Toluene-Sensing Abilities with Pt-Functionalized SnO2–ZnO Core–Shell Nanowires. ACS Appl. Mater. Interfaces 2015, 7, 17199–17208. [Google Scholar] [CrossRef] [PubMed]
- Spirig, J.V.; Ramamoorthy, R.; Akbar, S.A.; Routbort, J.L.; Singh, D.; Dutta, P. High temperature zirconia oxygen sensor with sealed metal/metal oxide internal reference. Sens. Actuators B Chem. 2007, 124, 192–201. [Google Scholar] [CrossRef]
- Bang, J.H.; Lee, N.; Mirzaei, A.; Choi, M.S.; Choi, H.; Jeon, H.; Kim, S.S.; Kim, H.W. Exploration of ZrO2-shelled nanowires for chemiresistive detection of NO2 gas. Sens. Actuators B Chem. 2020, 319, 128309. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, S.-H.; Kim, S.S. Chemiresistive sensing behavior of SnO2 (n)–Cu2O (p) core–shell nanowires. ACS Appl. Mater. Interfaces 2015, 7, 15351–15358. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-H.; Kim, S.S. CuO–TiO2 p–n core–shell nanowires: Sensing mechanism and p/n sensing-type transition. Appl. Surf. Sci. 2018, 448, 489–497. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Sun, G.-J.; Kim, H.W.; Kim, S.S. Mechanism and prominent enhancement of sensing ability to reducing gases in p/n core–shell nanofiber. Nanotechnology 2014, 25, 175501. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.-W.; Kim, S.S. A model for the enhancement of gas sensing properties in SnO2–ZnO core–shell nanofibres. J. Phys. D Appl. Phys. 2011, 44, 205403. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Ultra-sensitive benzene detection by a novel approach: Core-shell nanowires combined with the Pd-functionalization. Sens. Actuators B Chem. 2017, 239, 578–585. [Google Scholar] [CrossRef]
- Choi, M.S.; Mirzaei, A.; Bang, J.H.; Oum, W.; Jung Kwon, Y.; Kim, J.-H.; Choi, S.-W.; Kim, S.S.; Kim, H.W. Selective H2S-sensing performance of Si nanowires through the formation of ZnO shells with Au functionalization. Sens. Actuators B Chem. 2019, 289, 1–14. [Google Scholar] [CrossRef]
- Yu, Y.-T.; Dutta, P. Examination of Au/SnO2 core-shell architecture nanoparticle for low temperature gas sensing applications. Sens. Actuators B Chem. 2011, 157, 444–449. [Google Scholar] [CrossRef]
- Yanagimoto, T.; Yu, Y.-T.; Kaneko, K. Microstructure and CO gas sensing property of Au/SnO2 core–shell structure nanoparticles synthesized by precipitation method and microwave-assisted hydrothermal synthesis method. Sens. Actuators B Chem. 2012, 166, 31–35. [Google Scholar] [CrossRef]
- Song, H.-M.; Chon, B.-S.; Jeon, S.-H.; Rai, P.; Yu, Y.-T.; Dutta, P.K. Synthesis of Au@SnO2 core–shell nanoparticles with controllable shell thickness and their CO sensing properties. Mater. Chem. 2015, 166, 87–94. [Google Scholar] [CrossRef]
- Rai, P.; Khan, R.; Raj, S.; Majhi, S.M.; Park, K.-K.; Yu, Y.-T.; Lee, I.-H.; Sekhar, P.K. Au@Cu2O core–shell nanoparticles as chemiresistors for gas sensor applications: Effect of potential barrier modulation on the sensing performance. Nanoscale 2014, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Rai, P.; Raj, S.; Chon, B.-S.; Park, K.-K.; Yu, Y.-T. Effect of Au nanorods on potential barrier modulation in morphologically controlled Au@Cu2O core–shell nanoreactors for gas sensor applications. ACS Appl. Mater. Interfaces 2014, 6, 7491–7497. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Rai, P.; Yu, Y.-T. Facile approach to synthesize Au@ ZnO core–shell nanoparticles and their application for highly sensitive and selective gas sensors. ACS Appl. Mater. Intefaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Naik, G.K.; Lee, H.-J.; Song, H.-G.; Lee, C.-R.; Lee, I.-H.; Yu, Y. Au@NiO core-shell nanoparticles as a p-type gas sensor: Novel synthesis, characterization, and their gas sensing properties with sensing mechanism. Sens. Actuators B Chem. 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Majhi, S.M.; Lee, H.-J.; Choi, H.-N.; Cho, H.-Y.; Kim, J.-S.; Lee, C.-R.; Yu, Y.-T. Construction of novel hybrid PdO–ZnO p–n heterojunction nanostructures as a high-response sensor for acetaldehyde gas. CrystEngComm 2019, 21, 5084–5094. [Google Scholar] [CrossRef]
- Chava, R.K.; Oh, S.-Y.; Yu, Y.-T. Enhanced H2 gas sensing properties of Au@In2O3 core–shell hybrid metal–semiconductor heteronanostructures. CrystEngComm 2016, 18, 3655–3666. [Google Scholar] [CrossRef]
- Le, H.-J.; Van Dao, D.; Yu, Y.-T. Superfast and efficient hydrogen gas sensor using PdAuₐₗₗₒy@ ZnO core–shell nanoparticles. J. Mater. Chem. A 2020, 8, 12968–12974. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Matsushima, S.; Teraoka, Y.; Miura, N.; Yamazoe, N. Electronic interaction between metal additives and tin dioxide in tin dioxide-based gas sensors. Jpn. J. Appl. Phys. 1988, 27, 1798. [Google Scholar] [CrossRef]
- Rai, P.; Majhi, S.M.; Yu, Y.-T.; Lee, J.-H. Synthesis of plasmonic Ag@SnO2 core–shell nanoreactors for xylene detection. RSC Adv. 2015, 5, 17653–17659. [Google Scholar] [CrossRef]
- Rai, P.; Yoon, J.-W.; Kwak, C.-H.; Lee, J.-H. Role of Pd nanoparticles in gas sensing behaviour of Pd@ In2O3 yolk–shell nanoreactors. J. Mater. Chem. A 2016, 4, 264–269. [Google Scholar] [CrossRef]
- Rai, P.; Yoon, J.-W.; Jeong, H.-M.; Hwang, S.-J.; Kwak, C.-H.; Lee, J.-H. Design of highly sensitive and selective Au@NiO yolk–shell nanoreactors for gas sensor applications. Nanoscale 2014, 6, 8292–8299. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barreiro, S.; Gracia-Fernández, C.; López-Beceiro, J.; Artiaga, R. Rheological testing of a curing process controlled by Joule heating. Polym. Test. 2016, 55, 97–100. [Google Scholar] [CrossRef]
