Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends
Abstract
:1. Introduction
2. Materials and Methods
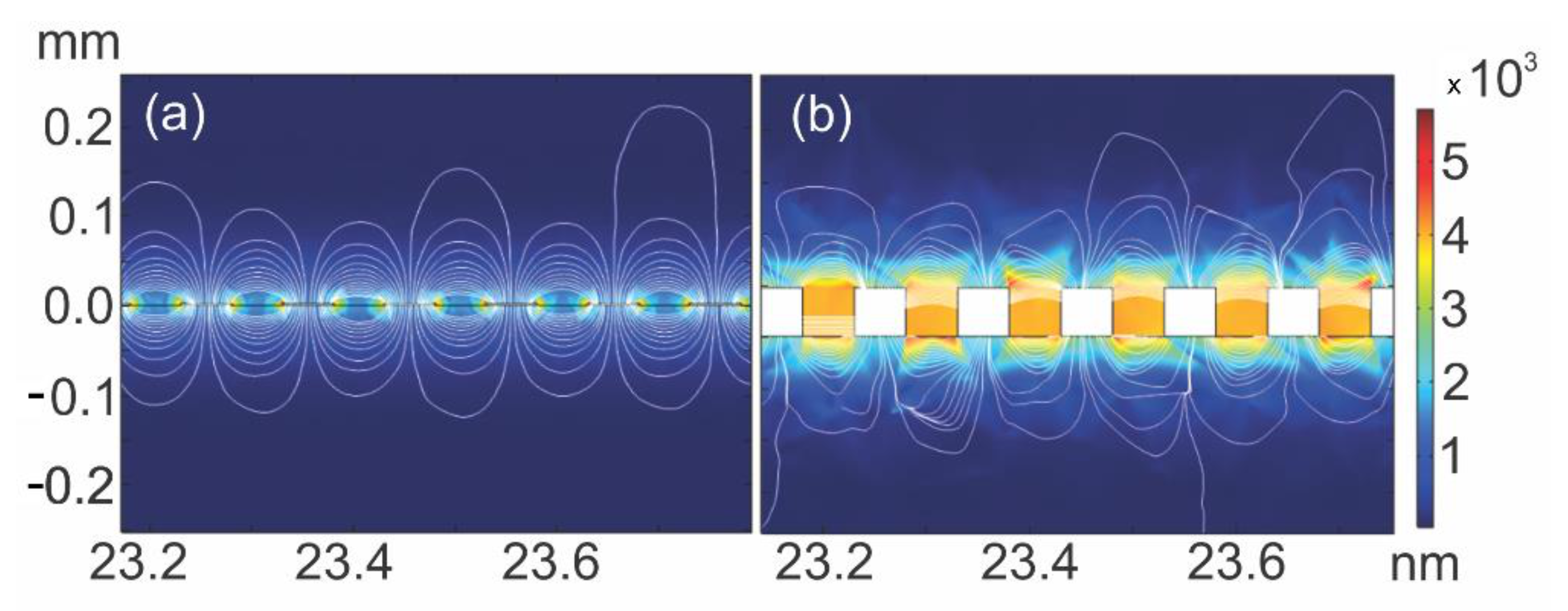
2.1. Interdigitated Electrode Design by the Finite Element Method
2.2. Chemical and Physical Characterization of the Interdigital Electrode
3. Results
3.1. Finite Element Analysis and Electrical Evaluation
3.2. Interdigitated Electrode Description
Structural Characterization
3.3. Electrical Characterization
Sensitivity of Electrodes to Different Fuels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Da Costa, R.P.M.; Khalila, T.C.; Dos Santosa, A.P.F.; De Andrade, D.F.; D’Avila, L.A. Determinação do teor de biodiesel em diesel empregando o ensaio colorimétrico do ácido hidroxâmico. Quim. Nova 2015, 38, 563–569. [Google Scholar]
- Scherer, M.D.; Oliveira, S.L.; Lima, S.M.; Andrade, L.H.C.; Caires, A.R.L. Determination of the biodiesel content in diesel/biodiesel blends: A method based on fluorescence spectroscopy. J. Fluoresc. 2011, 21, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.C.M.; Rezende, M.J.C.; Rezende, C.M.; Pinto, A.C. Desenvolvimento e validação de metodologia de análise de misturas biodiesel:Diesel utilizando cromatografia gasosa-espectrometria de massas. Quim. Nova 2007, 30, 1900–1905. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.C.C.; Brandão, C.R.R.; Ramalho, H.F.; da Costa, L.A.F.; Suarez, P.A.Z.; Rubim, J.C. Adulteration of diesel/biodiesel blends by vegetable oil as determined by Fourier transform (FT) near infrared spectrometry and FT-Raman spectroscopy. Anal. Chim. Acta 2007, 587, 194–199. [Google Scholar] [CrossRef]
- Ibrahim, M.; Claudel, J.; Kourtiche, D.; Nadi, M. Geometric parameters optimization of planar interdigitated electrodes for bioimpedance spectroscopy. J. Electr. Bioimpedance 2013, 4, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Diniz Carvalho, C.; Barros, A.K.; Lopes, M.V.; Silva, F.C.; Santana, E.E.; Sinfrônio, F.S.M. Determination of the composition of biodiesel-diesel blends using the dielectric constant. Instrum. Sci. Technol. 2016, 44, 377–385. [Google Scholar] [CrossRef]
- Kutia, M.; Mukhin, N.; Petrova, H.; Oseev, A.; Bakhchova, L.; Schmidt, M.P.; Aman, A.; Palis, S.; Tarasov, S.; Hirsch, S. Sensor for the evaluation of dielectric properties of sulfur-containing heteroatomic hydrocarbon compounds in petroleum based liquids at a microfluidic scale. AIP Adv. 2020, 10, 025006. [Google Scholar] [CrossRef]
- González Prieto, L.E.; Sorichetti, P.A.; Romano, S.D. Electric properties of biodiesel in the range from 20 Hz to 20 MHz. Comparison with diesel fossil fuel. Int. J. Hydrogen Energy 2008, 33, 3531–3537. [Google Scholar] [CrossRef]
- Yule, A.J.; Shrimpton, J.S.; Watkins, A.P.; Balachandran, W.; Hu, D. Electrostatically atomized hydrocarbon sprays. Fuel 1995, 74, 1094–1103. [Google Scholar] [CrossRef]
- Us, A.; Koehler, C.; Seitz, M.; Wooton, D. Patent Impedance Spectroscopy (Is) Methods and Systems for Characterizing Fuel. 2018. Available online: https://patents.google.com/patent/US20080172187?oq=ininventor:%22Richard+Hirthe%22 (accessed on 24 April 2021).
