Chemically Induced pH Perturbations for Analyzing Biological Barriers Using Ion-Sensitive Field-Effect Transistors
Abstract
:1. Introduction
2. Analysis of Cell Barriers for Nanomedicine
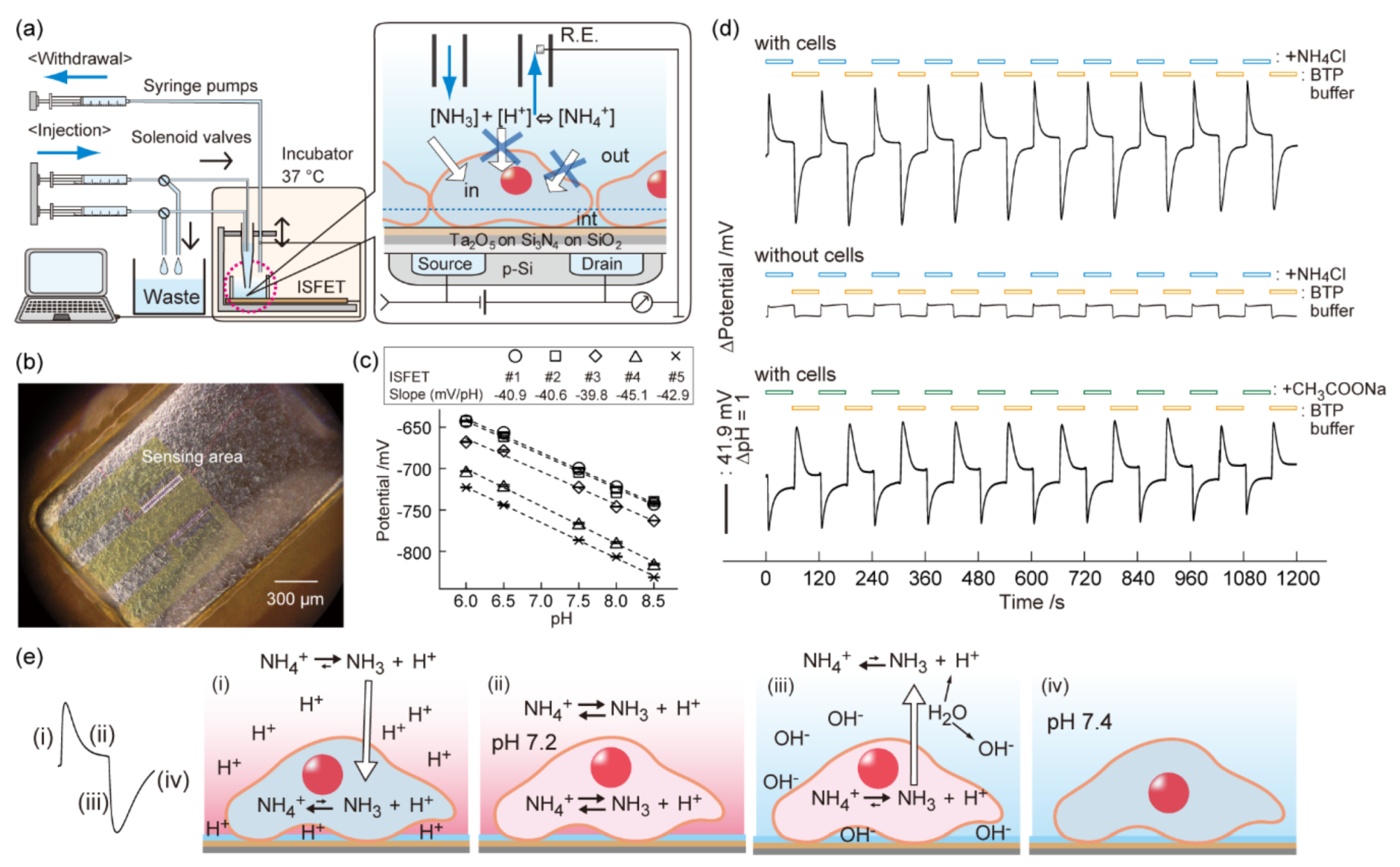
3. Weak Acid/Base-Induced pH Perturbation in a Cell Microenvironment
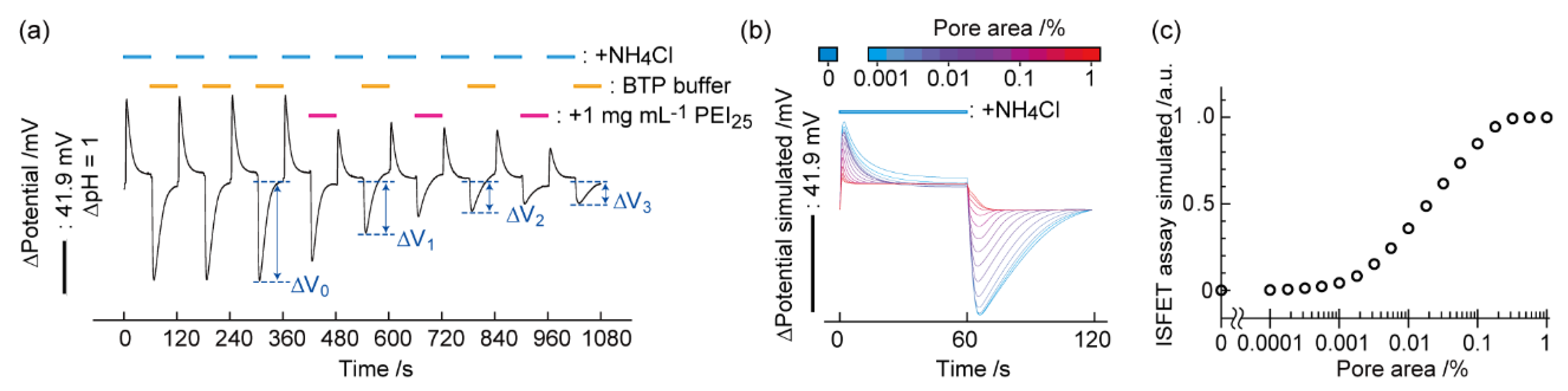
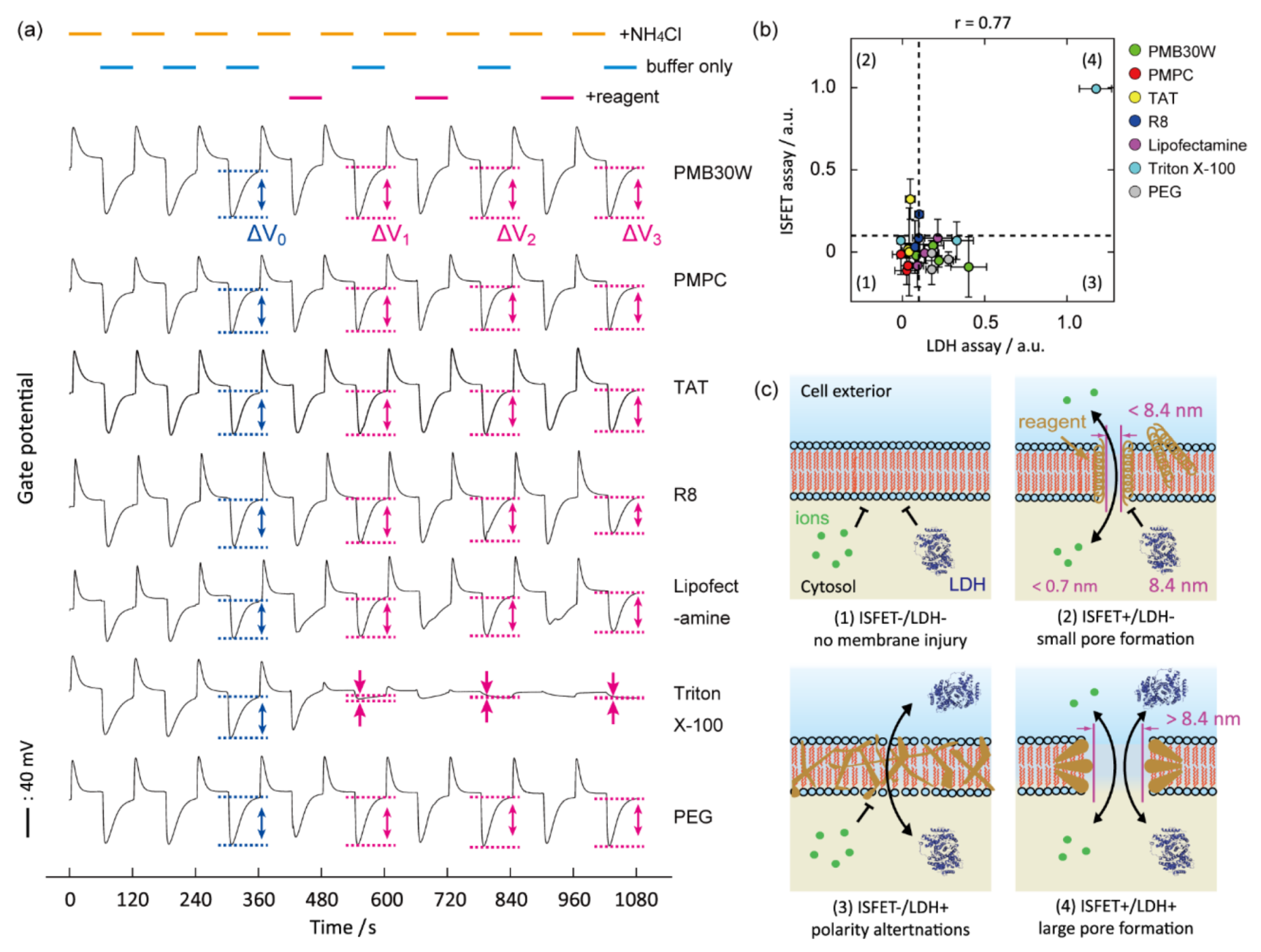
4. Detection of Pore Formation on Biomembranes
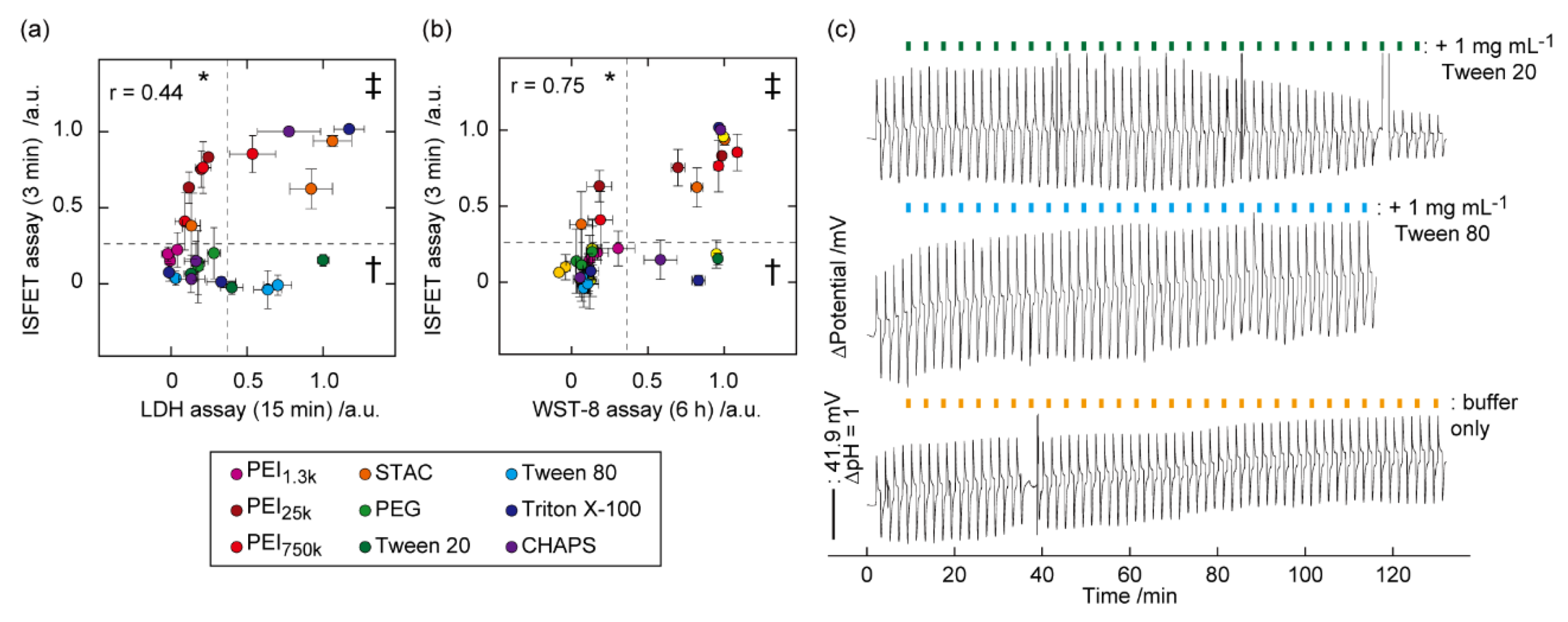
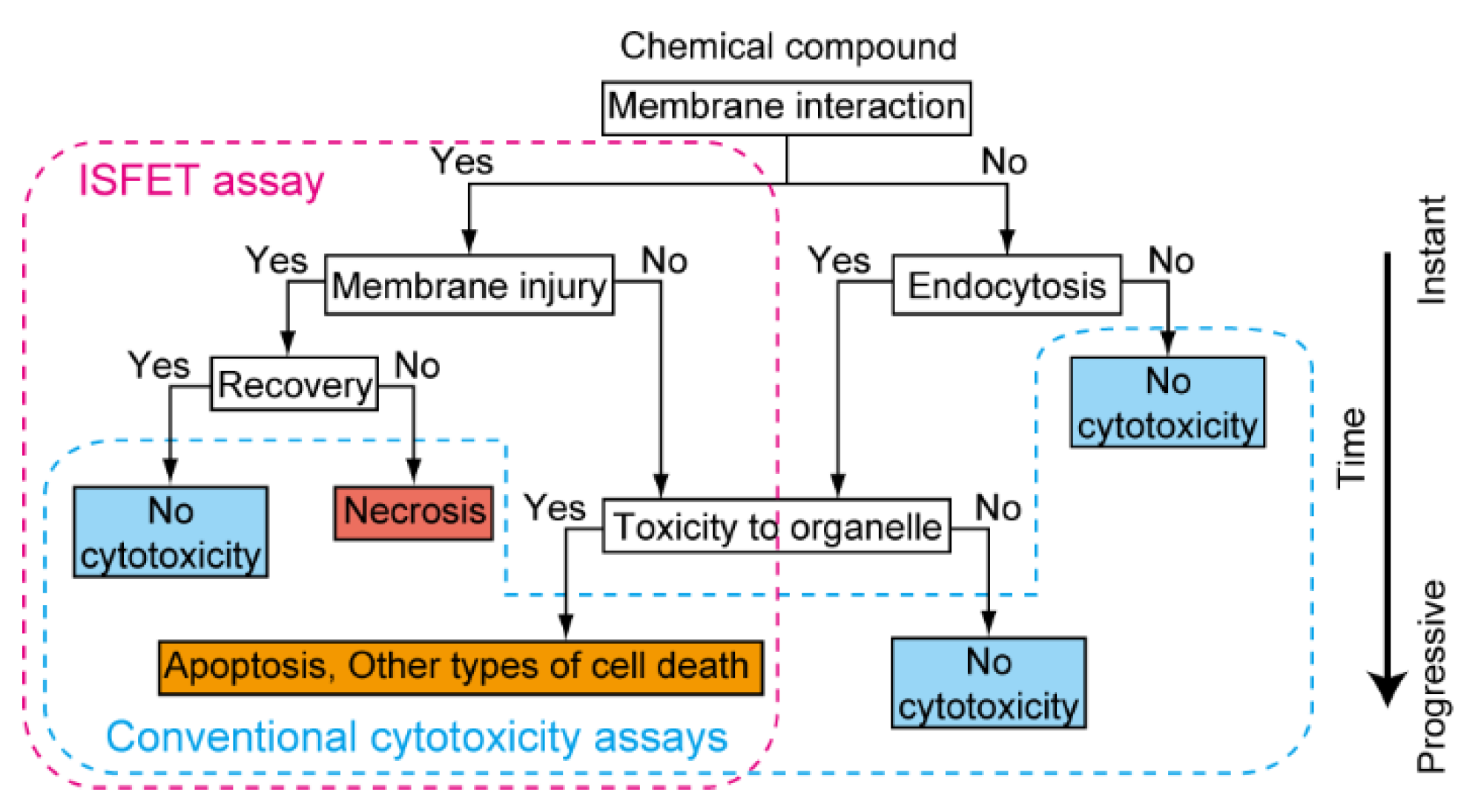
5. Identification of Biomembrane Injury Type and Cell Death
6. Identification of Type of Cell Death
7. Understanding the Permeation Mechanism of Nanocarrier
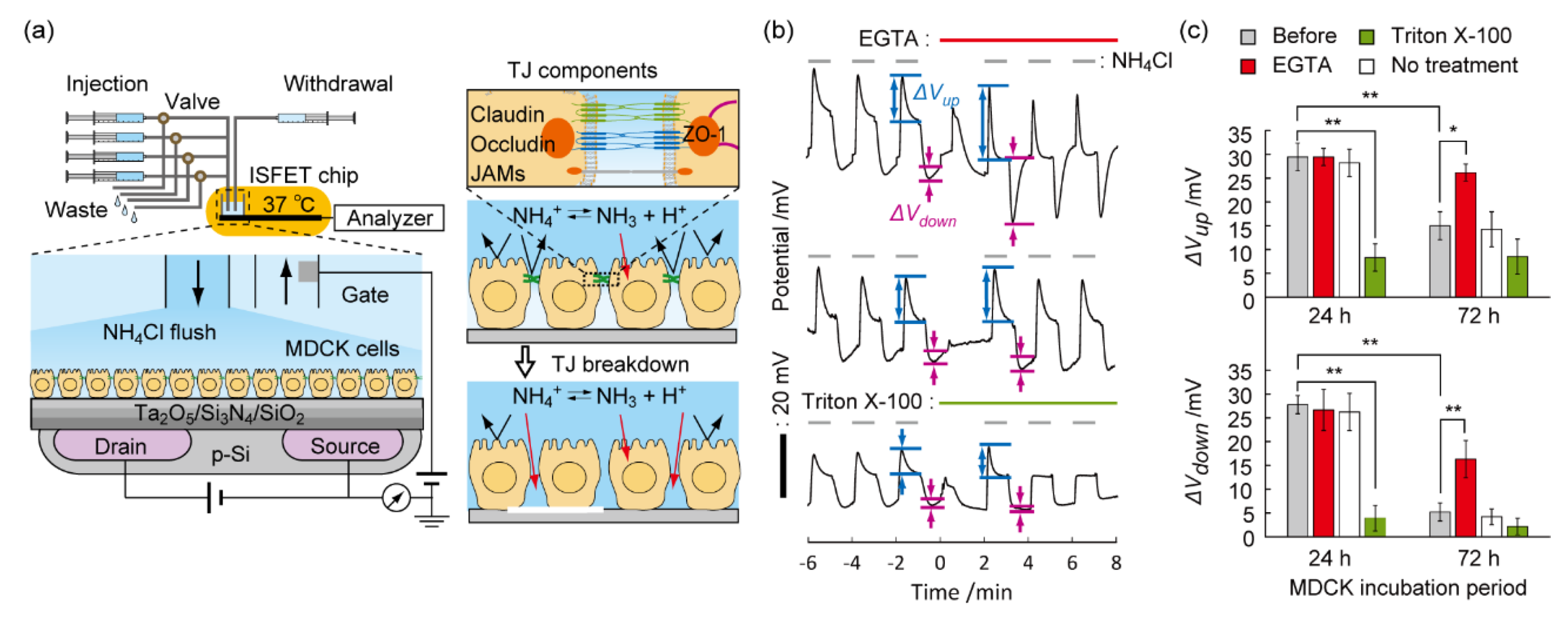
8. Detection of the Breach of the Tight Junction on the Epithelial Cell Layer
9. Conclusions
Funding
Conflicts of Interest
References
- Gatenby, R.A.; Gawlinski, E.T.; Gmitro, A.F.; Kaylor, B.; Gillies, R.J. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006, 66, 5216–5223. [Google Scholar] [CrossRef] [Green Version]
- Curley, G.; Contreras, M.M.; Nichol, A.D.; Higgins, B.D.; Laffey, J.G. Hypercapnia and acidosis in sepsis: A double-edged sword? Anesthesiology 2010, 112, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Ratanaporncharoen, C.; Tabata, M.; Kitasako, Y.; Ikeda, M.; Goda, T.; Matsumoto, A.; Tagami, J.; Miyahara, Y. pH Mapping on Tooth Surfaces for Quantitative Caries Diagnosis Using Micro Ir/IrOx pH Sensor. Anal. Chem. 2018, 90, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Ashby, B.S. pH studies in human malignant tumours. Lancet 1966, 2, 312–315. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, T.; Wang, C.; Nham, K.; Xiong, Y.; Gao, X.; Wang, Y.; Hao, G.; Ge, W.P.; Sun, X.; et al. PET imaging of occult tumours by temporal integration of tumour-acidosis signals from pH-sensitive (64)Cu-labelled polymers. Nat. Biomed. Eng. 2020, 4, 314–324. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Suzuki, R.; Mamiya, T.; Matsui, H.; Hirayama, A.; Nagasaki, Y. pH-sensitive radical-containing-nanoparticle (RNP) for the L-band-EPR imaging of low pH circumstances. Bioconj. Chem. 2009, 20, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, M.L.; Herigault, G.; Remy, C.; Farion, R.; Ballesteros, P.; Coles, J.A.; Cerdan, S.; Ziegler, A. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: Comparison with maps of metabolites. Cancer Res. 2001, 61, 6524–6531. [Google Scholar]