- Kim, J.-H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly selective sensing of CO, C6H6, and C7H8 gases by catalytic functionalization with metal nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.M.; Manh Hung, C.; Ngoc, T.M.; Nguyen, H.; Duc Hoa, N.; Van Duy, N.; Hieu, N. Novel self-heated gas sensors using on-chip networked nanowires with ultralow power consumption. ACS Appl. Mater. Interface 2017, 9, 6153–6162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd-functionalized core-shell composite nanowires for self-heating, sensitive, and benzene-selective gas sensors. Sens. Actuators A Phys. 2020, 308, 112011. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D materials for gas sensing applications: A review on graphene oxide, MoS2, WS2 and phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [Green Version]
- Late, D.J.; Kanawade, R.V.; Kannan, P.K.; Rout, C. Atomically thin WS2 nanosheets based gas sensor. Sens. Lett. 2016, 14, 1249–1254. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Flexible and low power CO gas sensor with Au-functionalized 2D WS2 nanoflakes. Sens. Actuators B Chem. 2020, 313, 128040. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mirzaei, A.; Kim, J.-H.; Kim, J.-Y.; Nasriddinov, A.F.; Rumyantseva, M.N.; Kim, H.W.; Kim, S.S. Gas-sensing behaviors of TiO2-layer-modified SnO2 quantum dots in self-heating mode and effects of the TiO2 layer. Sens. Actuators B Chem. 2020, 310, 127870. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synergistic effects of SnO2 and Au nanoparticles decorated on WS2 nanosheets for flexible, room-temperature CO gas sensing. Sens. Actuators B Chem. 2021, 332, 129493. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.-H.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of H2S sensing by Pd-functionalized networked CuO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low-voltage-driven sensors based on ZnO nanowires for room-temperature detection of NO2 and CO gases. ACS Appl. Mater. Interfaces 2019, 11, 24172–24183. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Self-heating effects on the toluene sensing of Pt-functionalized SnO2–ZnO core–shell nanowires. Sens. Actuators B Chem. 2017, 251, 781–794. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Sun, G.-J.; Kheel, H.; Lee, C. CO gas sensing properties of In4Sn3O12 and TeO2 composite nanoparticle sensors. J. Hazard. Mater. 2016, 305, 130–138. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Bang, J.H.; Kim, H.W.; Kim, S.S. Achievement of self-heated sensing of hazardous gases by WS2 (core)–SnO2 (shell) nanosheets. J. Hazard. Mater. 2021, 412, 125196. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B Chem. 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Park, Y.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Toluene-and benzene-selective gas sensors based on Pt-and Pd-functionalized ZnO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 294, 78–88. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Han, S.; Lee, H.Y.; Choi, S.W.; Kim, S.S.; Kim, H.W. Hybridization of silicon nanowires with TeO2 branch structures and Pt nanoparticles for highly sensitive and selective toluene sensing. Appl. Surf. Sci. 2020, 525, 146620. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Bang, J.H.; Kim, H.W.; Kim, S.S. Selective H2S sensing without external heat by a synergy effect in self-heated CuO-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B Chem. 2019, 300, 126981. [Google Scholar] [CrossRef]
- Yeo, S.; Lee, C.Y.; Kim, D.-S.; Hwang, Y.S.; Park, J.K.; Jung, M.-H.; Cho, W.-J.; Lee, J.S.; Kim, C. Sensing response enhancement of graphene gas sensors by ion beam bombardment. Thin Solid Films 2019, 677, 73–76. [Google Scholar] [CrossRef]
- Mintcheva, N.; Srinivasan, P.; Rayappan, J.B.B.; Kuchmizhak, A.A.; Gurbatov, S.; Kulinich, S. Room-temperature gas sensing of laser-modified anatase TiO2 decorated with Au nanoparticles. Appl. Surf. Sci. 2020, 507, 145169. [Google Scholar] [CrossRef]
- Lavanya, N.; Anithaa, A.; Sekar, C.; Asokan, K.; Bonavita, A.; Donato, N.; Leonardi, S.; Neri, G. Effect of gamma irradiation on structural, electrical and gas sensing properties of tungsten oxide nanoparticles. J. Alloys Compd. 2017, 693, 366–372. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Yu, J.; Moharram, D.; Chen, R.; Zhang, H.; Liu, Q.; Zhang, M.; Wang, J. HFIP-functionalized electrospun WO3 hollow nanofibers/rGO as an efficient double layer sensing material for dimethyl methylphosphonate gas under UV-Light irradiation. J. Alloys Compd. 2020, 832, 154999. [Google Scholar] [CrossRef]
- Rasmidi, R.; Duinong, M.; Chee, F.P. Radiation damage effects on zinc oxide (ZnO) based semiconductor devices—A review. Radiat. Phys. Chem. 2021, 184, 109455. [Google Scholar] [CrossRef]
- Kang, M.; Lee, D.H.; Kang, Y.-M.; Jung, H. Electron beam irradiation dose dependent physico-chemical and electrochemical properties of reduced graphene oxide for supercapacitor. Electrochim. Acta 2015, 184, 427–435. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kwon, Y.J.; Wu, P.; Kim, S.S.; Kim, H.W. Converting the conducting behavior of graphene oxides from n-type to p-type via electron-beam irradiation. ACS Appl. Mater. Interfaces 2018, 10, 7324–7333. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Wu, P.; Kim, S.S. Design of supersensitive and selective ZnO-nanofiber-based sensors for H2 gas sensing by electron-beam irradiation. Sens. Actuators B Chem. 2019, 293, 210–223. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Combination of Pd loading and electron beam irradiation for superior hydrogen sensing of electrospun ZnO nanofibers. Sens. Actuators B Chem. 2019, 284, 628–637. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Cho, H.Y.; Na, H.G.; Lee, B.C.; Kim, S.S.; Kim, H.W. Improvement of gas sensing behavior in reduced graphene oxides by electron-beam irradiation. Sens. Actuators B Chem. 2014, 203, 143–149. [Google Scholar] [CrossRef]