- Bueno, L.; Paixão, T.R.L.C. A copper interdigitated electrode and chemometrical tools used for the discrimination of the adulteration of ethanol fuel with water. Talanta 2011, 87, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Gaso, M.-I.; March-Iborra, C.; Montoya-Baides, Á.; Arnau-Vives, A. Surface Generated Acoustic Wave Biosensors for the Detection of Pathogens: A Review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef]
- Ramli, N.A.; Nordin, A.N. Design and modeling of MEMS SAW resonator on Lithium Niobate. In Proceedings of the 2011 4th International Conference on Mechatronics (ICOM), Kuala Lumpur, Malaysia, 17–19 May 2011; pp. 1–4. [Google Scholar]
- Mahameed, R.; El-Tanani, M.A.; Rebeiz, G.M. A zipper RF MEMS tunable capacitor with interdigitated RF and actuation electrodes. J. Micromechanics Microengineering 2010, 20, 035014. [Google Scholar] [CrossRef]
- Vakilian, M.; Majlis, B.Y. Study of interdigitated electrode sensor for lab-on-chip applications. In Proceedings of the 2014 IEEE International Conference on Semiconductor Electronics (ICSE2014), Kuala Lumpur, Malaysia, 27–29 August 2014; pp. 201–204. [Google Scholar]
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-free capacitive biosensor for sensitive detection of multiple biomarkers using gold interdigitated capacitor arrays. Biosens. Bioelectron. 2010, 25, 2318–2323. [Google Scholar] [CrossRef]
- Valera, E.; Ramón-Azcón, J.; Sanchez, F.J.; Marco, M.P.; Rodríguez, Á. Conductimetric immunosensor for atrazine detection based on antibodies labelled with gold nanoparticles. Sens. Actuators B Chem. 2008, 134, 95–103. [Google Scholar] [CrossRef]
- Ding, S. Highly Sensitive Biosensors with Interdigitated Electrode Arrays. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2018; p. 89. [Google Scholar]
- Ding, S.; Mosher, C.; Lee, X.Y.; Das, S.R.; Cargill, A.A.; Tang, X.; Chen, B.; McLamore, E.S.; Gomes, C.; Hostetter, J.M.; et al. Rapid and Label-Free Detection of Interferon Gamma via an Electrochemical Aptasensor Comprising a Ternary Surface Monolayer on a Gold Interdigitated Electrode Array. ACS Sens. 2017, 2, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Banqueri, J.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. A novel electrode structure compared with interdigitated electrodes as capacitive sensor. Sens. Actuators B Chem. 2014, 204, 552–560. [Google Scholar] [CrossRef]
- Bento Ribeiro, L.E.; Alcântara, G.P.; Gonçalves Andrade, C.M.; Fruett, F. Analysis of the Planar Electrode Morphology Applied to Zeolite Based Chemical Sensors. Sens. Transducers 2015, 193, 80–85. [Google Scholar]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Agudo-Acemel, M.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. Printed electrodes structures as capacitive humidity sensors: A comparison. Sens. Actuators A Phys. 2016, 244, 56–65. [Google Scholar] [CrossRef]
- Latimer, K.J.; Evans, J.W.; Cowell, M.A.; Wright, P.K. Modeling of interdigitated electrodes and supercapacitors with porous interdigitated electrodes. J. Electrochem. Soc. 2017, 164, A930–A936. [Google Scholar] [CrossRef]