- Cao, H.; Landge, V.; Tata, U.; Seo, Y.S.; Rao, S.; Tang, S.J.; Tibbals, H.F.; Spechler, S.; Chiao, J.C. An Implantable, Batteryless, and Wireless Capsule with Integrated Impedance and pH Sensors for Gastroesophageal Reflux Monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 3131–3139. [Google Scholar] [PubMed]
- Chen, L.Q.; Pagel, M.D. Evaluating pH in the Extracellular Tumor Microenvironment Using CEST MRI and Other Imaging Methods. Adv. Radiol. 2015, 2015, 206405. [Google Scholar] [CrossRef] [Green Version]
- Rothberg, J.M.; Hinz, W.; Rearick, T.M.; Schultz, J.; Mileski, W.; Davey, M.; Leamon, J.H.; Johnson, K.; Milgrew, M.J.; Edwards, M.; et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature 2011, 475, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Toumazou, C.; Shepherd, L.M.; Reed, S.C.; Chen, G.I.; Patel, A.; Garner, D.M.; Wang, C.J.A.; Ou, C.P.; Amin-Desai, K.; Athanasiou, P.; et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. Methods 2013, 10, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Sorensen, J.N.; Larsen, E.H.; Willumsen, N.J. Proton pump activity of mitochondria-rich cells. The interpretation of external proton-concentration gradients. J. Gen. Physiol. 1997, 109, 73–91. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, M.; Baumann, W.; Brischwein, M.; Ehret, R.; Kraus, M.; Schwinde, A.; Bitzenhofer, M.; Freund, I.; Wolf, B. Non-invasive measurement of cell membrane associated proton gradients by ion-sensitive field effect transistor arrays for microphysiological and bioelectronical applications. Biosens. Bioelectron. 2000, 15, 117–124. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Schoning, M.J. Light-addressable potentiometric sensors for cell monitoring and biosensing. Curr. Opin. Electrochem. 2021, 28, 100727. [Google Scholar] [CrossRef]
- Yang, C.M.; Yen, T.H.; Liu, H.L.; Lin, Y.J.; Lin, P.Y.; Tsui, L.S.; Chen, C.H.; Chen, Y.P.; Hsu, Y.C.; Lo, C.H.; et al. A real-time mirror-LAPS mini system for dynamic chemical imaging and cell acidification monitoring. Sens. Actuators B Chem. 2021, 341, 130003. [Google Scholar] [CrossRef]