- Choi, M.S.; Mirzaei, A.; Bang, J.H.; Oum, W.; Kim, S.S.; Kim, H.W. Improvement of NO2 sensing properties in Pd functionalized reduced graphene oxides by electron-beam irradiation. Front. Mater. 2019, 6, 197. [Google Scholar] [CrossRef]
- Avasthi, D.K.; Mehta, G.K. Swift Heavy Ions for Materials Engineering and Nanostructuring; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 145. [Google Scholar]
- Markwitz, A.; Leveneur, J.; Gupta, P.; Suschke, K.; Futter, J.; Rondeau, M. Transition metal ion implantation into diamond-like carbon coatings: Development of a base material for gas sensing applications. J. Nanomater. 2015, 16, 706417. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; He, Z.; Rao, J.; Fan, Z.; Wang, X.; Chen, Y. Applications of Ion Beam Irradiation in multifunctional oxide thin films: A Review. ACS Appl. Electron. Mater. 2021, 3, 1031–1042. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kang, S.Y.; Wu, P.; Peng, Y.; Kim, S.S.; Kim, H.W. Selective improvement of NO2 gas sensing behavior in SnO2 nanowires by Ion-Beam Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 13646–13658. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, J.-Y.; Lee, J.-H.; Kim, H.W.; Hishita, S.; Kim, S.S. Enhancement of gas sensing by implantation of Sb-ions in SnO2 nanowires. Sens. Actuators B Chem. 2020, 304, 127307. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-Y.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Hishita, S.; Kim, S.S. Indium-implantation-induced enhancement of gas sensing behaviors of SnO2 nanowires by the formation of homo-core–shell structure. Sens. Actuators B Chem. 2020, 321, 128475. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable flexible sensors: A review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef] [Green Version]
- Molina, A.; Escobar-Barrios, V.; Oliva, J. A review on hybrid and flexible CO2 gas sensors. Synth. Met. 2020, 270, 116602. [Google Scholar] [CrossRef]
- Mardonova, M.; Choi, Y. Review of wearable device technology and its applications to the mining industry. Energies 2018, 11, 547. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, J.; Kim, K.; Cheong, W.; Park, K.; Song, J.; Namgoong, G.; Kim, J.; Heo, J.; Bien, F.; et al. Wearable, wireless gas sensors using highly stretchable and transparent structures of nanowires and graphene. Nanoscale 2016, 8, 10591–10597. [Google Scholar] [CrossRef]
- Li, S.; Lin, P.; Zhao, L.; Wang, C.; Liu, D.; Liu, F.; Sun, P.; Liang, X.; Liu, F.; Yan, X. The room temperature gas sensor based on Polyaniline@flower-like WO3 nanocomposites and flexible PET substrate for NH3 detection. Sens. Actuators B Chem. 2018, 259, 505–513. [Google Scholar] [CrossRef]
- Young, S.-J.; Lin, Z.; Hsiao, C.; Huang, C. Ethanol gas sensors composed of carbon nanotubes with Au nanoparticles adsorbed onto a flexible PI substrate. ECS J. Electron. Sci. 2017, 6, M130. [Google Scholar] [CrossRef]
- Perillo, P.; Rodríguez, D. Low temperature trimethylamine flexible gas sensor based on TiO2 membrane nanotubes. J. Alloys Compd. 2016, 657, 765–769. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Y.; Wan, P.; Zhang, H.; Chen, X.; Sun, X. Flexible transparent electronic gas sensors. Nano-Micro Small 2016, 12, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A Phys. 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sens. Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.-J.; Lee, H.-K.; Lee, D.-S.; Shin, J.-H.; Jun, Y.; Yun, Y. Highly flexible, mechanically stable, and sensitive NO2 gas sensors based on reduced graphene oxide nanofibrous mesh fabric for flexible electronics. Sens. Actuators B Chem. 2018, 257, 846–852. [Google Scholar] [CrossRef]
- Jang, N.-S.; Kim, M.S.; Kim, S.-H.; Lee, S.-K.; Kim, J.-M. Direct growth of titania nanotubes on plastic substrates and their application to flexible gas sensors. Sens. Actuators B Chem. 2014, 199, 361–368. [Google Scholar] [CrossRef]
- Yi, J.; Lee, J.M.; Park, W.I. Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B Chem. 2011, 155, 264–269. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Yaqoob, U.; Phan, D.-T.; Chung, G.-S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2016, 222, 536–543. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Koo, W.-T.; Jang, J.-S.; Kim, D.-H.; Jeong, Y.J.; Kim, R.; Ahn, J.; Choi, S.-J.; Kim, I.-D. 2D layer assembly of Pt-ZnO nanoparticles on reduced graphene oxide for flexible NO2 sensors. Sens. Actuators B Chem. 2021, 331, 129371. [Google Scholar] [CrossRef]
- Duy, L.T.; Baek, J.-Y.; Mun, Y.-J.; Seo, H. Patternable production of SrTiO3 nanoparticles using 1-W laser directly on flexible humidity sensor platform based on ITO/SrTiO3/CNT. J. Mater. Sci. Tech. 2021, 71, 186–194. [Google Scholar] [CrossRef]
- Kim, J.-W.; Porte, Y.; Ko, K.Y.; Kim, H.; Myoung, J. Micropatternable double-faced ZnO nanoflowers for flexible gas sensor. ACS Appl. Mater. Interfaces 2017, 9, 32876–32886. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, J.-G.; Ryu, G.H.; Ko, K.Y.; Woo, W.J.; Kim, Y.; Kim, D.; Lim, J.H.; Lee, S.; Lee, Z.; et al. Low-temperature synthesis of 2D MoS2 on a plastic substrate for a flexible gas sensor. Nanoscale 2018, 10, 9338–9345. [Google Scholar] [CrossRef] [PubMed]
- Nogami, M.; Matsumura, M.; Daiko, Y. Hydrogen sensor prepared using fast proton-conducting glass films. Sens. Actuators B Chem. 2006, 120, 266–269. [Google Scholar] [CrossRef]