- Bowen, C.R.; Bowles, A.; Drake, S.; Johnson, N.; Mahon, S. Fabrication and finite element modelling of interdigitated electrodes. Ferroelectrics 1999, 228, 257–269. [Google Scholar] [CrossRef]
- Mizuguchi, J.; Piai, J.C.; De França, J.A.; De Morais França, M.B.; Yamashita, K.; Mathias, L.C. Fringing field capacitive sensor for measuring soil water content: Design, manufacture, and testing. IEEE Trans. Instrum. Meas. 2015, 64, 212–220. [Google Scholar] [CrossRef]
- Wang, Y.; Chong, N.; Cheng, Y.L.; Chan, H.L.W.; Choy, C.L. Dependence of capacitance on electrode configuration for ferroelectric films with interdigital electrodes. Microelectron. Eng. 2003, 66, 880–886. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Simorangkir, R.B.V.B.; Afsarimanesh, N.; Liu, H.; Mukhopadhyay, S.C.; Xu, Y.; Zhadobov, M.; Sauleau, R. Multifunctional flexible sensor based on laser-induced graphene. Sensors 2019, 19, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Bilican, I.; Guler, M.T.; Gulener, N.; Yuksel, M.; Agan, S. Capacitive solvent sensing with interdigitated microelectrodes. Microsyst. Technol. 2016, 22, 659–668. [Google Scholar] [CrossRef]
- Colniță, A.; Marconi, D.; Turcu, I. Fabrication of Interdigitated Electrodes using Molecular Beam Epitaxy and Optical Lithography. Anal. Lett. 2016, 49, 378–386. [Google Scholar] [CrossRef]
- Alexander, C.L.; Tribollet, B.; Orazem, M.E. Contribution of Surface Distributions to Constant-Phase-Element (CPE) Behavior: 1. Influence of Roughness. Electrochim. Acta 2015, 173, 416–424. [Google Scholar] [CrossRef]
- Eccher, J. Discotic liquid crystals as organic semiconductors for electronic applications. Ph.D. Thesis, Universidade de Santa Catarina, Florianópolis, Brazil, 2014. [Google Scholar]
- Manzoli, A.; De Almeida, G.F.B.; Filho, J.A.; Mattoso, L.H.C.; Riul, A.; Mendonca, C.R.; Correa, D.S. Femtosecond laser ablation of gold interdigitated electrodes for electronic tongues. Opt. Laser Technol. 2015, 69, 148–153. [Google Scholar] [CrossRef]
- Jung, H.W.; Chang, Y.W.; Lee, G.Y.; Cho, S.; Kang, M.J.; Pyun, J.C. A capacitive biosensor based on an interdigitated electrode with nanoislands. Anal. Chim. Acta 2014, 844, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wiziack, N.K.L. Desenvolvimento De Sistemas Multissensoriais Híbridos, Língua E Narizes Eletrônicos Para a Desenvolvimento De Sistemas Multissensoriais Híbridos, Língua E Narizes Eletrônicos Para a. Ph.D. Thesis, Universidade de São Paulo, Butantan, Brazil, 2010. [Google Scholar]
- Mizuguchi, J. Capacitive Edge Field Effect Sensors Applied to Quantification of Leaf Wetting and Soil Water. Master’s Thesis, Universidade Federal de Londrina, Parana, Brazil, 2014; p. 120. [Google Scholar]
- Dean, R.N.; Rane, A.K.; Baginski, M.E.; Richard, J.; Hartzog, Z.; Elton, D.J. A capacitive fringing field sensor design for moisture measurement based on printed circuit board technology. IEEE Trans. Instrum. Meas. 2012, 61, 1105–1112. [Google Scholar] [CrossRef]