- Schoning, M.J.; Poghossian, A. Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst 2002, 127, 1137–1151. [Google Scholar] [CrossRef] [Green Version]
- Bergveld, P. Thirty years of ISFETOLOGY—What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Kim, S.K.; Kim, M. Ion-sensitive field-effect transistor for biological sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schoning, M.J. Capacitive Field-Effect EIS Chemical Sensors and Biosensors: A Status Report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef]
- Lehmann, M.; Baumann, W.; Brischwein, M.; Gahle, H.J.; Freund, I.; Ehret, R.; Drechsler, S.; Palzer, H.; Kleintges, M.; Sieben, U.; et al. Simultaneous measurement of cellular respiration and acidification with a single CMOS ISFET. Biosens. Bioelectron. 2001, 16, 195–203. [Google Scholar] [CrossRef]
- Chiang, J.L.; Jan, S.S.; Chou, J.C.; Chen, Y.C. Study on the temperature effect, hysteresis and drift of pH-ISFET devices based on amorphous tungsten oxide. Sens. Actuators B Chem. 2001, 76, 624–628. [Google Scholar] [CrossRef]
- Poghossian, A.; Schoning, M.J. Recent progress in silicon-based biologically sensitive field-effect devices. Curr. Opin. Electrochem. 2021, 29, 100811. [Google Scholar] [CrossRef]
- Yang, Y.; Cuartero, M.; Goncales, V.R.; Gooding, J.J.; Bakker, E. Light-addressable ion sensing for real-time monitoring of extracellular potassium. Angew. Chem. Int. Ed. 2018, 57, 16801–16805. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays-A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef]
- Poghossian, A.; Ingebrandt, S.; Offenhausser, A.; Schoning, M.J. Field-effect devices for detecting cellular signals. Semin. Cell Dev. Biol. 2009, 20, 41–48. [Google Scholar] [CrossRef]
- Walsh, K.B.; DeRoller, N.; Zhu, Y.H.; Koley, G. Application of ion-sensitive field effect transistors for ion channel screening. Biosens. Bioelectron. 2014, 54, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Koppenhofer, D.; Susloparova, A.; Docter, D.; Stauber, R.H.; Ingebrandt, S. Monitoring nanoparticle induced cell death in H441 cells using field-effect transistors. Biosens. Bioelectron. 