- Sopiha, K.V.; Kim, J.-H.; Kim, S.S.; Wu, P. Gas sensing properties of standard soda-lime glass. Sens. Actuators B Chem. 2018, 266, 344–353. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Mirzaei, A.; Kim, J.-H.; Lee, J.-H.; Kim, H.W.; Kim, S.S. Incorporation of metal nanoparticles in soda-lime glass sensors for enhancing selective sensing. Sens. Actuators B Chem. 2019, 296, 126673. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.S.; Tonezzer, M. Selective gas detection and quantification using a resistive sensor based on Pd-decorated soda-lime glass. Sens. Actuators B Chem. 2021, 335, 129714. [Google Scholar] [CrossRef]
- Amato, M.; Rurali, R. Surface physics of semiconducting nanowires. Prog. Surf. Sci. 2016, 91, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Demami, F.; Ni, L.; Rogel, R.; Salaun, A.-C.; Pichon, L. Silicon nanowires-based resistors as gas sensors. Sens. Actuators B Chem. 2012, 170, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.; De Santiago, F.; Pérez, L.; Cruz-Irisson, M. Silicon nanowires as potential gas sensors: A density functional study. Sens. Actuators B Chem. 2017, 242, 1246–1250. [Google Scholar] [CrossRef]
- Qin, Y.; Zang, J. Stable clusters array of silicon nanowires developed by top-plating technique as a high-performance gas sensor. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 127, 114508. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.-W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Fabrication and gas sensing properties of vertically aligned Si nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, C.S.; Chou, C.J.; Yen, T. Morphological control of single-crystalline silicon nanowire arrays near room temperature. Adv. Mater. 2008, 20, 3811–3815. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Liu, W.; Yue, L.; Li, R.; Luo, Y.; Trevor, M.; Jiang, B.; Bai, F.; Fu, P. Metal-assisted chemical etching for designable monocrystalline silicon nanostructure. Mater. Res. Bull. 2016, 76, 436–449. [Google Scholar] [CrossRef]
- Ramírez-González, F.; García-Salgado, G.; Rosendo, E.; Díaz, T.; Nieto-Caballero, F.; Coyopol, A.; Romano, R.; Luna, A.; Monfil, K.; Gastellou, E. Porous silicon gas sensors: The role of the layer thickness and the silicon conductivity. Sensors 2020, 20, 4942. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Oum, W.; Han, S.; Kim, S.S.; Kim, H.W. Porous Si/SnO2 nanowires heterostructures for H2S gas sensing. Ceram. Int. 2020, 46, 604–611. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-Y.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd-decorated Si nano-horns as sensitive and selective hydrogen gas sensors. Mater. Res. Bull. 2020, 132, 110985. [Google Scholar] [CrossRef]
- Yao, M.S.; Tang, W.X.; Wang, G.E.; Nath, B.; Xu, G. MOF thin film-coated metal oxide nanowire array: Significantly improved chemiresistor sensor performance. Adv. Mater. 2016, 28, 5229–5234. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M. Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 2015, 39, 607–620. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Enviro. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Arul, C.; Moulaee, K.; Donato, N.; Iannazzo, D.; Lavanya, N.; Neri, G.; Sekar, C. Temperature modulated Cu-MOF based gas sensor with dual selectivity to acetone and NO2 at low operating temperatures. Sens. Actuators B Chem. 2021, 329, 129053. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Leroy, E.; Julbe, A.; Kim, S.S. Design and fabrication of highly selective H2 sensors based on SIM-1 nanomembrane-coated ZnO nanowires. Sens. Actuators B Chem. 2018, 264, 410–418. [Google Scholar] [CrossRef]
- Aguado, S.; Canivet, J.; Farrusseng, D. Facile shaping of an imidazolate-based MOF on ceramic beads for adsorption and catalytic applications. Chem. Comm. 2010, 46, 7999–8001. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.M.; Van, M.; Balkus, K. Tuning the crystal size and morphology of the substituted imidazole material, SIM-1. J. Porous Mater. 2014, 21, 889–902. [Google Scholar] [CrossRef]
- Weber, M.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Iatsunskyi, I.; Coy, E.; Drobek, M.; Julbe, A.; Bechelany, M.; Kim, S.S. High-performance nanowire hydrogen sensors by exploiting the synergistic effect of Pd nanoparticles and metal–organic framework membranes. ACS Appl. Mater. Interfaces 2018, 10, 34765–34773. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, B.; Lai, Z. Synthesis of ceramic hollow fiber supported zeolitic imidazolate framework-8 (ZIF-8) membranes with high hydrogen permeability. J. Memb. Sci. 2012, 421, 292–298. [Google Scholar] [CrossRef]
- Nguyen, D.-K.; Lee, J.-H.; Nguyen, T.-B.; Doan, T.L.H.; Phan, B.T.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of selective CO detection by Ni-incorporated metal-organic frameworks. Sens. Actuators B Chem. 2020, 315, 128110. [Google Scholar] [CrossRef]
- Lee, J.-H.; Nguyen, T.-B.; Nguyen, D.-K.; Kim, J.-H.; Kim, J.-Y.; Phan, B.T.; Kim, S.S. Gas Sensing Properties of Mg-Incorporated Metal–Organic Frameworks. Sensors 2019, 19, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, T.L.H.; Kim, J.Y.; Lee, J.H.; Nguyen, L.H.T.; Nguyen, H.T.T.; Pham, A.T.T.; Le, T.B.N.; Mirzaei, A.; Phan, T.B.; Kim, S.S. Facile synthesis of metal-organic framework-derived ZnO/CuO nanocomposites for highly sensitive and selective H2S gas sensing. Sens. Actuators B Chem. 2021, 349, 130741. [Google Scholar] [CrossRef]