- Tsori, Y.; Tournilhac, F.; Leibler, L. Demixing in simple fluids induced by electric field gradients. Nature 2004, 430, 544–547. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Pereira, T.C. Development of an electroanalytical method for the determination of biodiesel content by electrochemical impedance spectroscopy. Master’s Thesis, Universidade Federal do Maranhão, Maranhão, Brazil, 2014. [Google Scholar]
- Djatna, T.; Noor, E. An identification and characterization of biodiesel fatty acid based by using dielectric sensor. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012004. [Google Scholar]
- Pereira, T.C.; Delfino, J.R.; Ferreira, A.A.P.; Barros, F.J.S.; Marques, E.P.; Zhang, J.; Marques, A.L.B. Stainless Steel Electrodes to Determine Biodiesel Content in Petroleum Diesel Fuel by Electrochemical Impedance Spectroscopy. Electroanalysis 2017, 29, 814–820. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Abramova, N.; Uria, N.; Bratov, A. Impedimetric transducers based on interdigitated electrode arrays for bacterial detection—A review. Anal. Chim. Acta 2019, 1088, 1–19. [Google Scholar] [CrossRef]
- Ulrich, C.; Petersson, H.; Sundgren, H.; Björefors, F.; Krantz-Rülcker, C. Simultaneous estimation of soot and diesel contamination in engine oil using electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2007, 127, 613–618. [Google Scholar] [CrossRef]
- Delfino, J.R.; Pereira, T.C.; Costa Viegas, H.D.; Marques, E.P.; Pupim Ferreira, A.A.; Zhang, L.; Zhang, J.; Brandes Marques, A.L. A simple and fast method to determine water content in biodiesel by electrochemical impedance spectroscopy. Talanta 2018, 179, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Bocian, P.; Biernat, K.; Matuszewska, A.; Sharma, P.S.; Bukrejewski, P.; Noworyta, K.R. Electrochemical impedance spectroscopy studies of gasoline oxidative stability—Attempt to devise new gasolines chemical stability index. Fuel 2021, 288, 119620. [Google Scholar] [CrossRef]
- Biernat, K.; Bocian, P.; Bukrejewski, P.; Noworyta, K.R. Application of the impedance spectroscopy as a new tool for studying biodiesel fuel aging processes. Energies 2019, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Souza, J.E.; Scherer, M.D.; Cáceres, J.A.S.; Caires, A.R.L.; M’Peko, J.C. A close dielectric spectroscopic analysis of diesel/biodiesel blends and potential dielectric approaches for biodiesel content assessment. Fuel 2013, 105, 705–710. [Google Scholar] [CrossRef]
- Pereira, F. Impedance spectroscopy applied to the characterization of parasitized cells on a micro-fluidics platform. Ph.D. Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2012. [Google Scholar]
- Lvovich, V.F.; Liu, C.C.; Smiechowski, M.F. Optimization and fabrication of planar interdigitated impedance sensors for highly resistive non-aqueous industrial fluids. Sens. Actuators B Chem. 2006, 119, 490–496. [Google Scholar] [CrossRef]
- Frey, J.J. Current trends in contraception. Br. Med. J. 1979, 2, 866–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolouei, N.E.; Ghamari, S.; Shavezipur, M. Development of circuit models for electrochemical impedance spectroscopy (EIS) responses of interdigitated MEMS biochemical sensors. J. Electroanal. Chem. 2020, 878, 114598. [Google Scholar] [CrossRef]