2013, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.M.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.X.; Xia, Y.N. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.L.; Smith, J.C. Biological Membrane Organization and Cellular Signaling. Chem. Rev. 2019, 119, 5849–5880. [Google Scholar] [CrossRef]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Claudin-based barrier in simple and stratified cellular sheets. Curr. Opin. Cell Biol. 2002, 14, 531–536. [Google Scholar] [CrossRef]
- Yamada, K.M.; Geiger, B. Molecular interactions in cell adhesion complexes. Curr. Opin. Cell Biol. 1997, 9, 76–85. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Kondoh, M.; Yoshida, T.; Kakutani, H.; Yagi, K. Targeting tight junction proteins-significance for drug development. Drug Discov. Today 2008, 13, 180–186. [Google Scholar] [CrossRef]
- Lee, J.H.; Sahu, A.; Choi, W.I.; Lee, J.Y.; Tae, G. ZOT-derived peptide and chitosan functionalized nanocarrier for oral delivery of protein drug. Biomaterials 2016, 103, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.P.; Lau, J.; Deng, L.M.; Shen, Z.Y.; Chen, X.Y. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Dedoussis, G.V.; Spanakos, G.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J. Immunol. Methods 1994, 177, 101–111. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Alonso, A.; Goni, F.M. Detergent solubilization of lipid bilayers: A balance of driving forces. Trends Biochem. Sci. 2013, 38, 85–93. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Orr, B.G.; Holl, M.M.B. Detergent Induction of HEK 293A Cell Membrane Permeability Measured under Quiescent and Superfusion Conditions Using Whole Cell Patch Clamp. J. Phys. Chem. B 2014, 118, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Goda, T.; Schaffhauser, D.F.; Okada, J.; Matsumoto, A.; Miyahara, Y. Proton-sensing transistor systems for detecting ion leakage from plasma membranes under chemical stimuli. Acta Biomater. 2017, 50, 502–509. [Google Scholar] [CrossRef]