- Doan, T.L.H.; Kim, J.Y.; Lee, J.H.; Nguyen, L.H.T.; Dang, Y.T.; Bui, K.B.T.; Pham, A.T.T.; Mirzaei, A.; Phan, T.B.; Kim, S.S. Preparation of n-ZnO/p-Co3O4 heterojunctions from zeolitic imidazolate frameworks (ZIF-8/ZIF-67) for sensing low ethanol concentrations. Sens. Actuators B Chem. 2021, 348, 130684. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, S.; Choi, J.; Choi, J.; Ji, H.; Kim, G.; Cao, G.; Lee, J. Synthesis and gas sensing characteristics of highly crystalline ZnO–SnO2 core–shell nanowires. Sens. Actuators B Chem. 2010, 148, 595–600. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 4285–4292. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Woo, H.; Na, C.W.; Kim, I.D.; Lee, J.H. Highly sensitive and selective trimethylamine sensor using one-dimensional ZnO–Cr2O3 hetero-nanostructures. Nanotechnology 2012, 23, 245501. [Google Scholar] [CrossRef]
- Jang, Y.G.; Kim, W.S.; Kim, D.H.; Hong, S.H. Fabrication of Ga2O3/SnO2 core–shell nanowires and their ethanol gas sensing properties. J. Mater. Res. 2011, 26, 2322–2327. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Dao, D.V.; Kim, D.S.; Lee, H.J.; Oh, S.Y.; Lee, I.H.; Yu, Y.T. Effect of core and surface area toward hydrogen gas sensing performance using Pd@ZnO core-shell nanoparticles. J. Collide Interfaces Sci. 2021, 587, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kim, T.H.; Kim, S.M.; Oh, S.; Kim, K.K. Ultraviolet light-emitting diode-assisted highly sensitive room temperature NO2 gas sensors based on low-temperature solution-processed ZnO/TiO2 nanorods decorated with plasmonic Au nanoparticles. Nanoscale 2021, 13, 12177–12184. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.; Sun, G.J.; Lee, W.I.; Kim, K.K.; Lee, C. Fabrication and NO2 gas sensing performance of TeO2-core/CuO-shell heterostructure nanorod sensors. Nanoscale Res. Lett. 2014, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.D.; Dao, D.V.; Lee, I.H.; Yu, Y.T.; Oh, S.Y. High response and selectivity toward hydrogen gas detection by In2O3 doped Pd@ZnO core-shell nanoparticles. J. Alloys Compd. 2021, 854, 157280. [Google Scholar] [CrossRef]
- Dao, D.V.; Nguyen, T.T.D.; Kim, D.S.; Yoon, J.W.; Yu, Y.T.; Lee, I.H. Core and dopant effects toward hydrogen gas sensing activity using Pd@N-CeO2 core–shell nanoflatforms. J. Ind. Eng. Chem. 2021, 95, 325–332. [Google Scholar] [CrossRef]
- Yun, J.; Jin, C.Y.; Ahn, J.H.; Jeon, S.; Park, I. A self-heated silicon nanowire array: Selective surface modification with catalytic nanoparticles by nanoscale Joule heating and its gas sensing applications. Nanoscale 2013, 5, 6851–6856. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yun, J.; Moon, D.I.; Choi, Y.K.; Park, I. Self-heated silicon nanowires for high performance hydrogen gas detection. Nanotechnology 2015, 26, 095501–095511. [Google Scholar] [CrossRef]
- Seo, J.Y.; Shin, H.J. Self-heating hydrogen gas sensor based on an array of single suspended carbon nanowires functionalized with palladium nanoparticles. Sens. Actuators B Chem. 2017, 247, 564–572. [Google Scholar] [CrossRef]
- Moon, H.G.; Shim, Y.S.; Kim, D.H.; Jeong, H.Y.; Jeong, M.; Jung, J.Y.; Han, S.M.; Kim, J.K.; Kim, J.S.; Park, H.H.; et al. Self-activated ultrahigh chemosensitivity of oxide thin film nanostructures for transparent sensors. Sci. Rep. 2012, 2, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Na, H.G.; Kim, H.W.; Kulish, V.; Wu, P. Promotion of acceptor formation in SnO2 nanowires by e-beam bombardment and impacts to sensor application. Sci. Rep. 2015, 5, 10723. [Google Scholar]
- Byoun, Y.; Park, S.; Jin, C.; Song, Y.J.; Choi, S.W. Highly sensitive and selective ethanol detection at room temperature utilizing holey SWCNT-Sn/SnO2 nanocomposites synthesized by microwave irradiation. Sens. Actuators B Chem. 2019, 290, 467–476. [Google Scholar] [CrossRef]
- Bae, G.; Song, D.S.; Lim, Y.R.; Jeon, I.S.; Jang, M.; Yoon, Y.; Jeon, C.; Song, W.; Myung, S.; Lee, S.S.; et al. Chemical patterning of graphene via metal-assisted highly energetic electron irradiation for graphene homojunction-based gas sensors. ACS Appl. Mater. Interfaces 2020, 12, 47802–47810. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, D.S.; Choi, H.K.; Lee, D.H.; Kim, J.-E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S.-Y. Flexible room-temperature NO2 gas sensors based on carbon nanotube/reduced graphene oxide hybrid films. Appl. Phys. Lett. 2010, 96, 213105. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Ahn, J.; Lee, K.B.; Kim, D.; Kim, J. Graphene-based flexible NO2 chemical sensors. Thin Solid Films 2012, 520, 5459–5462. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.J.; Kim, Y.-J.; Shim, Y.-S.; Kim, S.Y.; Hong, B.H.; Jang, H.W. Self-activated transparent all-graphene gas sensor with endurance to humidity and mechanical bending. ACS Nano 2015, 9, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.J.; Hong, W.G.; Choi, N.J.; Kim, B.H.; Jun, Y.; Lee, H.K. Ultrasensitive and highly selective graphene-based single yarn for use in wearable gas sensor. Sci. Rep. 2015, 5, 10904. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.A.; Ji, S.; Kim, S.; Park, C.Y.; Myung, S.; Song, W.; Lee, S.S.; Lim, J.; An, K.-S. Highly sensitive and wearable gas sensors consisting of chemically functionalized graphene oxide assembled on cotton yarn. RSC Adv. 2018, 8, 11991–11996. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Jung, H.G.; Jang, J.W.; Park, D.; Lee, D.; Kim, I.; Kim, Y.; Cheong, D.Y.; Hwang, K.S.; Lee, G.; et al. Graphene-based electronic textile sheet for highly sensitive detection of NO2 and NH3. Sens. Actuators B Chem. 2021, 345, 130361. [Google Scholar] [CrossRef]
- Ugale, A.D.; Umarji, G.G.; Jung, S.H.; Deshpande, N.G.; Lee, W.; Cho, H.K.; Yoo, J.B. ZnO decorated flexible and strong graphene fibers for sensing NO2 and H2S at room temperature. Sens. Actuators B Chem. 2020, 308, 127–690. [Google Scholar] [CrossRef]
- Lee, S.H.; Eom, W.; Shin, H.; Ambade, R.B.; Bang, J.H.; Kim, H.W.; Han, T.H. Room-temperature, highly durable Ti3C2Tx MXene/Graphene hybrid fibers for NH3 gas sensing. ACS Appl. Mater. Interfaces 2020, 12, 10434–10442. [Google Scholar] [CrossRef]