- Teruya, M. Impedance spectroscopy in ionic solutions and ethanol/water mixture. Ph.D. Thesis, Universidade Estadual Paulista, Presidente Prudente, Brazil, 2008. [Google Scholar]
- Davanse, L.G. Aplicação de Espectroscopia de Impedância no Estudo de Blendas de Biodiesel/diesel. Master’s Thesis, Universidade Estadual De Maringa, Maringá, Brazil, 2010. [Google Scholar]
- Mamishev, A.V.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–844. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, F.; Savard, S.; Maldague, X.; Filleter, T.; Bendada, A.H. Non-Destructive Testing of Materials by Capacitive Sensing. Automot. Eng. 2021. [Google Scholar] [CrossRef]
- Pajkossy, T.; Jurczakowski, R. Electrochemical impedance spectroscopy in interfacial studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. EIS study of corrosion behavior of metallic materials in ethanol blended gasoline containing water as a contaminant. Fuel 2011, 90, 1181–1187. [Google Scholar] [CrossRef]












| Sensor Coding | Comb Spacing (µm) | Effective Area (mm²) | Number of Electrodes (Units) |
|---|---|---|---|
| Ba1810 | 10 | 1.25 | 20 |
| Ba1820 | 20 | 1.65 | 20 |
| Ba1830 | 30 | 2.00 | 20 |
| Ba1850 | 50 | 2.80 | 20 |
| Circuit Element | B0 | B10 | B100 |
|---|---|---|---|
| Rs | 5421 | 3984 | 3530 |
| Rct | 5.08 × 108 | 4.02 × 108 | 3.16 × 108 |
| CPEDL—T | 3.52 × 10−12 | 3.56 × 10−12 | 3.59 × 10−12 |
| CPEDL—p | 0.97 | 0.97 | 0.98 |
| CPEW—T | 5.33 × 10−10 | 5.40 × 10−10 | 5.44 × 10−10 |
| CPEW—P | 0.49 | 0.46 | 0.45 |
| 0.003 | 0.007 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Neto, I.S.d.; Carvalho, C.D.; Filho, G.B.A.; Andrade, C.D.S.S.; Santos, G.C.d.O.; Barros, A.K.; Neto, J.V.d.F.; Casas, V.L.P.; Alencar, L.M.R.; Lopes, A.J.O.; et al. Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends. Sensors 2021, 21, 7288. https://doi.org/10.3390/s21217288
Santos-Neto ISd, Carvalho CD, Filho GBA, Andrade CDSS, Santos GCdO, Barros AK, Neto JVdF, Casas VLP, Alencar LMR, Lopes AJO, et al. Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends. Sensors. 2021; 21(21):7288. https://doi.org/10.3390/s21217288
Chicago/Turabian StyleSantos-Neto, Inocêncio Sanches dos, Christian Diniz Carvalho, Gilberto Balby Araújo Filho, Cassio Daniel Salomão Silva Andrade, Giselle Cutrim de Oliveira Santos, Allan Kardec Barros, João Viana da Fonseca Neto, Vicente Leonardo Paucar Casas, Luciana Magalhães Rebelo Alencar, Alberto Jorge Oliveira Lopes, and et al. 2021. "Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends" Sensors 21, no. 21: 7288. https://doi.org/10.3390/s21217288
APA StyleSantos-Neto, I. S. d., Carvalho, C. D., Filho, G. B. A., Andrade, C. D. S. S., Santos, G. C. d. O., Barros, A. K., Neto, J. V. d. F., Casas, V. L. P., Alencar, L. M. R., Lopes, A. J. O., Silva, F. C., & Sinfrônio, F. S. M. (2021). Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends. Sensors, 21(21), 7288. https://doi.org/10.3390/s21217288