- Dantism, S.; Rohlen, D.; Wagner, T.; Wagner, P.; Schoning, M.J. A LAPS-Based Differential Sensor for Parallelized Metabolism Monitoring of Various Bacteria. Sensors 2019, 19, 4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.; Gu, C.L.; Gan, Y.; Wu, Q.; He, C.J.; Tu, J.W.; Pan, Y.X.; Qiu, Y.; Kong, L.B.; Wan, H.; et al. Microfluidic chip system integrated with light addressable potentiometric sensor (LAPS) for real-time extracellular acidification detection. Sens. Actuators B Chem. 2019, 301, 127004. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.L.; Chen, F.M.; Chen, W.; Du, L.P.; He, Z.Y.; Wu, C.S.; Zhang, D.W. Scanning Electrochemical Photometric Sensors for Label-Free Single-Cell Imaging and Quantitative Absorption Analysis. Anal. Chem. 2020, 92, 9739–9744. [Google Scholar] [CrossRef]
- Saito, A.; Sakata, T. Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Non-Optical and Live Monitoring of Sperm Capacitation. Sensors 2019, 19, 1784. [Google Scholar] [CrossRef] [Green Version]
- Sakata, T.; Sugimoto, H.; Saito, A. Live Monitoring of Microenvironmental pH Based on Extracellular Acidosis around Cancer Cells with Cell-Coupled Gate Ion-Sensitive Field-Effect Transistor. Anal. Chem. 2018, 90, 12731–12736. [Google Scholar] [CrossRef]
- Schaffhauser, D.F.; Patti, M.; Goda, T.; Miyahara, Y.; Forster, I.C.; Dittrich, P.S. An Integrated field-effect microdevice for monitoring membrane transport in Xenopus laevis oocytes via lateral proton diffusion. PLoS ONE 2012, 7, e39238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, S.E.; Taylor, D.; Kozlov, E.; Prokop, A.; Cliffel, D.E. A microphysiometer for simultaneous measurement of changes in extracellular glucose, lactate, oxygen, and acidification rate. Anal. Chem. 2004, 76, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Miyahara, Y. Noninvasive monitoring of transporter-substrate interaction at cell membrane. Anal. Chem. 2008, 80, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Schaffhauser, D.; Fine, M.; Tabata, M.; Goda, T.; Miyahara, Y. Measurement of rapid amiloride-dependent pH changes at the cell surface using a proton-sensitive field-effect transistor. Biosensors 2016, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Ray, N.G.; Shuler, M.L. A computer model for intracellular pH regulation in Chinese hamster ovary cells. Biotechnol. Prog. 1993, 9, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Goda, T.; Matsumoto, A.; Miyahara, Y. Identification