- Li, H.Y.; Lee, C.S.; Kim, D.H.; Lee, J.H. Flexible room-temperature NH3 sensor for ultrasensitive, selective, and humidity-independent gas detection. ACS Appl. Mater. Interfaces 2018, 10, 27858–27867. [Google Scholar] [CrossRef]
- Na, C.W.; Kim, J.H.; Kim, H.J.; Woo, H.S.; Gupta, A.; Kim, H.K.; Lee, J.H. Highly selective and sensitive detection of NO2 using rGO-In2O3 structure on flexible substrate at low temperature. Sens. Actuators B Chem. 2018, 255, 1671–1679. [Google Scholar] [CrossRef]
- Yaqoob, U.; Phan, D.T.; Iftekhar Uddin, A.S.M.; Chung, G.S. Highly flexible room temperature NO2 sensor based on MWCNTs-WO3 nanoparticles hybrid on a PET substrate. Sens. Actuators B Chem. 2015, 221, 760–768. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.D.; Kim, J.H.; Shin, D.H.; Kim, S.; Choi, S.-H. Graphene/Si-nanowire heterostructure molecular sensors. Sci. Rep. 2014, 4, 5384. [Google Scholar] [CrossRef] [Green Version]
- Eom, N.; Cho, H.B.; Lim, H.R.; Hwan, T.Y.; Song, Y.; Choa, Y.H. Ultrasensitive detection of low-ppm H2S gases based on palladium-doped porous silicon sensors. RSC Adv. 2018, 8, 29995. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.S.; Kim, H.; Kim, B.S.; Lee, E.; Cho, H.H.; Lee, W. High-performance vertical hydrogen sensors using Pd-coated rough Si nanowires. J. Mater. Chem. 2011, 21, 15935–15939. [Google Scholar] [CrossRef]
- Lee, J.H.; Nguyen, T.T.T.; Nguyen, L.; Phan, T.B.; Kim, S.S.; Doan, T.L.H. Functionalization of zirconium-based metal–organic frameworks for gas sensing applications. J. Hazard. Mater. 2021, 403, 124104. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.T.; Qiao, S.; Ogata, A.F.; Jha, G.; Jang, J.S.; Chen, V.T.; Kim, I.D.; Penner, R.M. Accelerating palladium nanowire H2 sensors using engineered nanofiltration. ACS Nano 2017, 11, 9276–9285. [Google Scholar] [CrossRef]
- Koo, W.T.; Cha, J.H.; Jung, J.W.; Choi, S.J.; Jang, J.S.; Kim, D.H.; Kim, I.D. Few-layered WS2 nanoplates confined in Co, N-doped hollow carbon nanocages: Abundant WS2 edges for highly sensitive gas sensors. Adv. Funct. Mater. 2018, 28, 1802575. [Google Scholar] [CrossRef]
- Jo, Y.M.; Kim, T.H.; Lee, C.S.; Lim, K.; Na, C.W.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.H. Metal–organic framework-derived hollow hierarchical Co3O4 nanocages with tunable size and morphology: Ultrasensitive and highly selective detection of methylbenzenes. ACS Appl. Mater. Interfaces 2018, 10, 8860–8868. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.T.; Yu, S.; Choi, S.J.; Jang, J.S.; Cheong, J.Y.; Kim, I.D. Nanoscale PdO catalyst functionalized Co3O4 hollow nanocages using MOF templates for selective detection of acetone molecules in exhaled breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar] [CrossRef]
- Jang, J.S.; Koo, W.T.; Kim, D.H.; Kim, I.D. In situ coupling of multidimensional MOFs for heterogeneous metal-oxide architectures: Toward sensitive chemiresistors. ACS Cent. Sci. 2018, 4, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.; Abuzalat, O.; Mostafa, S.; Park, S.S.; Kim, S. Intense pulsed light-based synthesis of hybrid TiO2-SnO2/MWCNT doped Cu-BTC for room temperature ammonia sensing. J. Mater. Chem. C 2020, 8, 7567–7574. [Google Scholar] [CrossRef]
- Jo, Y.M.; Lim, K.; Yoon, J.W.; Jo, Y.K.; Moon, Y.K.; Jang, H.W.; Lee, J.H. Visible-light-activated Type II heterojunction in Cu3(hexahydroxytriphenylene)2/Fe2O3 hybrids for reversible NO2 sensing: Critical role of π–π* transition. ACS Cent. Sci. 2021, 7, 1176–1182. [Google Scholar] [CrossRef]

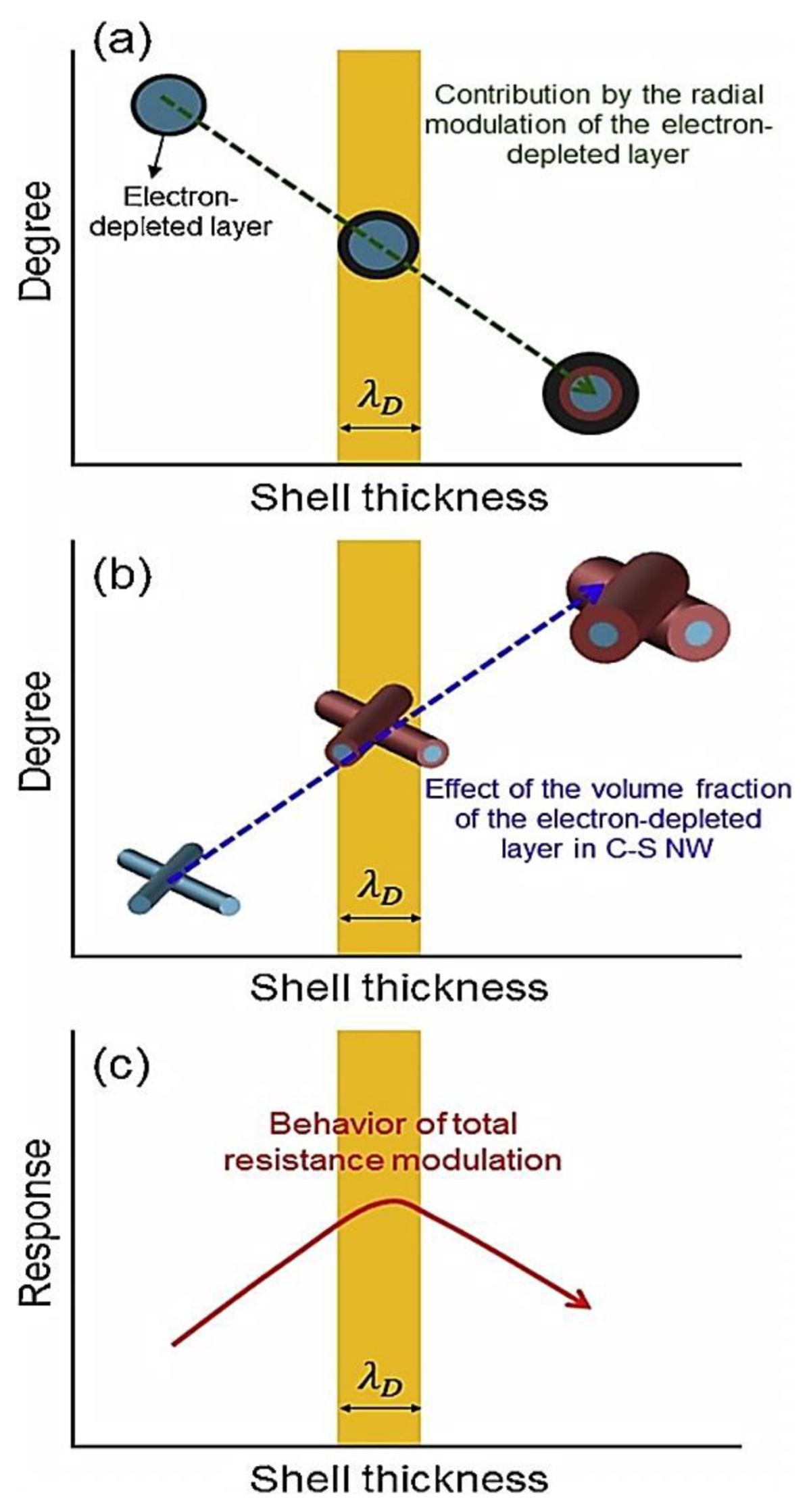

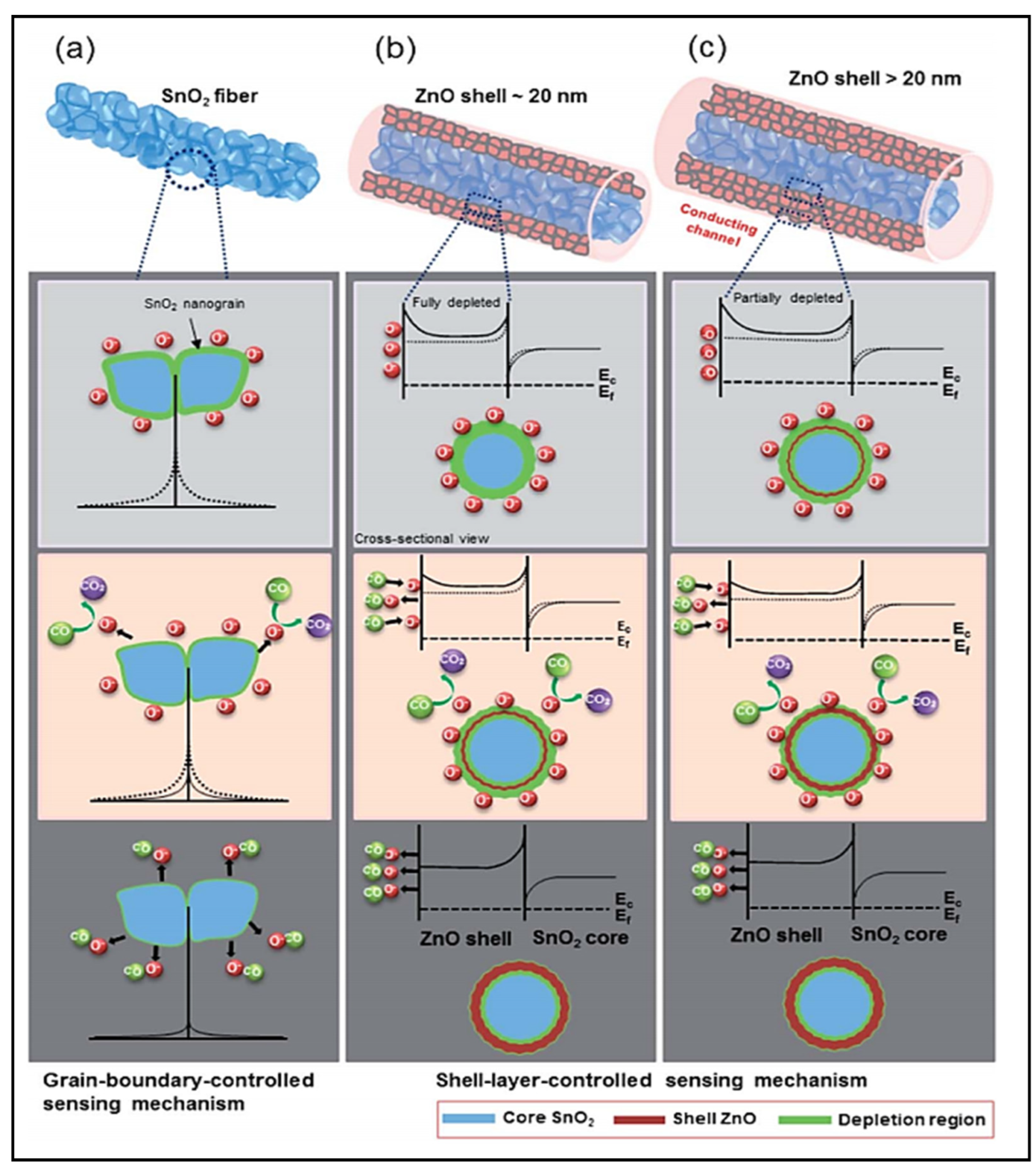
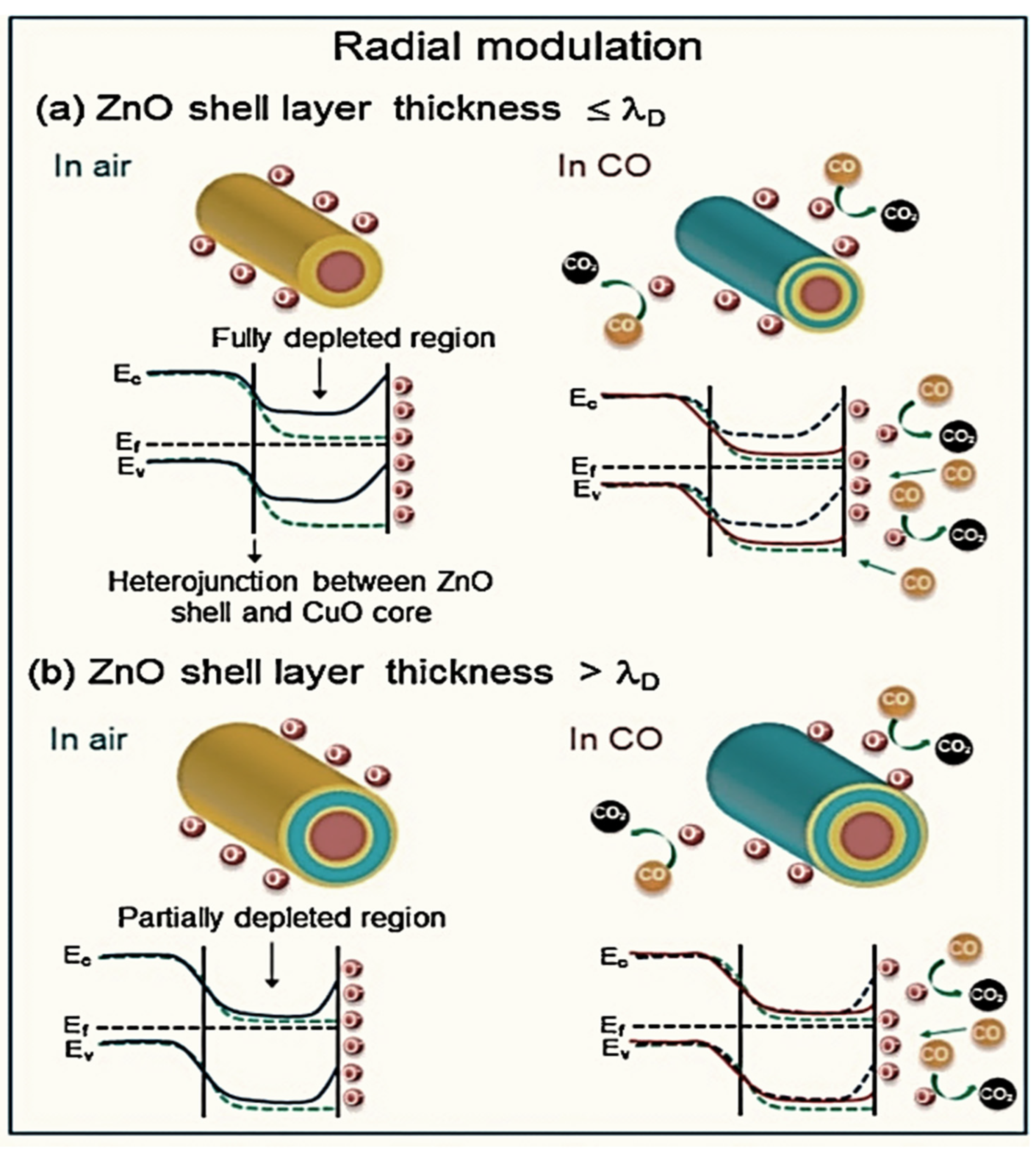
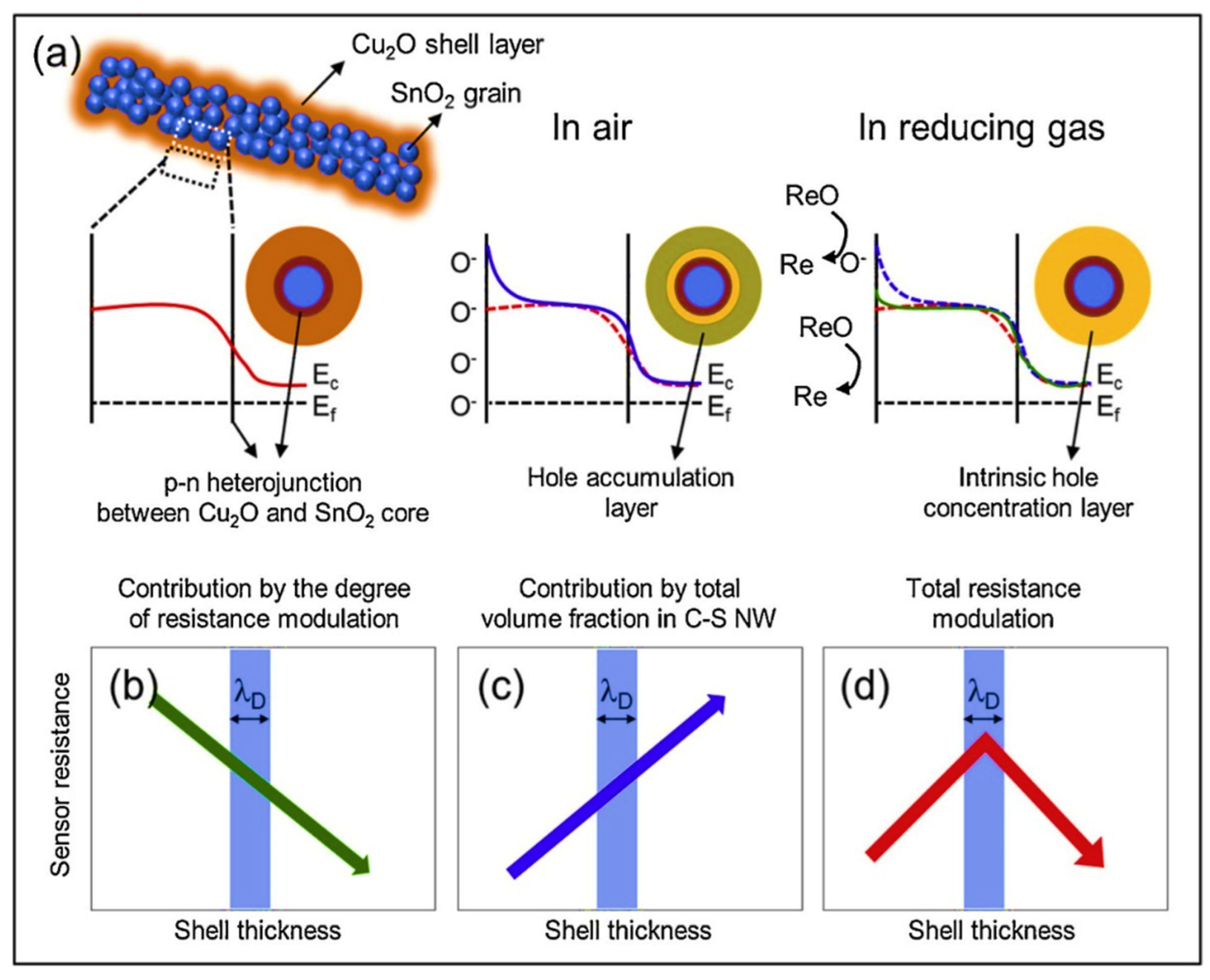
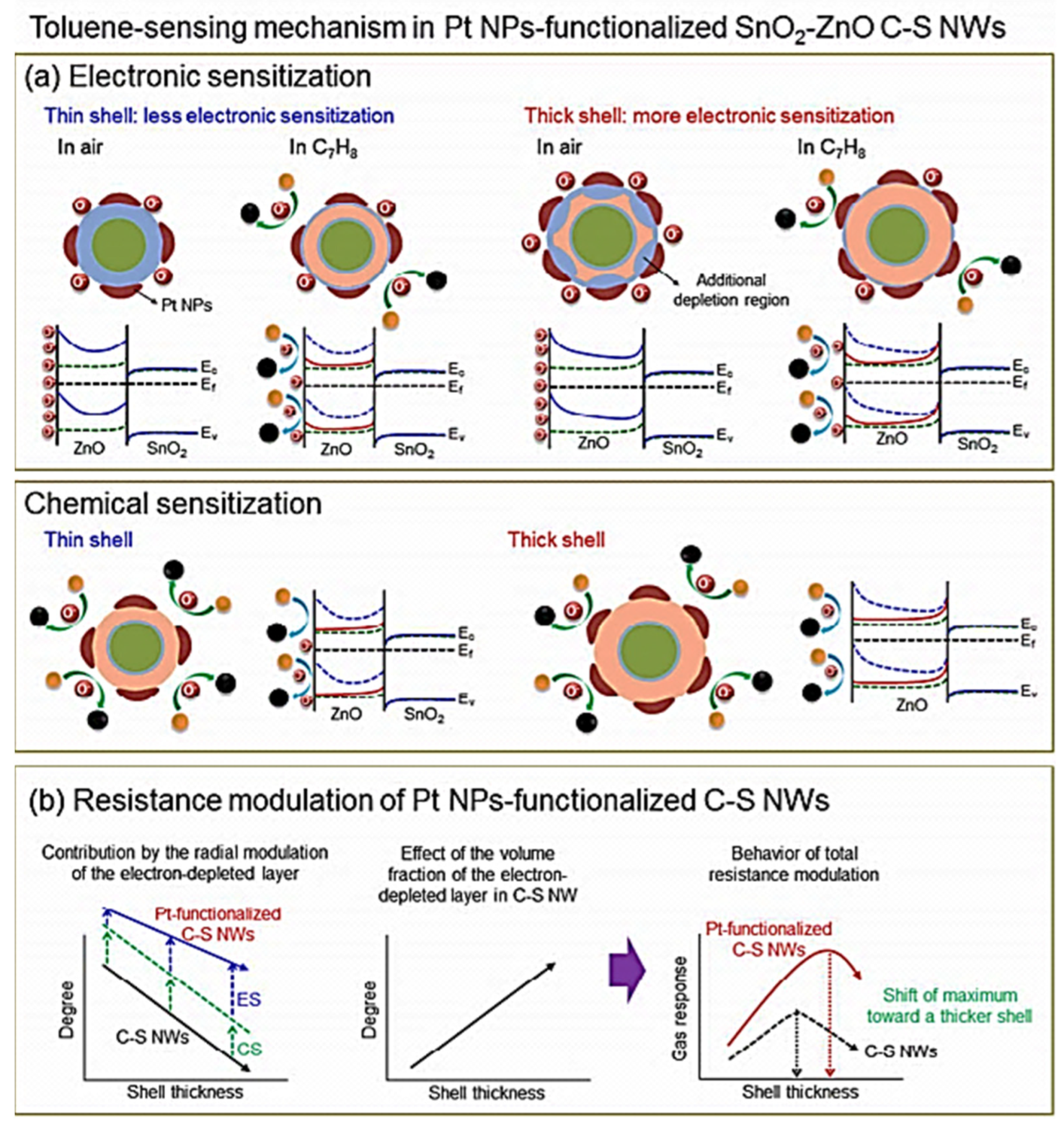
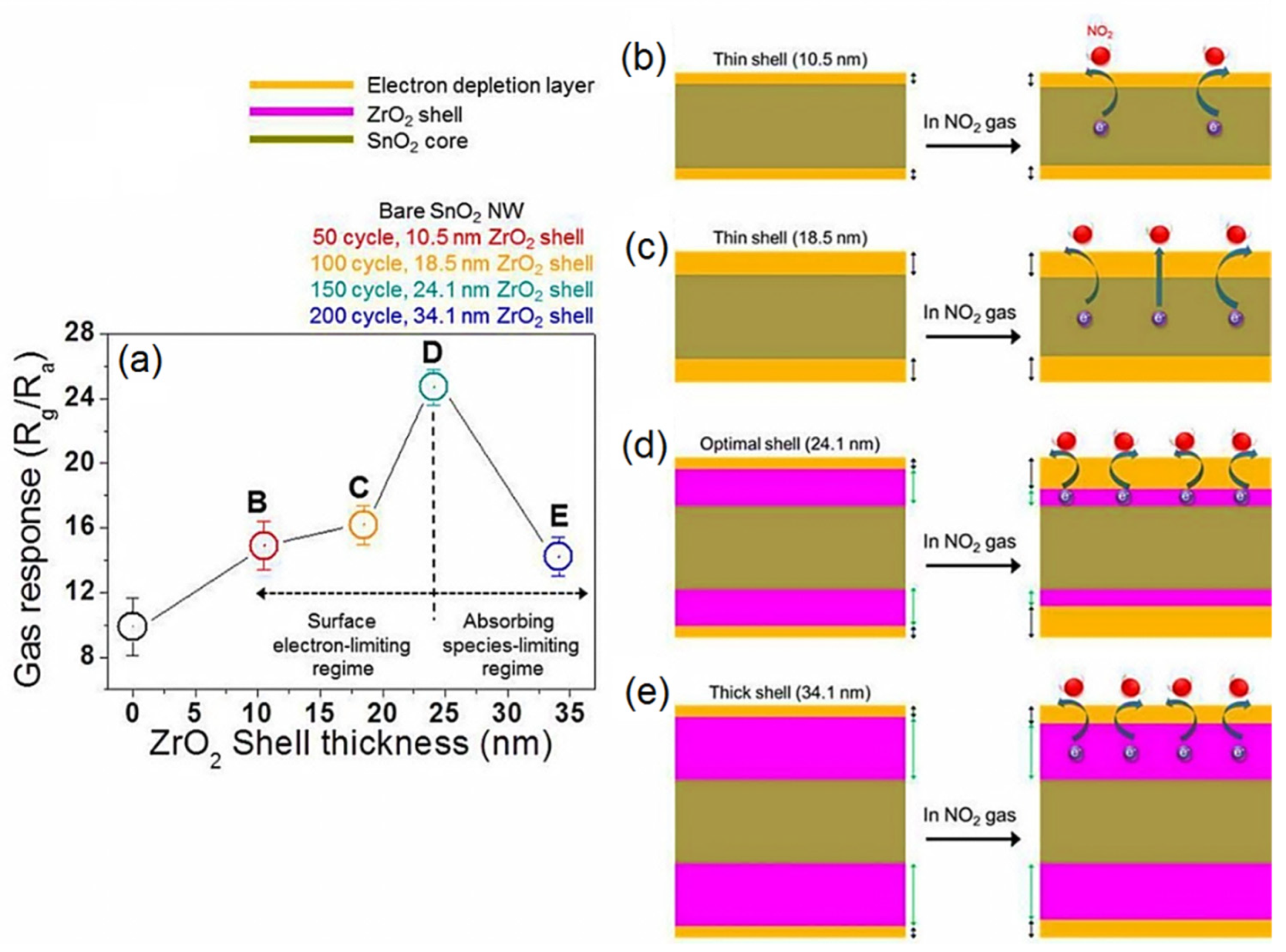
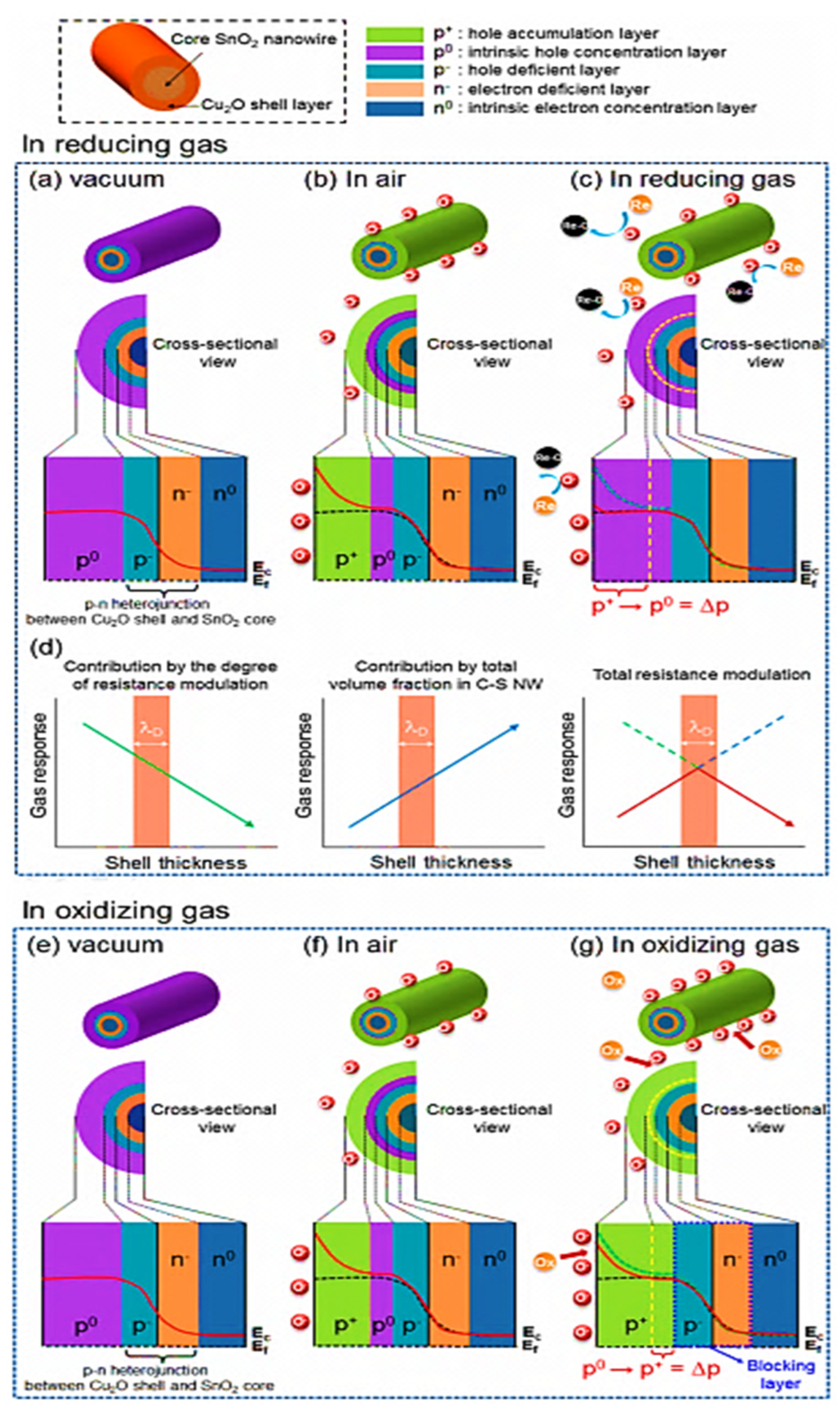
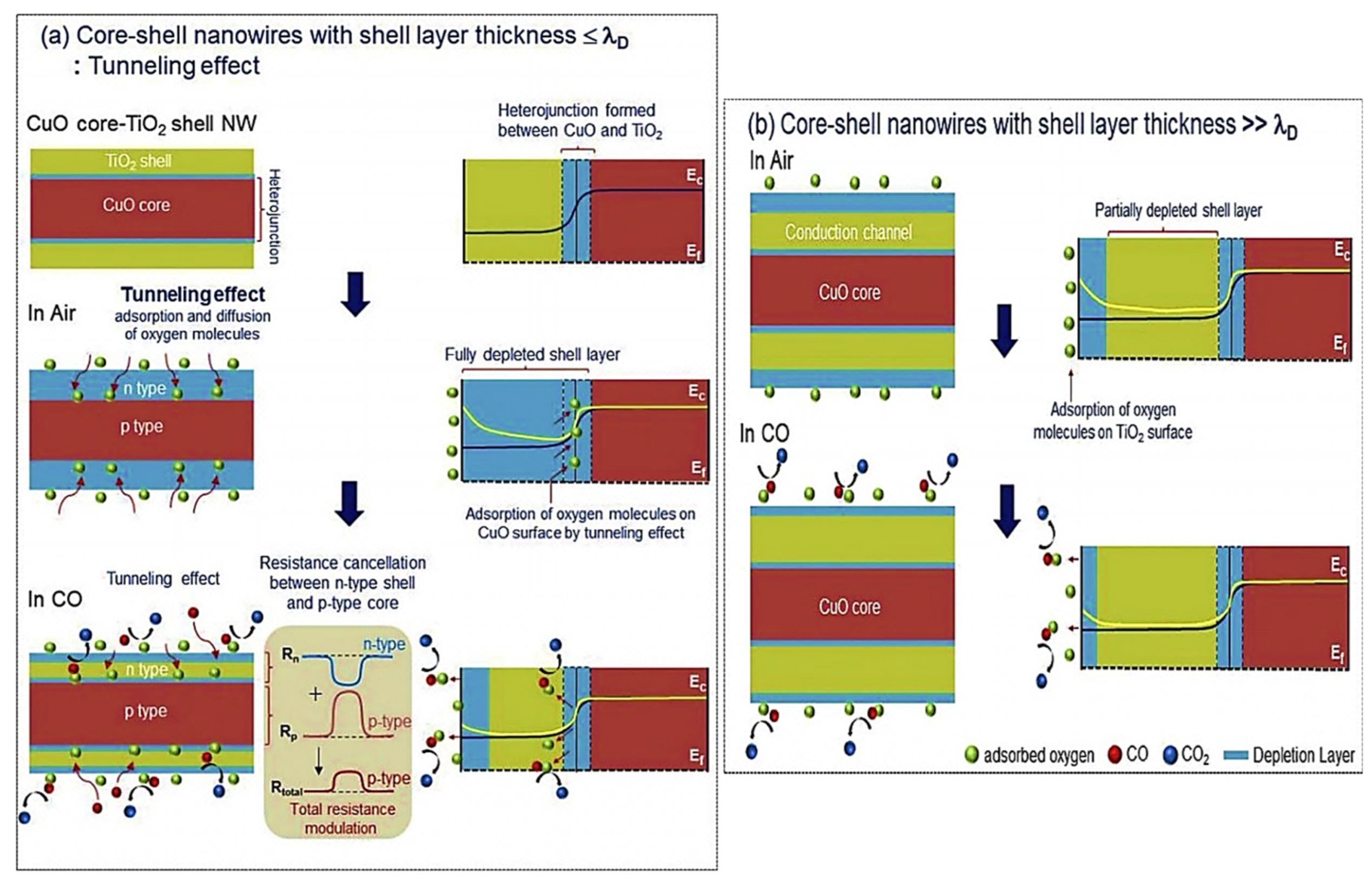
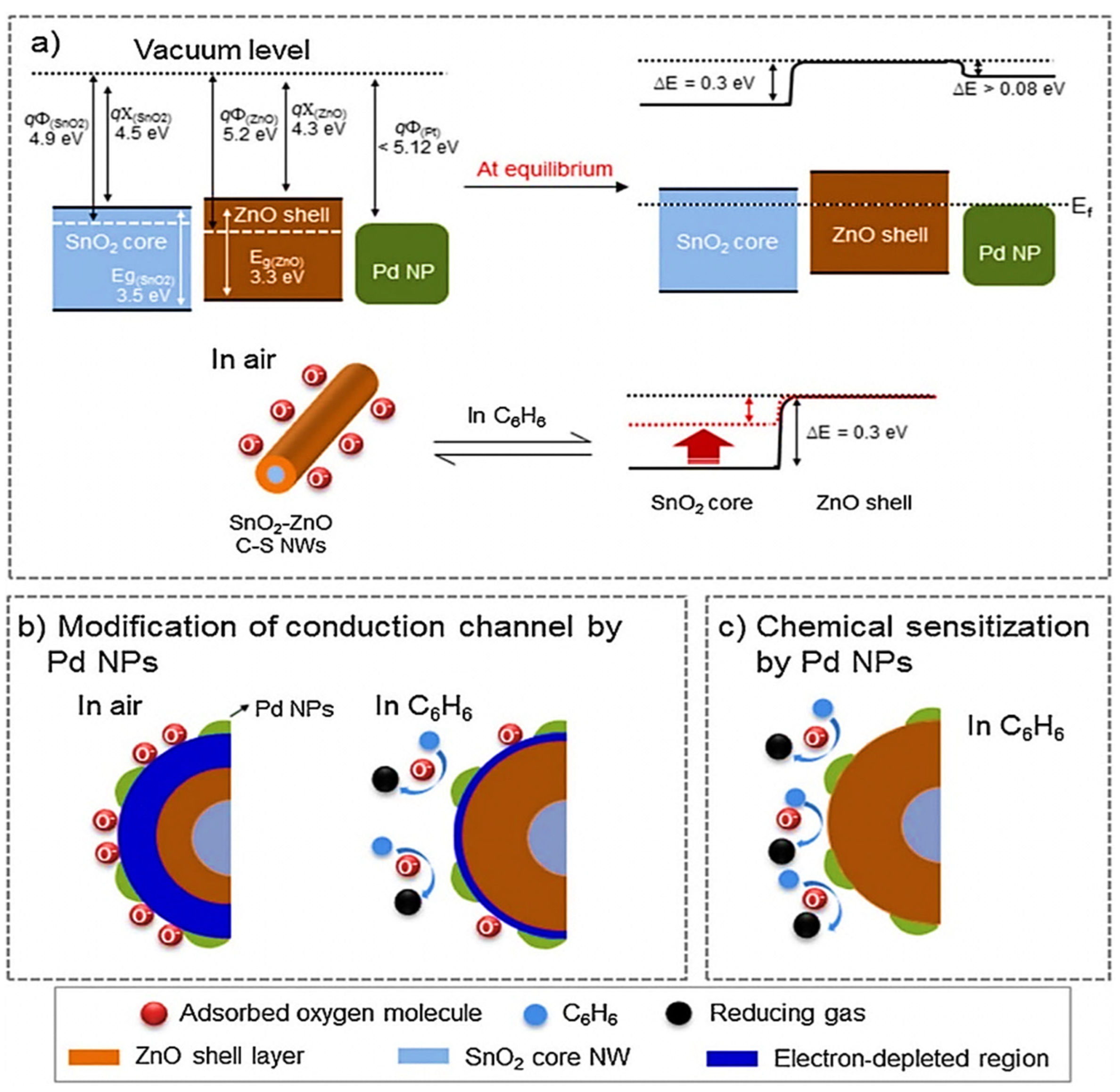
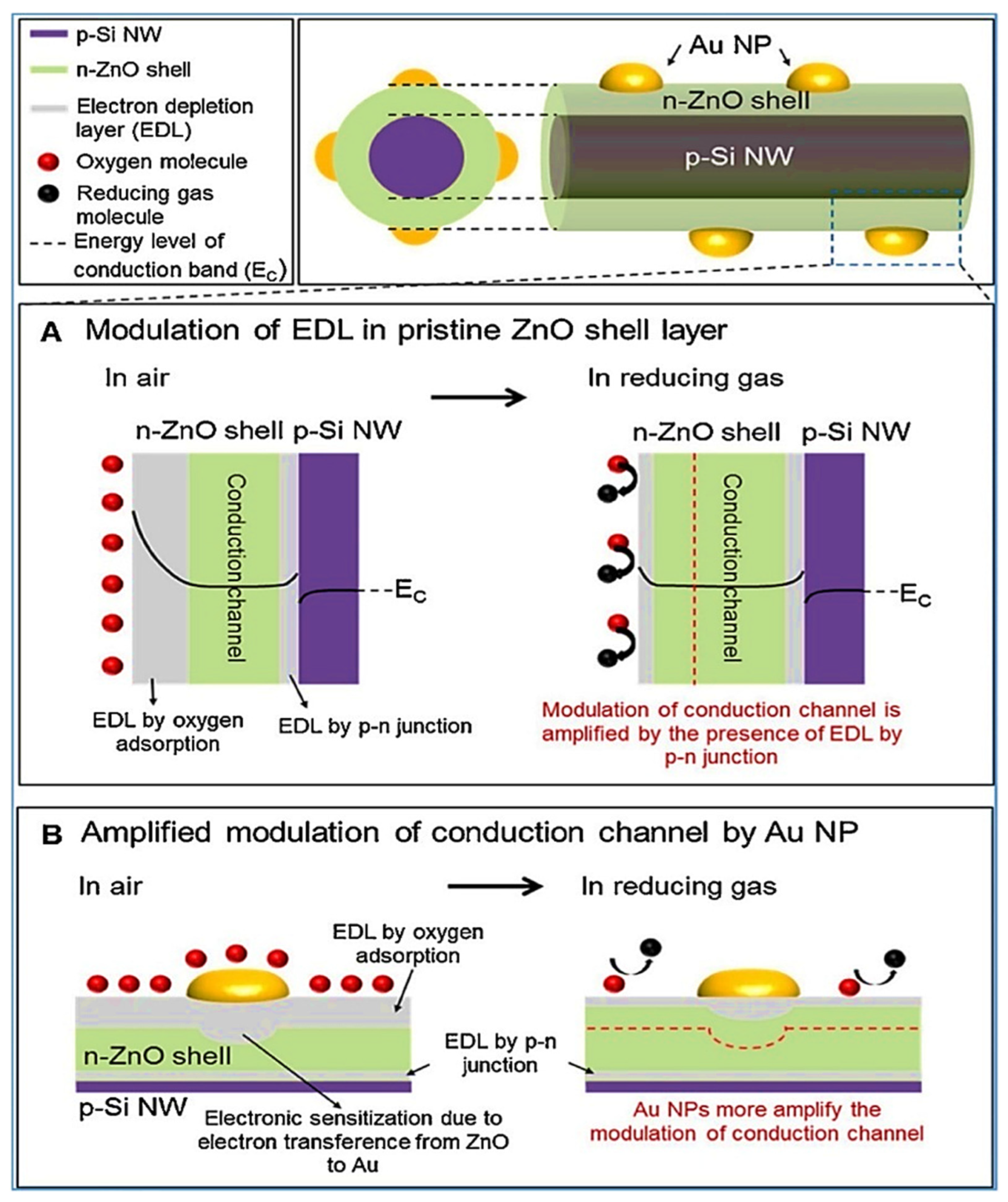
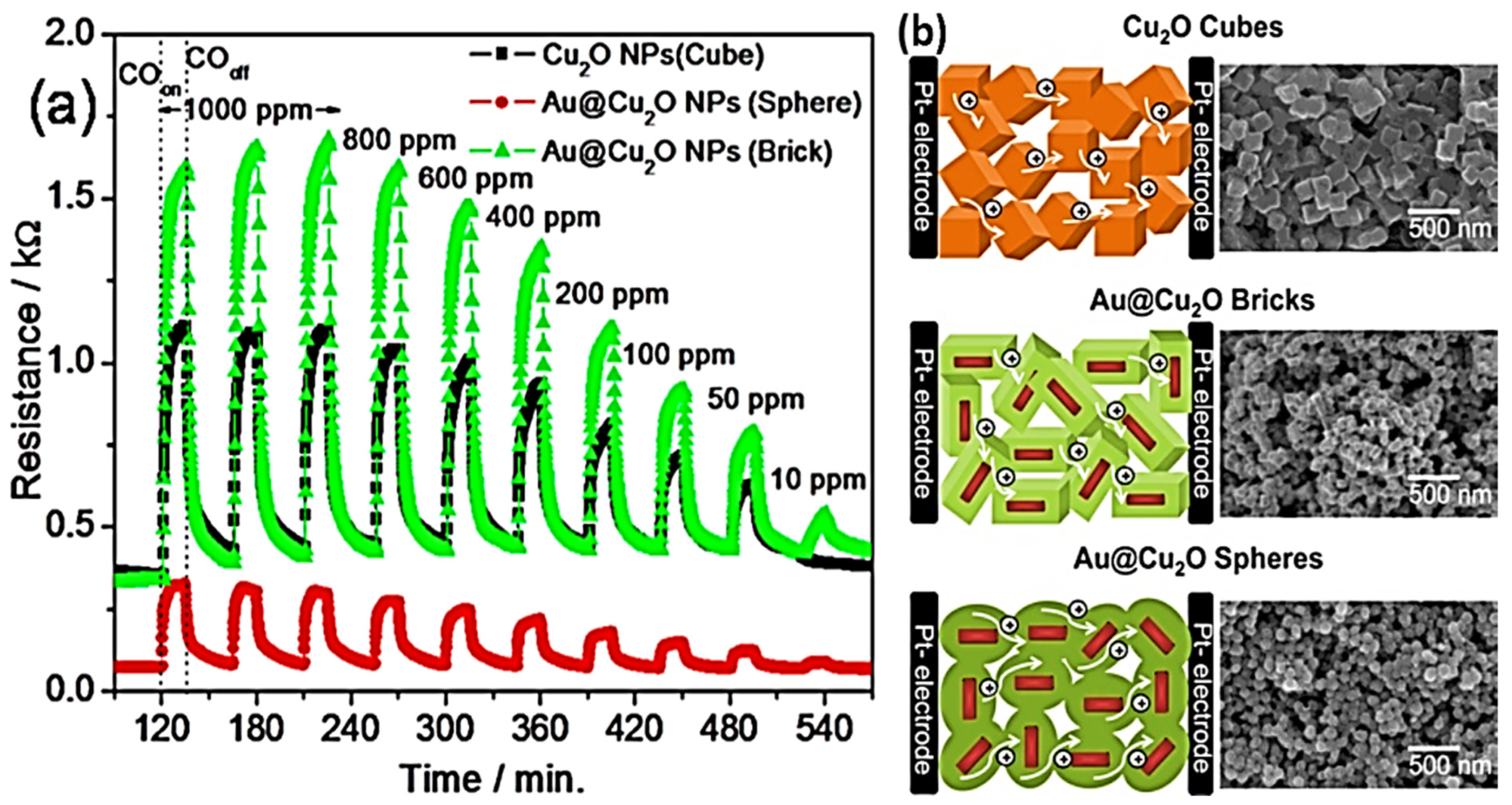
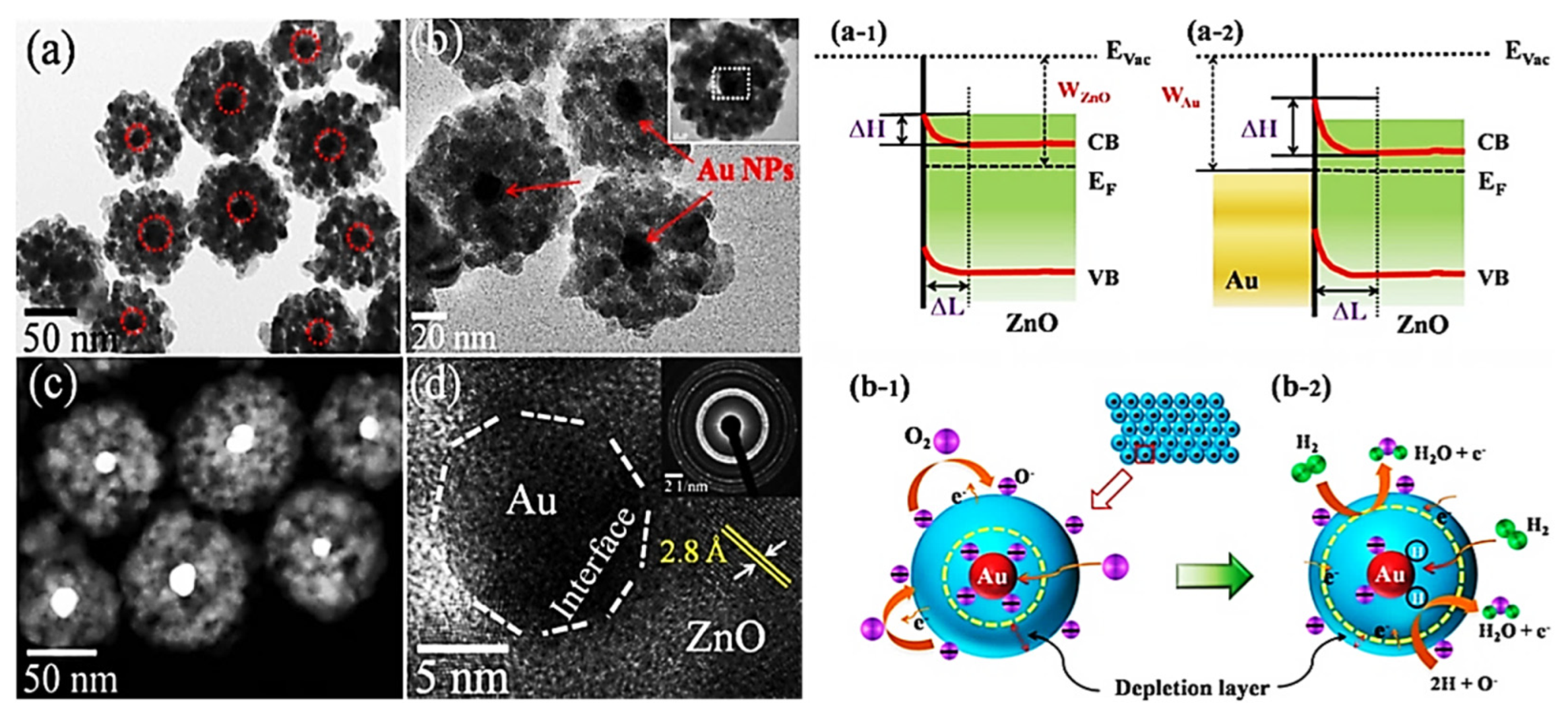

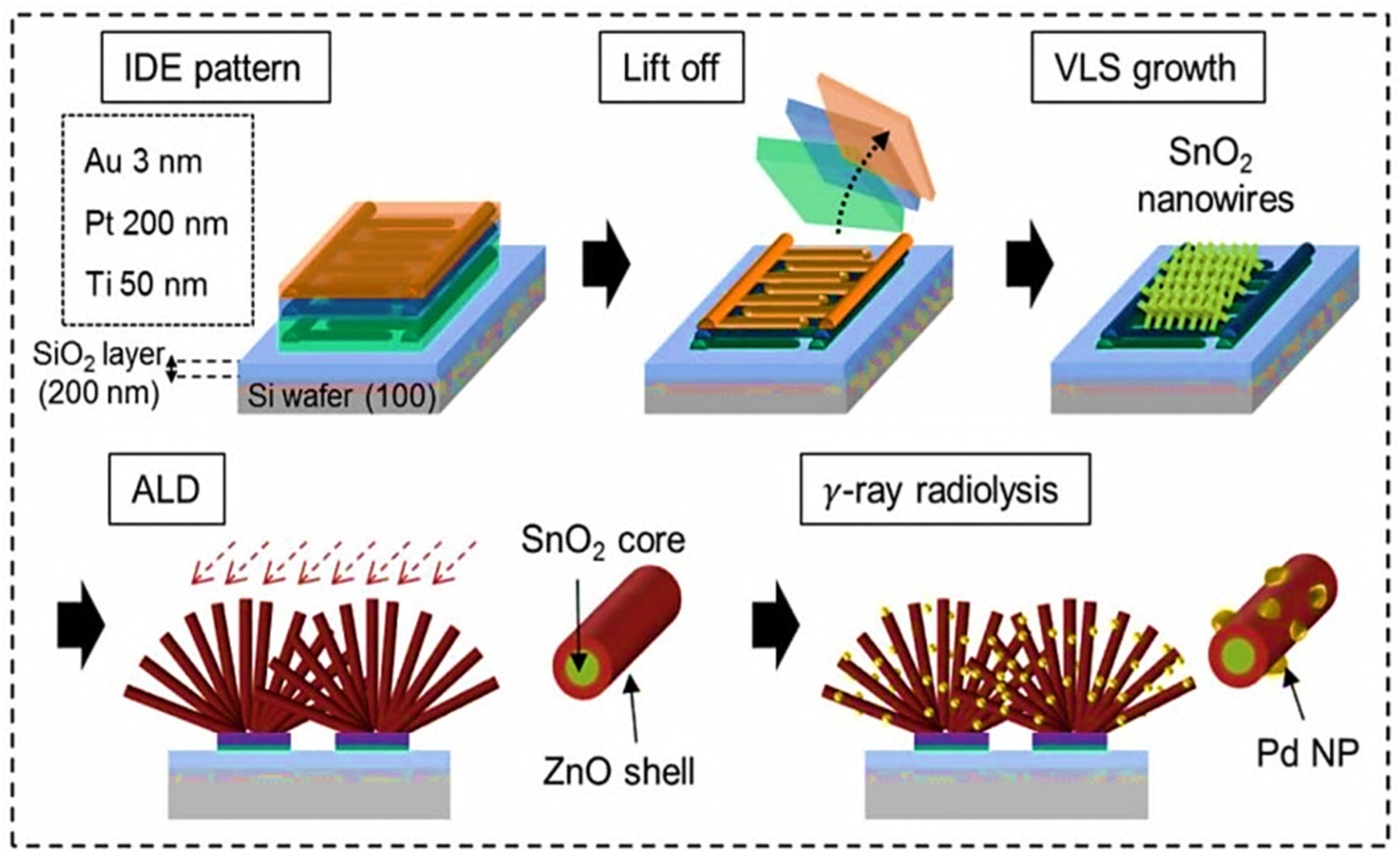
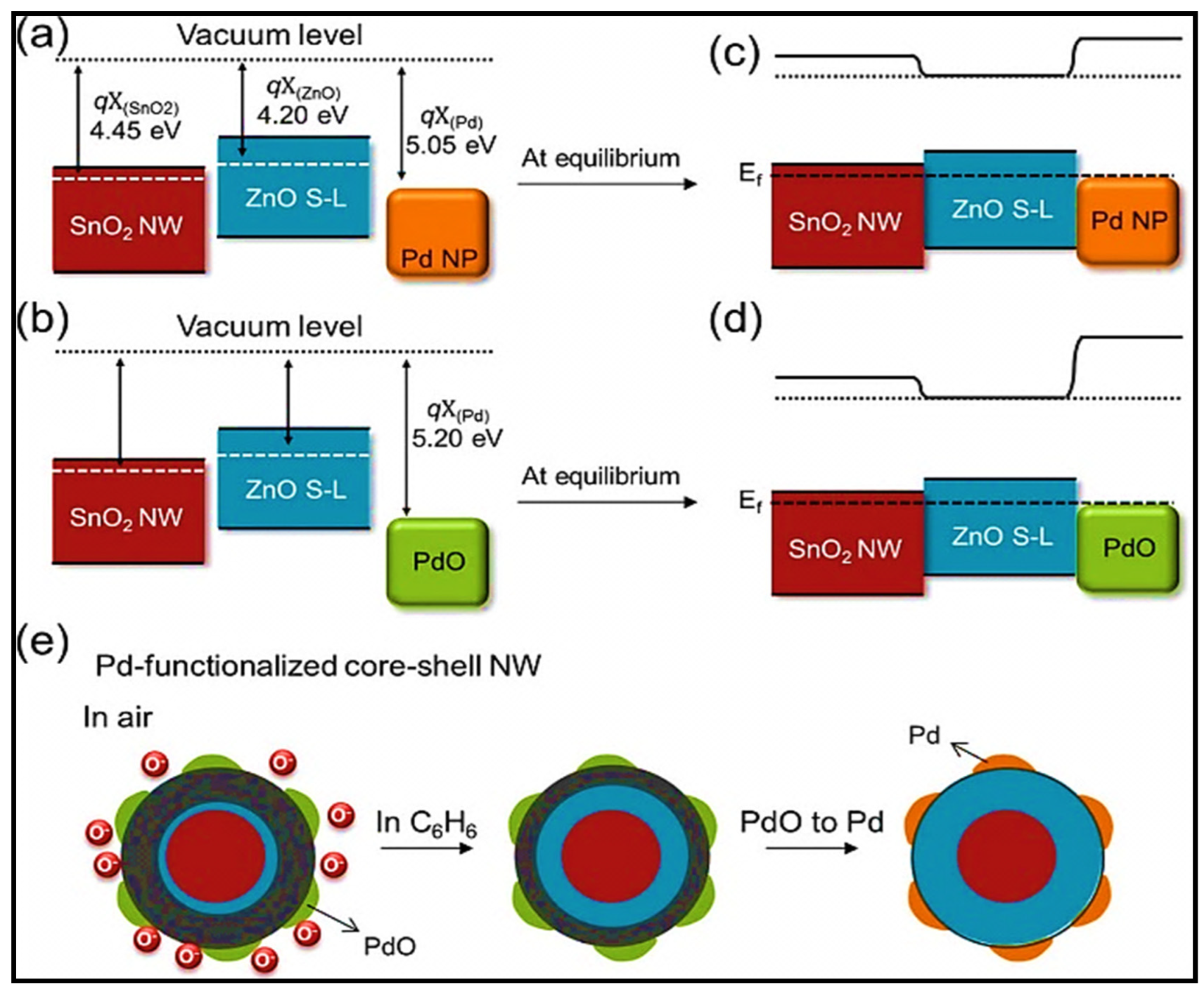

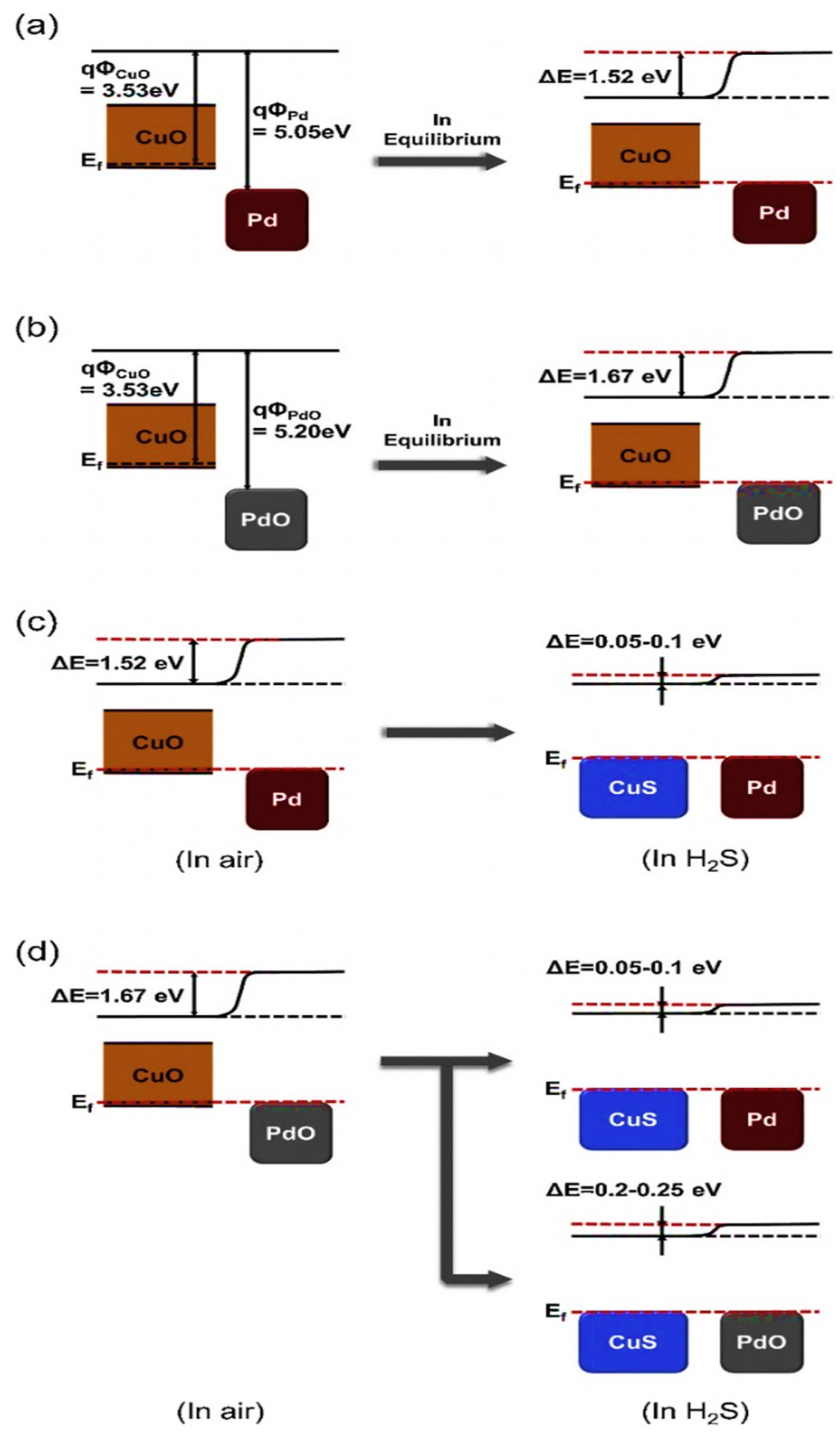
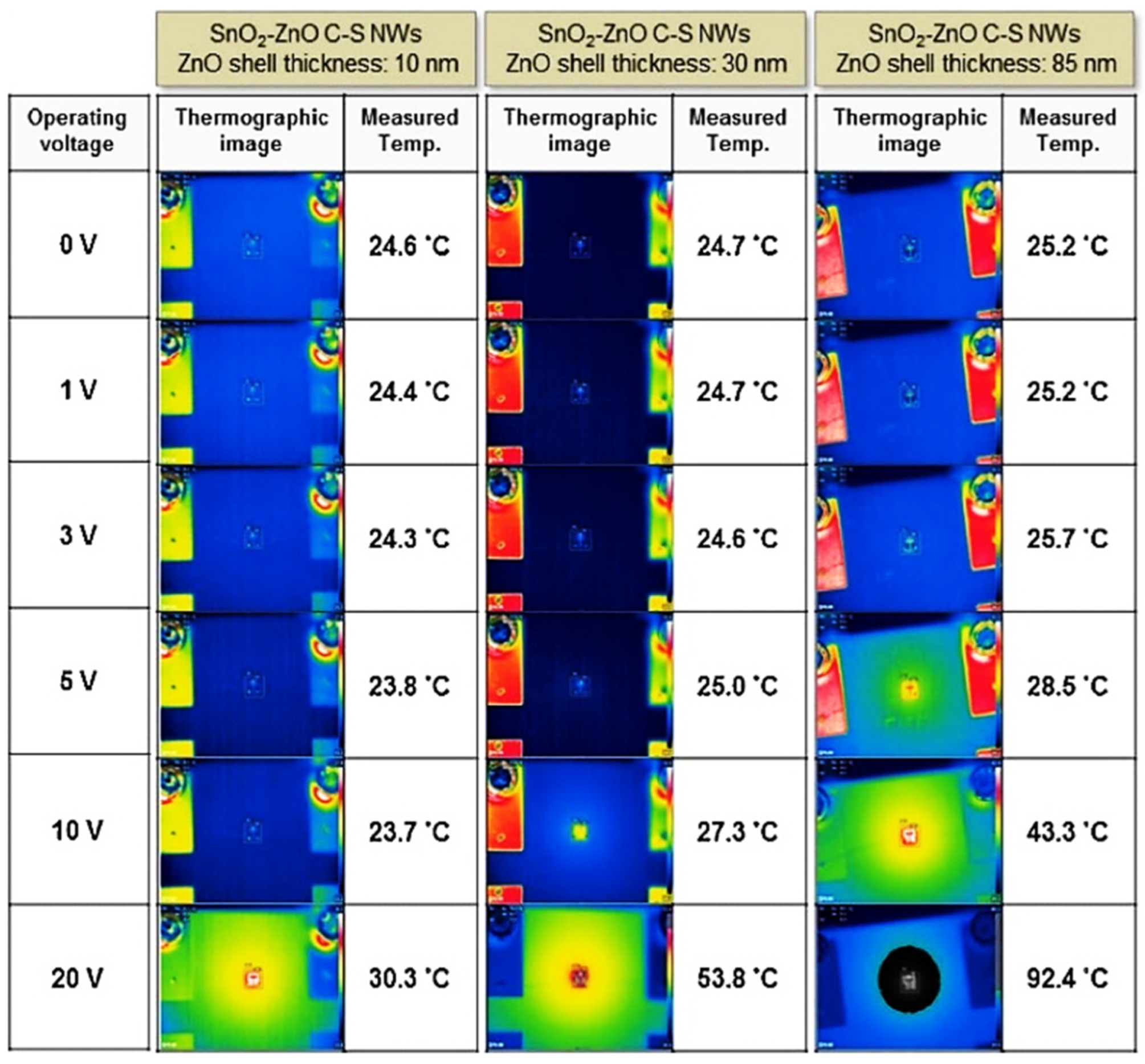
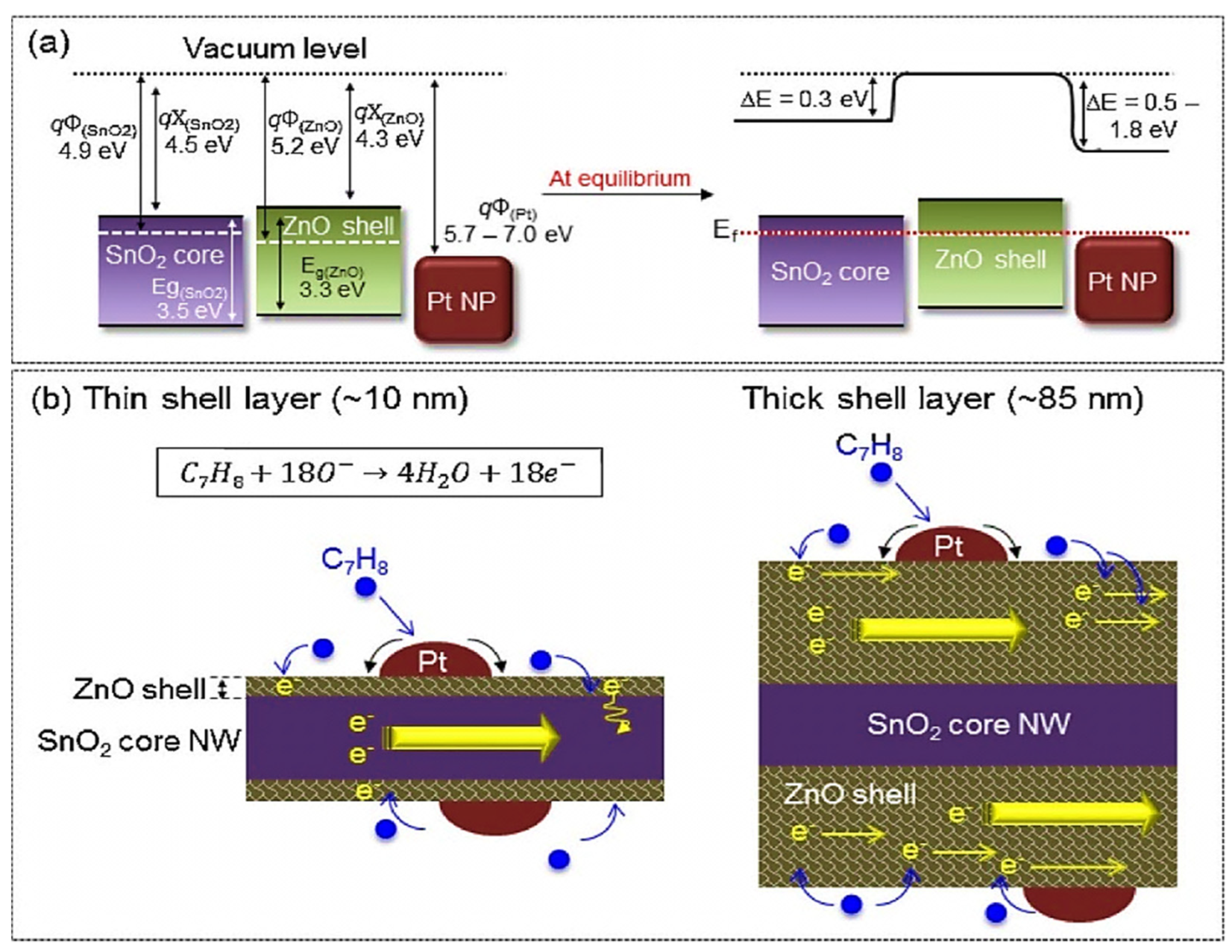
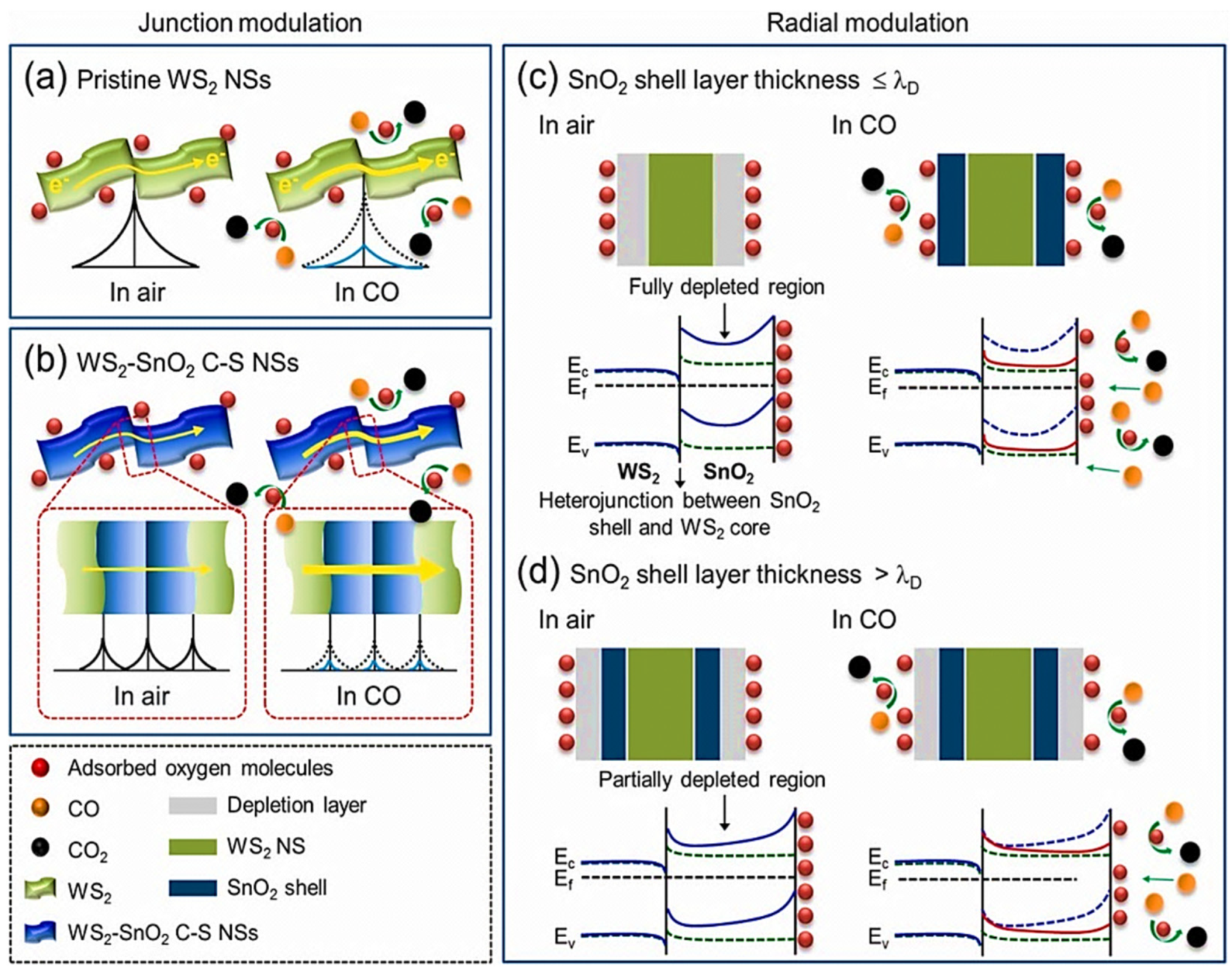
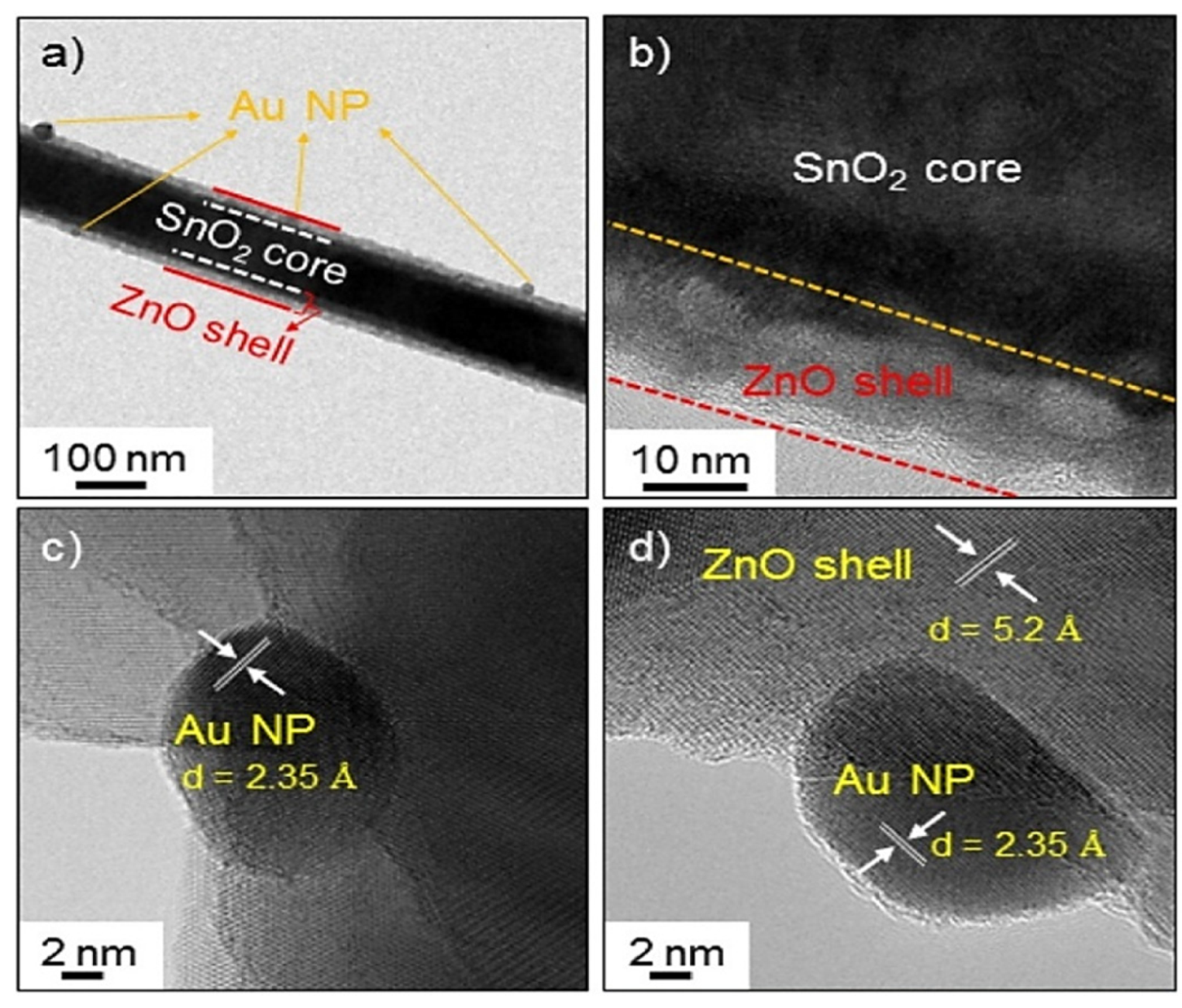
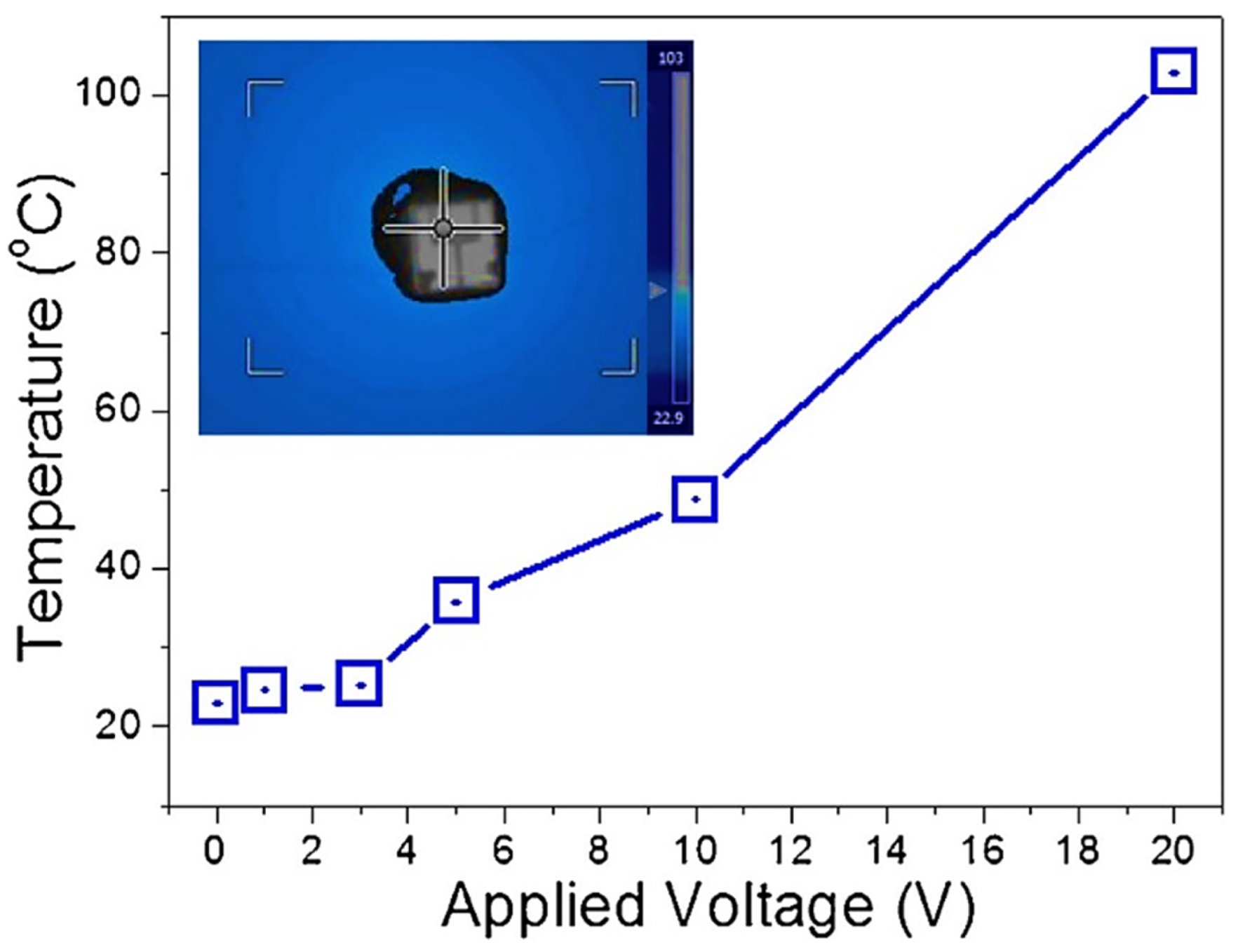

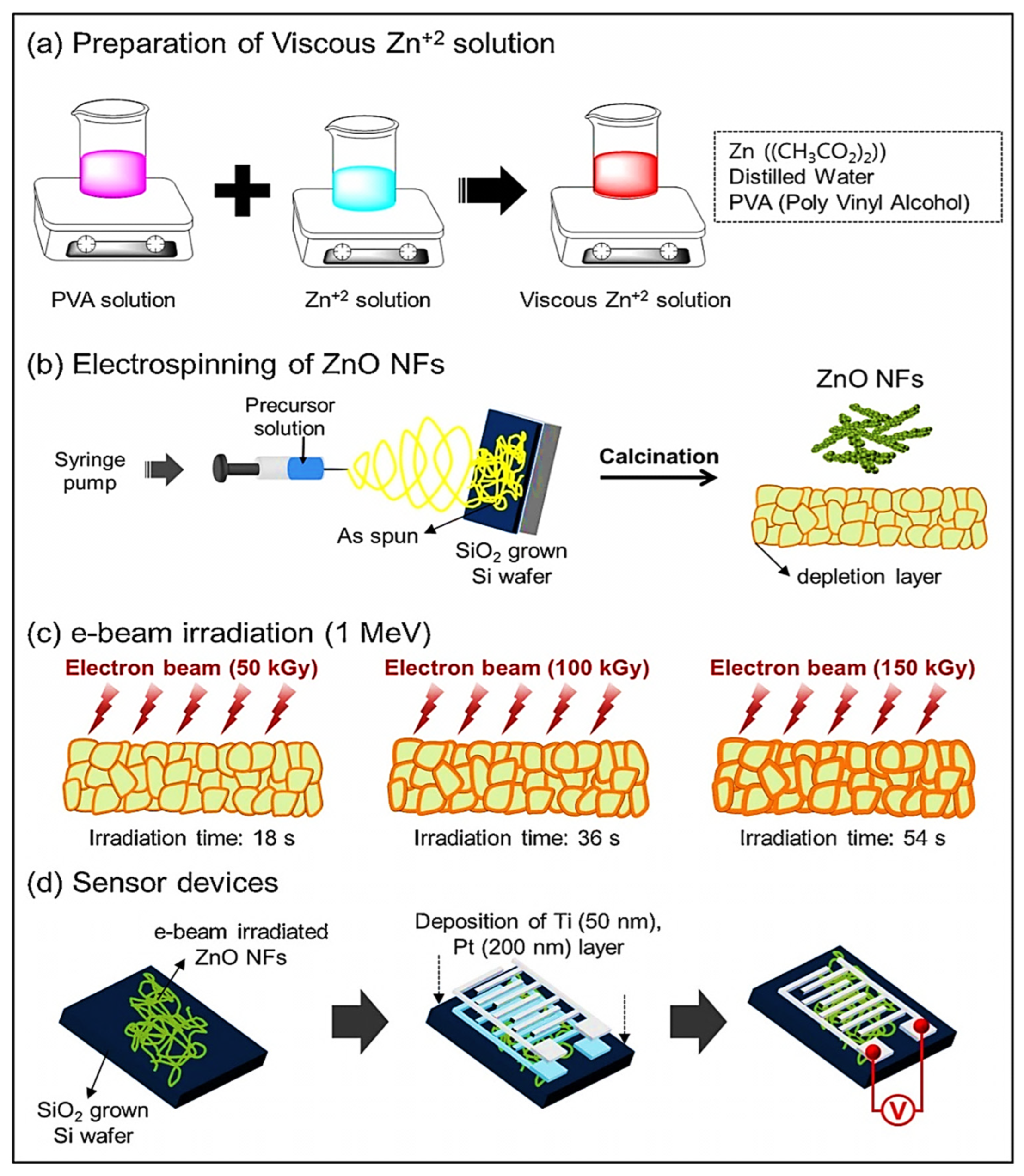
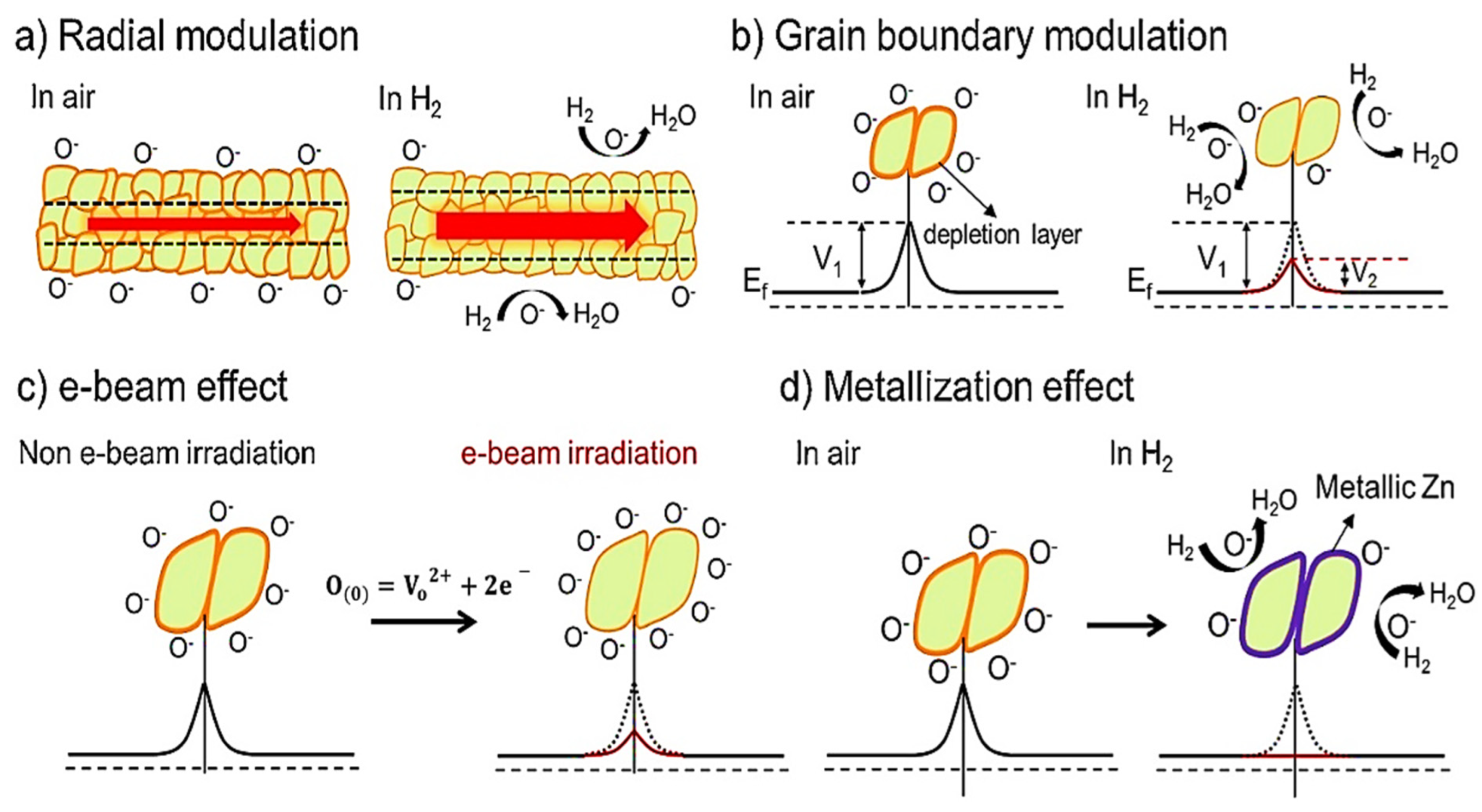
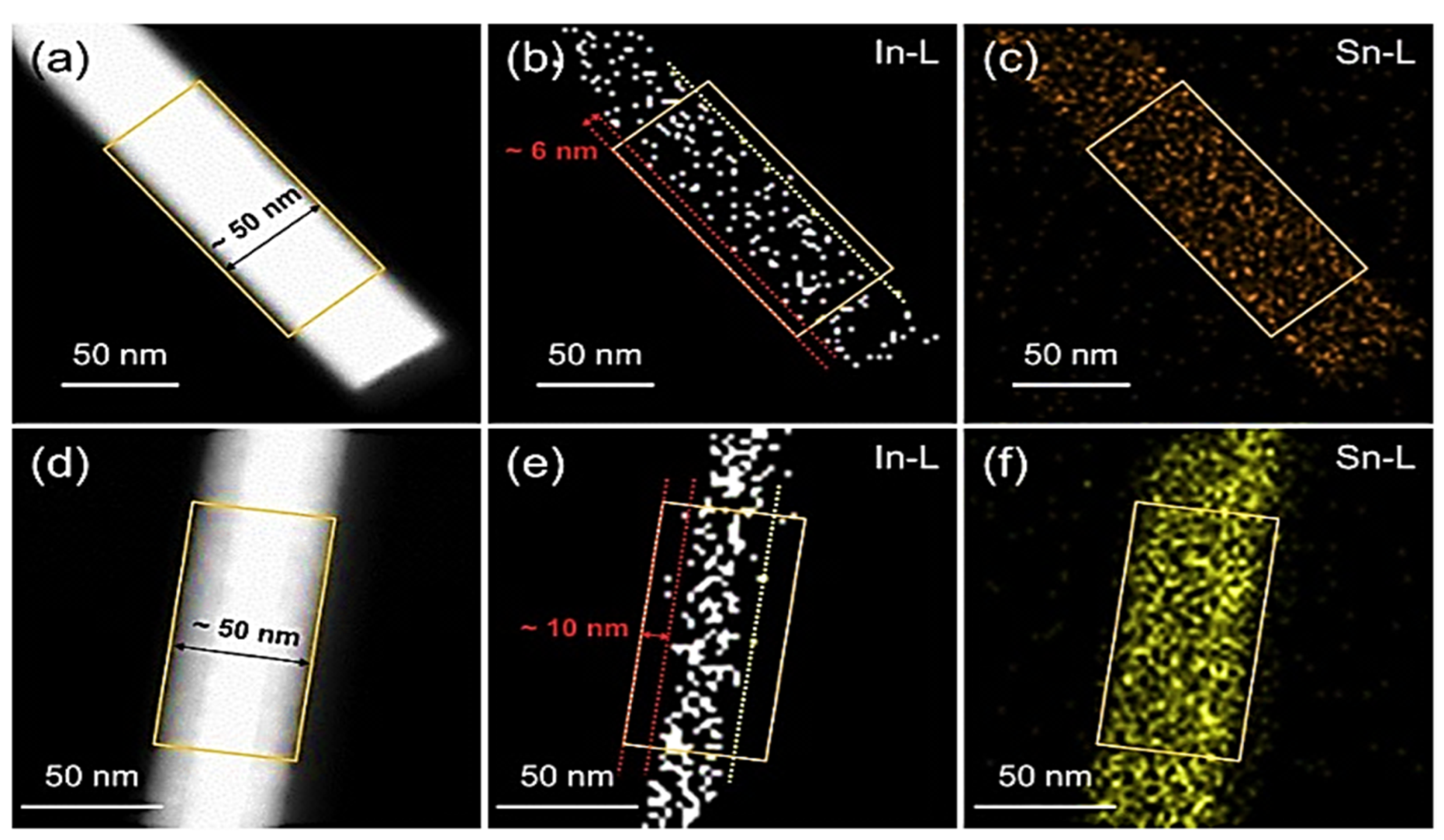
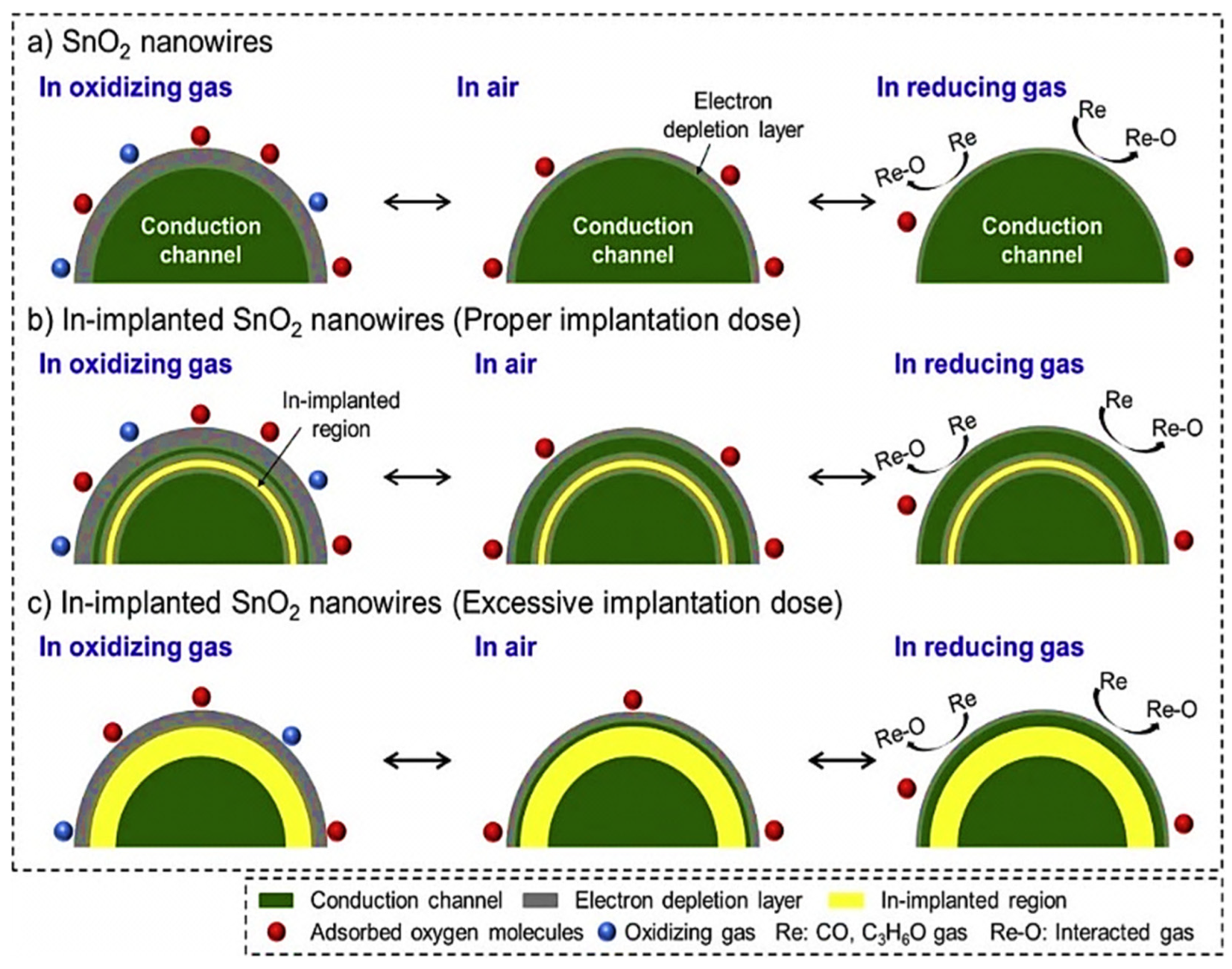
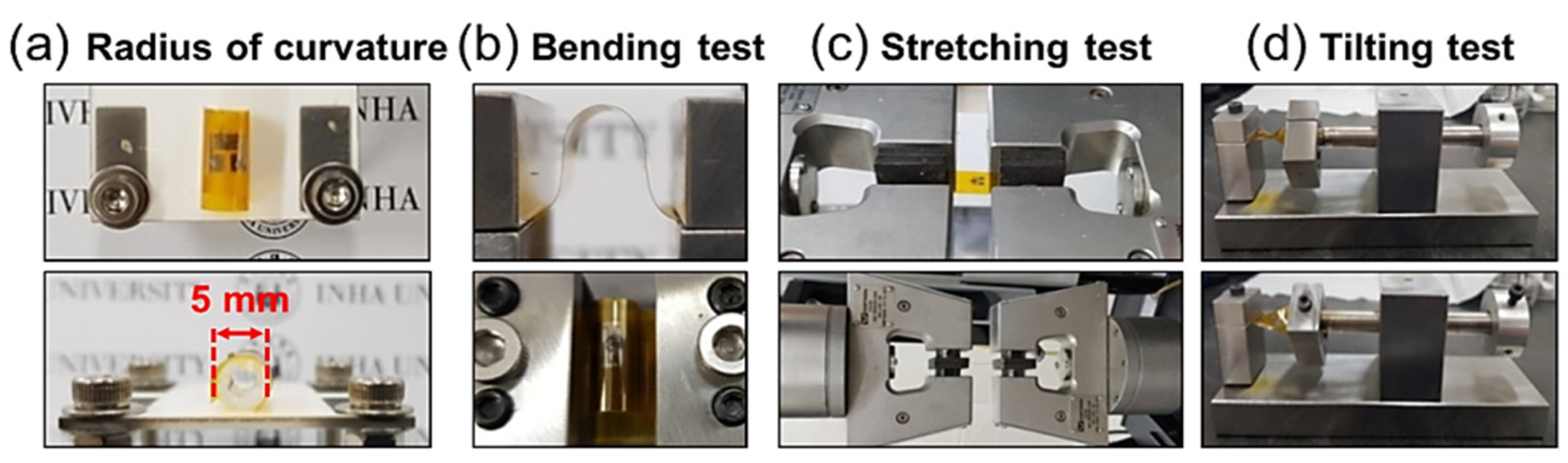
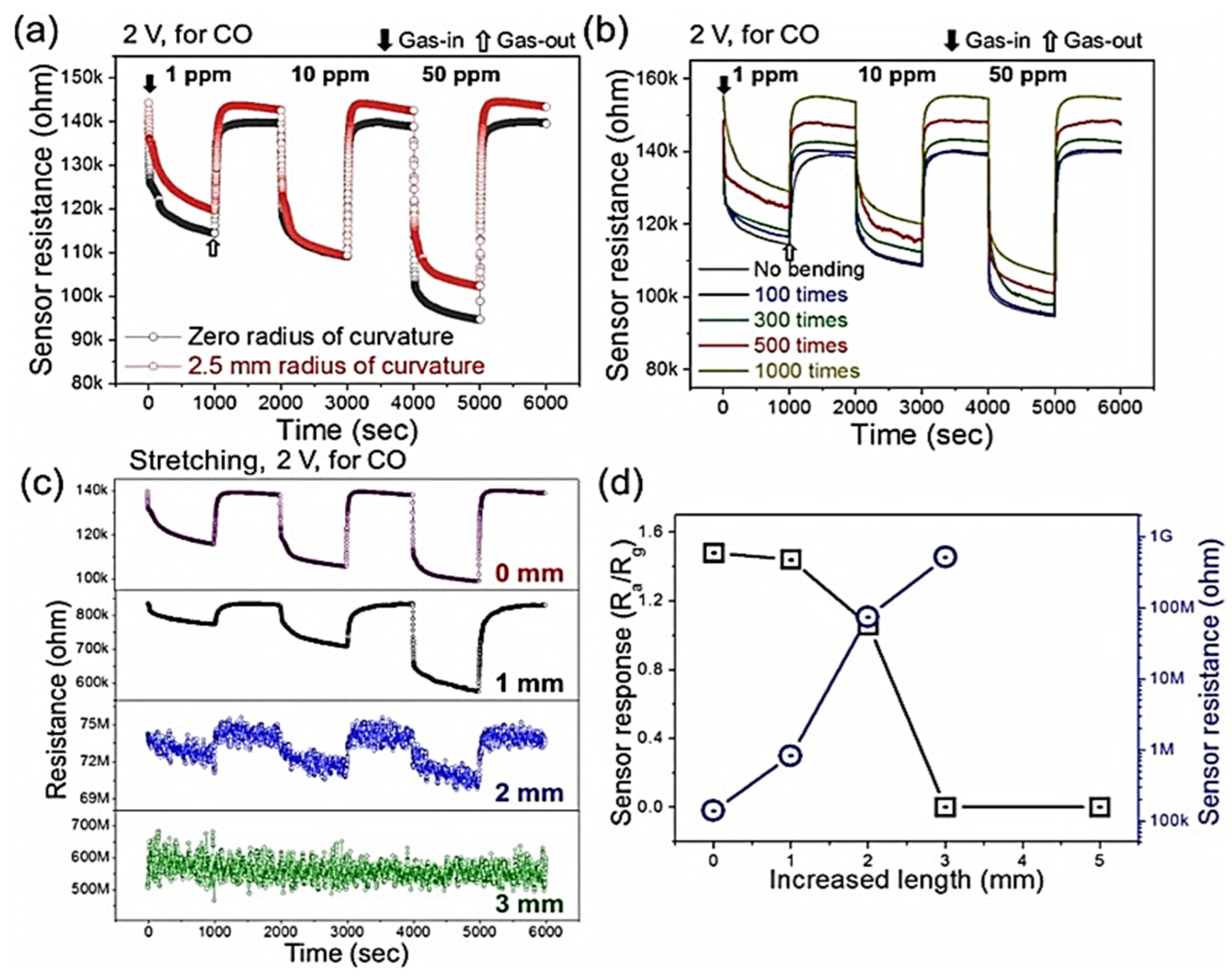


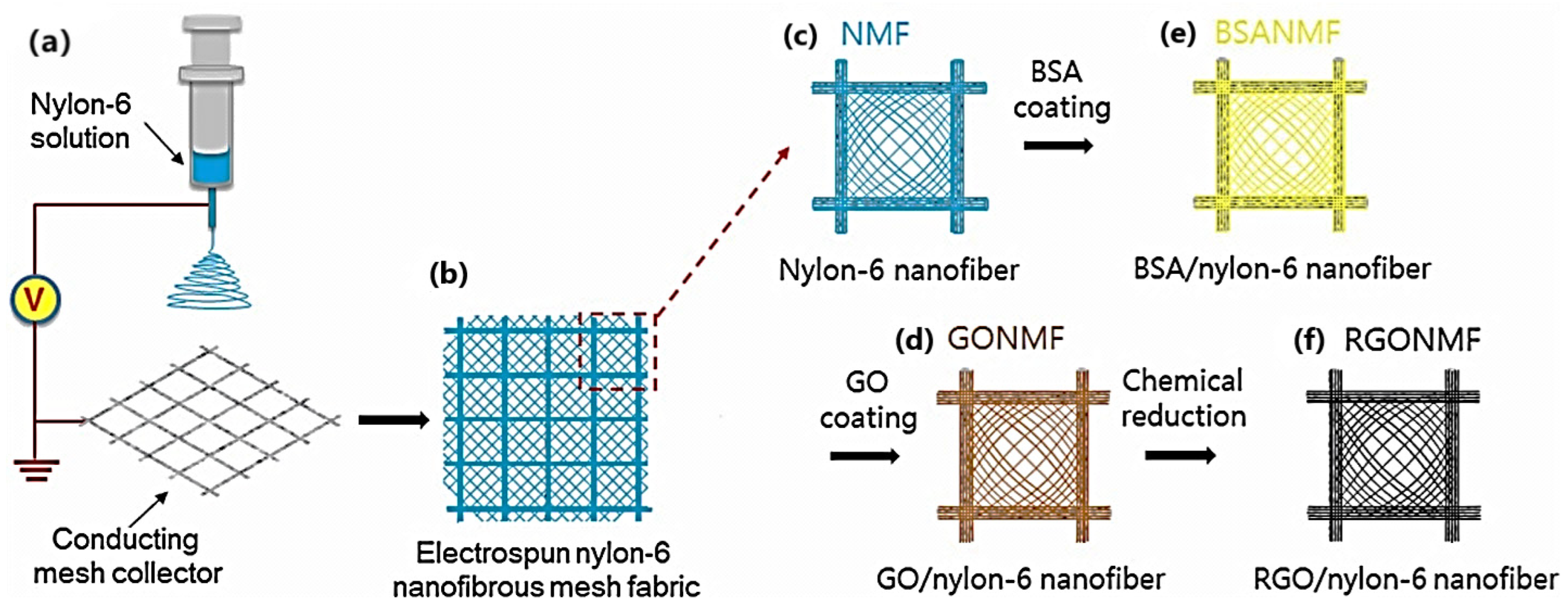
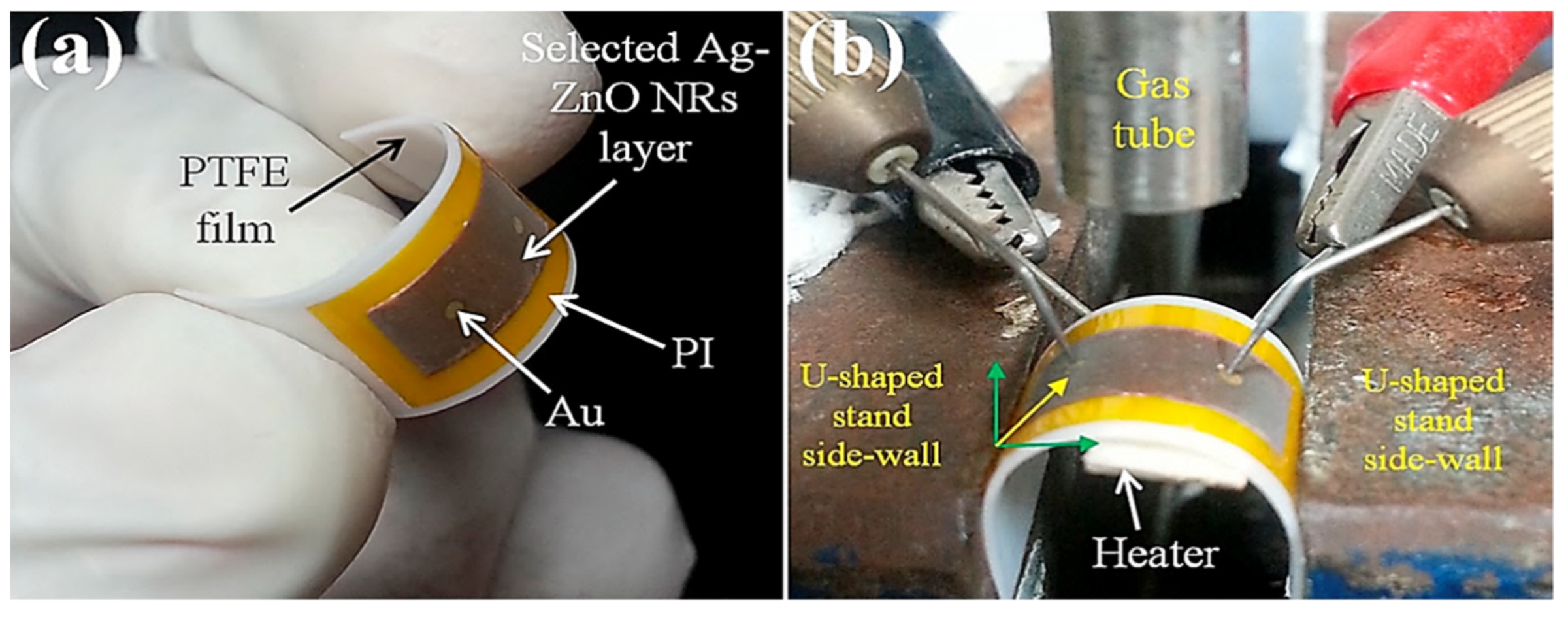



| Year | Milestone | Ref. |
|---|---|---|
| 1953 | Brattain and Bardeen reported the effects of gases on the electrical conductivity of Ge-based devices | [10] |
| 1954 | Heiland reported a change in the electrical properties of ZnO in the presence of various gases | [11] |
| 1962 | Seiyama et al. reported the first resistive-based gas sensor using ZnO | [12] |
| 1962 | Taguchi patented the first SnO2 gas sensor | [13] |
| 1963 | Taguchi investigated effect of noble metals on the gas-sensing properties of SnO2 | [14] |
| 1968 | Taguchi commercialized the first resistive-based gas sensors using SnO2 | [15] |
| 2003 | Salehi reported the first self-heated gas sensor based on SnO2 | [16] |
| 2007 | Schedin et al. for the first time reported the gas-sensing properties of graphene | [17] |
| Dimensions | Sensing Material | Target Gas | Gas Conc. (ppm) | T (°C) | Response (Ra/Rg) or (Rg/Ra) or [(Ra − Rg)/Ra]*100% | Res (s)/Rec (s) | LDL (ppm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 0D | Fe2O3 NPs | CH3COCH3 | 100 | 300 | 11.6 | 4/10 | 0.5 | [19] |
| In2O3 NPs | HCHO | 10 | 280 | 20 | 4/8 | NA | [20] | |
| ZnO NPs | Cl2 | 200 | 200 | 1278% | 6/64 | 5 | [21] | |
| SnO2 NPs | C2H5OH | 250 | 100 | 30 | 16/25 | NA | [22] | |
| NiO NPs | HCHO | 1 | 230 | 80 | ≈54/≈14 | NA | [23] | |
| WO3 NPs | NO2 | 100 | 200 | 34% | 24/300 | 5 | [24] | |
| Co3O4 NPs | CH3COCH3 | 100 | 200 | 8.61 | 43/92 | 0.1 | [25] | |
| CeO2 NPs | H2S | 40 | RT | 5.5 | 64/62 | NA | [26] | |
| TiO2 NPs | CH3COCH3 | 1000 | 270 | 15.24 | 10/9 | 0.5 | [27] | |
| CuO NPs | H2S | 5 | 40 | 4.9 | 297/54 | 0.2 | [28] | |
| 1D | SnO2 NWs | CO | 20 | RT | 4 | NA | NA | [29] |
| ZnO NRs | CH3COCH3 | 100 | 300 | 32 | 5/15 | 1 | [30] | |
| In2O3 MRs | C2H5OH | 100 | 300 | 18.33 | 15/20 | 1 | [31] | |
| Co3O4 MRs | C2H5OH | 100 | 220 | 9.8 | ≈1/≈11 | NA | [32] | |
| WO3 NFs | CH3COCH3 | 50 | 270 | 55.6 | 13/9 | 0.1 | [33] | |
| ZnO NWs | C2H5OH | 500 | 340 | 10.68 | 6/26 | NA | [34] | |
| Fe2O3 NRs | CH3COCH3 | 100 | 280 | 23.5 | ≈1/≈3 | NA | [35] | |
| NiO NWs | NH3 | 50 | RT | 0.19 | 36/NA | NA | [36] | |
| WO3 NWs | NO | 500 | 300 | 37 | 63/88 | 50 | [37] | |
| TiO2 NTs | C7H8 | 50 | 500 | 3 | 110/800 | NA | [38] | |
| WO3 NWs | C2H2 | 200 | 300 | 58 | 6/7 | NA | [39] | |
| NiO nanochains | HCHO | 50 | 210 | NA | 1/10 | 1 | [40] | |
| CuO NWs | n-propanol | 100 | 190 | 6.2 | ≈2/≈7 | 1 | [41] | |
| CuO NTs | CO | 100 | 175 | 1.55 | 24/29 | 0.6 | [42] | |
| V2O5 NWs | C2H5OH | 1000 | 330 | 9.03 | NA | NA | [43] | |
| 2D | ZnO NSs | C2H2 | 100 | 400 | 101.1 | 11/5 | 1 | [44] |
| CuO NSs | H2S | 10 ppb | RT | 1.25 | 234/76 | 10 | [45] | |
| Co3O4 NSs | CH3COCH3 | 1000 | 111 | 36.5 | NA | 20 | [46] | |
| NiO NSs | C2H5OH | 50 | 240 | 11.15 | 4/7 | 1 | [47] | |
| α-Fe2O3 NSs | TEA | 100 | 300 | 520 | NA | 1 | [48] | |
| V2O5 NSs | CH3COCH3 | 100 | 300 | ~3.2 | 25/13 | 5 | [49] | |
| TiO2 NSs | CH3OH | 1 | 100 | 17.46% | NA | 1 | [50] | |
| WO3 NSs | NO2 | 10 | 100 | 460 | 54/63 | 1 | [51] | |
| SnO2 NSs | CH3COCH3 | 1 | 280 | 10.4 | NA | 0.2 | [52] | |
| In2O3 NSs | NOx | 97 | RT | 89.48 | 16/NA | 0.48 | [53] | |
| 3D | Co3O4 nanocubes | CH3COCH3 | 500 | 240 | 4.9 | 2/5 | 10 | [54] |
| Fe2O3 MFs | CH3COCH3 | 100 | 220 | 52 | 8/19 | NA | [55] | |
| ZnO NFs | C2H2 | 200 µL/L | 375 | 48.2 | 8/11 | NA | [56] | |
| SnO2 nanocages | C7H8 | 20 | 250 | 33.4 | ≈3/≈6 | NA | [57] | |
| In2O3 MSs | C7H8 | 50 | 350 | 85% | 12/25 | 0.5 | [58] | |
| NiO nanotetrahedra | HCHO | 50 | 250 | 11.6 | NA | NA | [59] | |
| CuO MSs | HCHO | 100 | 300 | 3.2 | 26/28 | NA | [60] | |
| WO3 urchin-like structures | C2H5OH | 100 | 350 | 68.56 | 28/12 | NA | [61] | |
| V2O5 hollow spheres | H2 | 200 | RT | 2.8 | 50/10 | 10 | [62] | |
| TiO2 Bowl-like structure | C8H10 | 100 | 302 | 1.8 | 12/2 | NA | [63] |
| Sensing Material | Target Gas | Gas Conc. (ppm) | T (°C) | Response (Ra/Rg) or (Rg/Ra) or [(Ra − Rg)/Ra]*100% | Res (s)/Rec (s) | LDL (ppm) | Ref. |
|---|---|---|---|---|---|---|---|
| Core–shell (C-S) Gas Sensors | |||||||
| SnO2-ZnO C-S NFs | NO2 | 5 | 300 | ~0.45 | NA | 1 | [70] |
| ZnO-SnO2 C-S NWs | CO | 10 | 300 | 42 | 9/57 | 1 | [71] |
| SnO2-ZnO C-S NWs | NO2 | 10 | 300 | ~155 | NA | 1 | [72] |
| SnO2-ZnO C-S NFs | CO | 1 | 300 | ~48 | NA | 1 | [73] |
| CuO-ZnO C-S NWs | C6H6 | 1 | 300 | ~6 | NA | 1 | [74] |
| SnO2-Cu2O C-S NFs | CO | 10 | 300 | 5 | 14/14 | 1 | [75] |
| Pt@SnO2-ZnO C-S NWs | C7H8 | 0.1 | 300 | 279 | NA | 0.1 | [76] |
| SnO2-ZrO2 C-S NWs | NO2 | 10 | 150 | 24.7 | NA | NA | [78] |
| SnO2-Cu2O C-S NWs | C6H6 | 10 | 300 | 12.5 | 4/4 | 1 | [79] |
| CuO-TiO2 C-S NWs | CO | 10 | 300 | ~16 | NA | 1 | [80] |
| CuO-ZnO C-S NFs | CO | 10 | 300 | ~8 | NA | 0.1 | [81] |
| SnO2-ZnO C-S NFs | CO | 150 | 300 | ~17 | NA | 3 | [82] |
| Pd@SnO2-ZnO C-S NWs | C6H6 | 0.1 | 300 | 71 | 33/114 | 0.1 | [83] |
| Au-decorated Si NW-ZnO C-S | H2S | 50 | 300 | 11.22 | 48/64 | 10 | [84] |
| Au/SnO2 C-S NPs | CO | 1000 | 100 | ~1 | NA | NA | [85] |
| Au/SnO2 C-S NPs | CO | 1000 | 200 | ~2.2 | NA | NA | [87] |
| Au@Cu2O C-S NPs | CO | 1000 | 200 | ~5.8 | NA | NA | [88] |
| Au@Cu2O C-S NPs | CO | 1000 | 250 | 5.67 | NA | 10 | [89] |
| Au@ZnO C-S NPs | H2 | 100 | 300 | 103.9 | NA | 0.5 | [90] |
| Au@NiO C-S NPs | C2H5OH | 100 | 200 | 2.54 | NA | 2 | [91] |
| Au@In2O3 C-S NPs | H2 | 100 | 300 | 34.38 | 31/600 | 2 | [92] |
| AuPdalloy-ZnO C-S NPs | H2 | 100 | 300 | 80 | 36/720 | NA | [94] |
| Au@SnO2 C-S NPs | C8H10 | 5 | 300 | 16.17 | NA | NA | [97] |
| Pd@In2O3 yoll-shell NPs | C2H5OH | 5 | 350 | 159.02 | NA | NA | [98] |
| Au@NiO yoll-shell NPs | H2S | 5 | 300 | 108.90 | NA | 1.25 | [99] |
| ZnO-SnO2 C-S NWs | C2H5OH | 200 | 400 | 280 | NA | 0.5 | [184] |
| SnO2-ZnO C-S NWs | NO2 | 5 | 25 | 6.18 | NA | 1 | [185] |
| Ag-Fe2O3 C-S NPs | NO2 | 4 | 150 | 3.6 | 280 | 340 | [186] |
| ZnO-Cr2O3 C-S nanocables | TMA | 5 | 400 | 17.8 | NA | 0.05 | [187] |
| Ga2O3-SnO2 C-S NWs | C2H5OH | 1000 | 400 | 66 | NA | NA | [188] |
| Pd@ZnO C-S NPs | H2 | 100 | 350 | 22 | 84/468 | 5 | [189] |
| ZnO-TiO2 C-S NRs | NO2 | 50 | RT | 7.50 | NA | NA | [190] |
| TeO2-CuO C-S NRs | NO2 | 10 | 150 | 425% | NA | 0.5 | [191] |
| Pd@ZnO–In2O3 C-S NPs | H2 | 100 | 300 | 42 | 24/240 | NA | [192] |
| Pd@N-CeO2 C-S nanoflatforms | H2 | 100 | 350 | 19 | 60/360 | 0.5 | [193] |
| Self-Heated Gas Sensors | |||||||
| Pd@SnO2-ZnO C-S NWs | C6H6 | 50 | 20 V (RT) | 1.62 | NA | 0.1 | [104] |
| Au@WS2 nanoflakes | CO | 50 | 2 V (RT) | 1.48 | 174/30 | 1 | [107] |
| TiO2-layer-modified SnO2 QDs | NO2 | 1 | 20 V (RT) | ~90% | NA | 1 | [108] |
| Au-SnO2-decorated WS2 NSs | CO | 50 | 4.7 V (RT) | 3.68 | NA | 0.3 | [109] |