of types of membrane injuries and cell death using whole cell-based proton-sensitive field-effect transistor systems. Analyst 2017, 142, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Goda, T.; Toya, Y.; Matsumoto, A.; Miyahara, Y. Oleyl group-functionalized insulating gate transistors for measuring extracellular pH of floating cells. Sci. Technol. Adv. Mater. 2016, 17, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.P.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R.; Holl, M.M.B. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconj. Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Sikor, M.; Sabin, J.; Keyvanloo, A.; Schneider, M.F.; Thewalt, J.L.; Bailey, A.E.; Frisken, B.J. Interaction of a Charged Polymer with Zwitterionic Lipid Vesicles. Langmuir 2010, 26, 4095–4102. [Google Scholar] [CrossRef]
- Rothe, G.M.; Purkhanbaba, H. Determination of molecular weights and Stokes’ radii of non-denatured proteins by polyacrylamide gradient gel electrophoresis. 1. An equation relating total polymer concentration, the molecular weight of proteins in the range of 104–106, and duration of electrophoresis. Electrophoresis 1982, 3, 33–42. [Google Scholar]
- Tamba, Y.; Ariyama, H.; Levadny, V.; Yamazaki, M. Kinetic Pathway of Antimicrobial Peptide Magainin 2-Induced Pore Formation in Lipid Membranes. J. Phys. Chem. B 2010, 114, 12018–12026. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.Y.; Balogh, L.; Orr, B.G.; Baker, J.R.; Holl, M.M.B. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconj. Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef]
- Tan, A.M.; Ziegler, A.; Steinbauer, B.; Seelig, J. Thermodynamics of sodium dodecyl sulfate partitioning into lipid membranes. Biophys. J. 2002, 83, 1547–1556. [Google Scholar] [CrossRef] [Green Version]
- Deo, N.; Somasundaran, P. Electron spin resonance study of phosphatidyl choline vesicles using 5-doxyl stearic acid. Colloids Surf. B 2002, 25, 225–232. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef]
- Cronican, J.J.; Beier, K.T.; Davis, T.N.; Tseng, J.C.; Li, W.D.; Thompson, D.B.; Shih, A.F.; May, E.M.; Cepko, C.L.; Kung, A.L.; et al. A Class of Human Proteins that Deliver Functional Proteins into Mammalian Cells In Vitro and In Vivo. Chem. Biol. 2011, 18, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goda, T.; Goto, Y.; Ishihara, K. Cell-penetrating macromolecules: Direct penetration of amphipathic phospholipid polymers across plasma membrane of living cells. Biomaterials 2010, 31, 2380–2387. [Google Scholar] [CrossRef]
- Goda, T.; Miyahara, Y.; Ishihara, K. Phospholipid-mimicking cell-penetrating polymers: Principles and applications. J. Mater. Chem. B 2020, 8, 7633–7641. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Ishihara, K.; Miyahara, Y. Critical update on 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer science. J. Appl. Polym. Sci. 2015, 132, 41766. [Google Scholar] [CrossRef]
- Du, S.; Liew, S.S.; Li, L.; Yao, S.Q. Bypassing Endocytosis: Direct Cytosolic Delivery of Proteins. J. Am. Chem. Soc. 2018, 140, 15986–15996. [Google Scholar] [CrossRef] [PubMed]
- Marie, E.; Sagan, S.; Cribier, S.; Tribet, C. Amphiphilic Macromolecules on Cell Membranes: From Protective Layers to Controlled Permeabilization. J. Membr. Biol. 2014, 247, 861–881. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Imaizumi, Y.; Hatano, H.; Matsumoto, A.; Ishihara, K.; Miyahara, Y. Translocation Mechanisms of Cell-Penetrating Polymers Identified by Induced Proton Dynamics. Langmuir 2019, 35, 8167–8173. [Google Scholar] [CrossRef]
- Werner, M.; Sommer, J.U. Translocation and Induced Permeability of Random Amphiphilic Copolymers Interacting with Lipid Bilayer Membranes. Biomacromolecules 2015, 16, 125–135. [Google Scholar] [CrossRef]
- Goda, T.; Hatano, H.; Yamamoto, M.; Miyahara, Y.; Morimoto, N. Internalization Mechanisms of Pyridinium Sulfobetaine Polymers Evaluated by Induced Protic Perturbations on Cell Surfaces. Langmuir 2020, 36, 9977–9984. [Google Scholar] [CrossRef]
- Boonstra, E.; Hatano, H.; Miyahara, Y.; Uchida, S.; Goda, T.; Cabral, H. A proton/macromolecule-sensing approach distinguishes changes in biological membrane permeability during polymer/lipid-based nucleic acid delivery. J. Mater. Chem. B 2021, 9, 4298–4302. [Google Scholar] [CrossRef]