| Pd@CuO NWs | H2S | 100 | 5 V (RT) | 1.89 | NA | 1 | [110] |
| Au@ZnO NWs | NO2 | 10 | 7 V (RT) | 3.07 | NA | 0.1 | [111] |
| Pt@SnO2–ZnO C-S NWs | C7H8 | 50 | 20 V (RT) | 3.14 | NA | 0.1 | [112] |
| WS2-SnO2 C-S NSs | CO | 10 | 3.4 V | 8 | NA | NA | [114] |
| Au@SnO2–ZnO C-S NWs | CO | 50 | 20 V (RT) | 1.62 | NA | 0.1 | [115] |
| Pt@ZnO NWs | C7H8 | 50 | 20 V (RT) | 2.86 | NA | NA | [117] |
| Pd@ZnO NWs | C6H6 | 50 | 20 V (RT) | 2.20 | NA | NA | [117] |
| CuO@SnO2-ZnO C-S NWs | H2S | 10 | 1V (RT) | ~1.9 | NA | 1 | [119] |
| Pd@Si NWs | H2 | 0.5% | 30 V (RT) | 106% | 24/590 | NA | [194] |
| Pd@Si NWs | H2 | 1% | 1.7 V (RT) | 1.6% | NA | NA | [195] |
| Pd@C NWs | H2 | 1000 | 6 V (RT) | ~95% | NA | 10 | [196] |
| Nanocolumnar WO3 thin films | NO2 | 1 | 5 V (RT) | ~130 | NA | 1 | [197] |
| Irradiated Gas Sensors | |||||||
| ZnO NFs (e-beam, 150 kGy) | H2 | 10 | 350 | 150 | 23/114 | 0.1 | [127] |
| Pd@ZnO NFs (e-beam, 150 kGy) | H2 | 10 | 350 | 236.82 | NA | 0.1 | [128] |
| RGO (e-beam, 500 kGy) | NO2 | 50 | RT | 5.280 | 84/1592 | 10 | [129] |
| Pd@RGO (e-beam, 500 kGy | NO2 | 10 | RT | 1.047 | 345/816 | 2 | [130] |
| SnO2 NWs (1 × 1016 ions/cm2) | NO2 | 2 | 150 | ~14 | 292/228 | NA | [134] |
| Sb@SnO2 NWs (2 × 1013 ion/cm2) | NO2 | 1 | 300 | 111.58 | NA | 0.05 | [135] |
| In@SnO2 NWs (2 × 1014 ion/cm2) | NO2 | 1 | 300 | ~4.9 | NA | 0.1 | [136] |
| SnO2 NWs (e-beam, 150 kGy) | NO2 | 10 | NA | 1.03 | 16/230 | NA | [198] |
| SWCNT-Sn/SnO2 composites (1KW) | C2H5OH | 10 | RT | ~6.5 | NA | 1 | [199] |
| Graphene (1000 kGy) | NO2 | 100 | RT | 40.68 | 86/499 | 5 | [200] |
| Flexible Gas Sensors | |||||||
| Au@WS2 nanoflakes | CO | 50 | RT | 1.48 | 174/30 | 1 | [107] |
| Au-SnO2 decorated WS2 NSs | CO | 50 | RT | 3.68 | NA | 0.3 | [109] |
| RGO nanofibrous mesh fabrics | NO2 | 8 | RT | 26.5% | NA | 8 | [147] |
| Titania NTs | NH3 | 200 | 350 | ~80% | NA | NA | [148] |
| ZnO NRs | C2H5OH | 50 | 300 | ~90 | NA | 10 | [149] |
| Ag@ZnO NRs | C2H2 | 1000 | 200 | 27.2 | 62/39 | 3 | [150] |
| Pt-ZnO@RGO | NO2 | 5 | RT | 43.28% | 528/702 | 0.1 | [151] |
| ZnO nanoflowers | NO2 | 500 | 270 | 218.1 | ~31/~14 | NA | [153] |
| MoS2 | NO2 | 500 | RT | ~300% | NA | 25 | [154] |
| CNTs/RGO flexible film | NO2 | 10 | RT | 20% | NA | 0.5 | [201] |
| Flexible graphene films | NO2 | 200 | RT | 23% | NA | NA | [202] |
| Flexible graphene | NO2 | 5 | RT | 13% | NA | 1 | [203] |
| Flexible RGO cotton yarn | NO2 | 1.25 | RT | 12% | NA | 0.25 | [204] |
| GO on cotton yarn | NO2 | 3 | RT | 65% | NA | 0.15 | [205] |
| Graphene-based electronic sheet | NO2 | 100 | RT | ~38% | NA | 1 | [206] |
| ZnO-decorated rGO fibers | H2S | 20 | RT | 2.68% | 404/275 | 1.5 | [207] |
| Ti3C2Tx/Graphene fibers | NH3 | 50 | RT | 6.8% | NA | 10 | [208] |
| CeO2-CuBr | NH3 | 5 | RT | 68 | NA | 0.02 | [209] |
| RGO-In2O3 | NO2 | 0.5 | 150 | 22.3 | 112/175 | 0.5 | [210] |
| WO3/MWCNTs | NO2 | 5 | RT | 14 | 600/1620 | NA | [211] |
| Glass/Si/MOF-Based Gas Sensors | |||||||
| Soda-lime glass | CO2 | 4000 | 350 | 0.4 | NA | NA | [156] |
| Pd@Soda-lime glass | CO2 | 100 | 350 | 8.160 | NA | 0.2 | [157] |
| Si NWs | H2 | 50 | 100 | 17.1 | 505/150 | 10 | [163] |
| Si/SnO2 NWs | H2S | 50 | 100 | 3.5 | NA | NA | [167] |
| Pd@Si nanohorns | H2 | 10 | 400 | ~2300 | NA | 0.1 | [168] |
| Graphene/Si NWs | H2 | NA | 25 | 1280% | 12/0.15 | NA | [212] |
| Pd@p-Si | H2S | 3 | RT | ~88% | NA | 0.3 | [213] |
| Pd@Si NWs | H2 | 20000 | RT | ~300% | NA | 5 | [214] |
| ZnO@ZIF-8 MOFs | H2 | 50 | 300 | 1.44 | NA | NA | [174] |
| SIM-1 nanomembrane@ZnO NWs | H2 | 50 | 300 | ~2.5 | NA | NA | [175] |
| Pd@ZnO NWs | H2 | 50 | 200 | 6.7 | NA | NA | [178] |
| Ni-MOFs | CO | 50 | 200 | 1.7 | NA | 1 | [180] |
| Mg-MOFs | NO2 | 50 | 200 | ~1.35 | 167/92 | 1 | [181] |
| MOF derived ZnO-CuO | H2S | 10 | 350 | 10.99 | 58/273 | 1 | [182] |
| n-ZnO/p-Co3O4 derived from MOFs | C2H5OH | 10 | 300 | 34.9 | 57/235 | 1 | [183] |
| Pd@Zr-MOFs | H2 | 100 | 150 | 1.94 | NA | 10 | [215] |
| Pd NWs @ZIF-8 | H2 | 0.1% | RT | 0.7% | 30/8 | 0.6 | [216] |
| ZIF-67 derived WS2@carbon composites | NO2 | 1 | RT | 18% | NA | 0.1 | [217] |
| ZIF-67 derived hollow Co3O4 nanocages | p-Xylene | 5 | 225 | 78.6 | NA | 0.25 | [218] |
| Pd@ZIF-67 derived PdO@Co3O4 | CH3COCH3 | 5 | 350 | 2.51 | NA | 0.1 | [219] |
| MOF derived ZnO-Co3O4 | CH3COCH3 | 5 | 450 | 29 | NA | 1 | [220] |
| TiO2-SnO2/MWCNTs@Cu-BTC | NH3 | 10 | RT | 0.58 | 80/15 | 0.77 | [221] |
| Cu3(HHTP)2/Fe2O3 | NO2 | 5 | 20 | 89.4% | NA | 0.2 | [222] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navale, S.; Mirzaei, A.; Majhi, S.M.; Kim, H.W.; Kim, S.S. State-of-the-Art Research on Chemiresistive Gas Sensors in Korea: Emphasis on the Achievements of the Research Labs of Professors Hyoun Woo Kim and Sang Sub Kim. Sensors 2022, 22, 61. https://doi.org/10.3390/s22010061
Navale S, Mirzaei A, Majhi SM, Kim HW, Kim SS. State-of-the-Art Research on Chemiresistive Gas Sensors in Korea: Emphasis on the Achievements of the Research Labs of Professors Hyoun Woo Kim and Sang Sub Kim. Sensors. 2022; 22(1):61. https://doi.org/10.3390/s22010061
Chicago/Turabian StyleNavale, Sachin, Ali Mirzaei, Sanjit Manohar Majhi, Hyoun Woo Kim, and Sang Sub Kim. 2022. "State-of-the-Art Research on Chemiresistive Gas Sensors in Korea: Emphasis on the Achievements of the Research Labs of Professors Hyoun Woo Kim and Sang Sub Kim" Sensors 22, no. 1: 61. https://doi.org/10.3390/s22010061
APA StyleNavale, S., Mirzaei, A., Majhi, S. M., Kim, H. W., & Kim, S. S. (2022). State-of-the-Art Research on Chemiresistive Gas Sensors in Korea: Emphasis on the Achievements of the Research Labs of Professors Hyoun Woo Kim and Sang Sub Kim. Sensors, 22(1), 61. https://doi.org/10.3390/s22010061