- Miyata, K.; Oba, M.; Nakanishi, M.; Fukushima, S.; Yamasaki, Y.; Koyama, H.; Nishiyama, N.; Kataoka, K. Polyplexes from Poly(aspartamide) Bearing 1,2-Diaminoethane Side Chains Induce pH-Selective, Endosomal Membrane Destabilization with Amplified Transfection and Negligible Cytotoxicity. J. Am. Chem. Soc. 2008, 130, 16287–16294. [Google Scholar] [CrossRef]
- Funhoff, A.M.; van Nostrum, C.F.; Koning, G.A.; Schuurmans-Nieuwenbroek, N.M.E.; Crommelin, D.J.A.; Hennink, W.E. Endosomal escape of polymeric gene delivery complexes is not always enhanced by polymers buffering at low pH. Biomacromolecules 2004, 5, 32–39. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. Jala-J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltra-Noguera, D.; Mangas-Sanjuan, V.; Centelles-Sanguesa, A.; Gonzalez-Garcia, I.; Sanchez-Castano, G.; Gonzalez-Alvarez, M.; Casabo, V.G.; Merino, V.; Gonzalez-Alvarez, I.; Bermejo, M. Variability of permeability estimation from different protocols of subculture and transport experiments in cell monolayers. J. Pharmacol. Toxicol. Methods 2015, 71, 21–32. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Varadi, J.; Feher, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyes, M.; Bacskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Hatano, H.; Goda, T.; Matsumoto, A.; Miyahara, Y. Induced Proton Perturbation for Sensitive and Selective Detection of Tight Junction Breakdown. Anal. Chem. 2019, 91, 3525–3532. [Google Scholar] [CrossRef]
- Hatano, H.; Goda, T.; Matsumoto, A.; Miyahara, Y. Induced Proton Dynamics on Semiconductor Surfaces for Sensing Tight Junction Formation Enhanced by an Extracellular Matrix and Drug. ACS Sens. 2019, 4, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, T. Chemically Induced pH Perturbations for Analyzing Biological Barriers Using Ion-Sensitive Field-Effect Transistors. Sensors 2021, 21, 7277. https://doi.org/10.3390/s21217277
Goda T. Chemically Induced pH Perturbations for Analyzing Biological Barriers Using Ion-Sensitive Field-Effect Transistors. Sensors. 2021; 21(21):7277. https://doi.org/10.3390/s21217277
Chicago/Turabian StyleGoda, Tatsuro. 2021. "Chemically Induced pH Perturbations for Analyzing Biological Barriers Using Ion-Sensitive Field-Effect Transistors" Sensors 21, no. 21: 7277. https://doi.org/10.3390/s21217277





