An Array of On-Chip Integrated, Individually Addressable Capacitive Field-Effect Sensors with Control Gate: Design and Modelling
Abstract
:1. Introduction
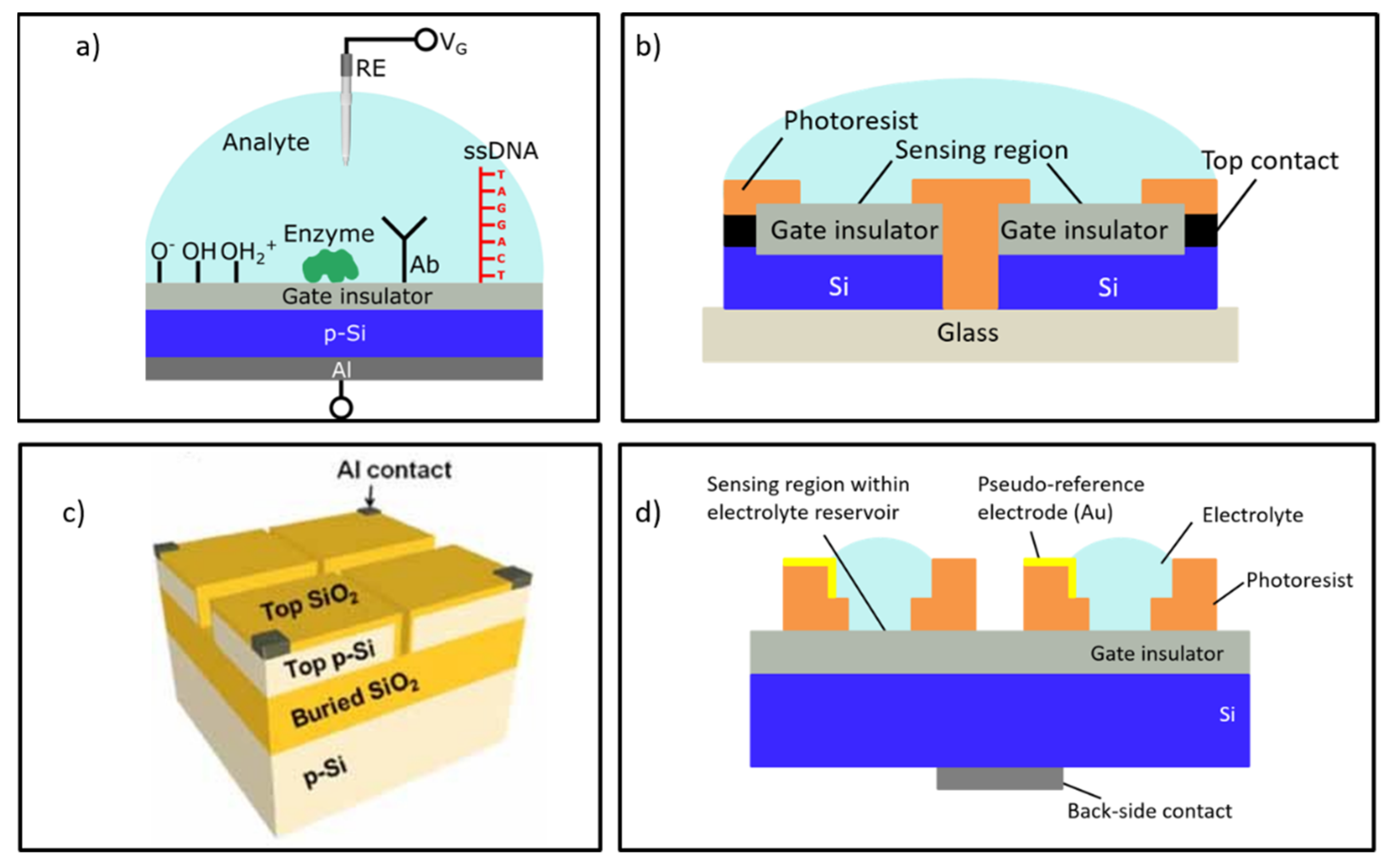
2. Design of On-Chip Integrated, Individually Addressable CG-EISCAPs
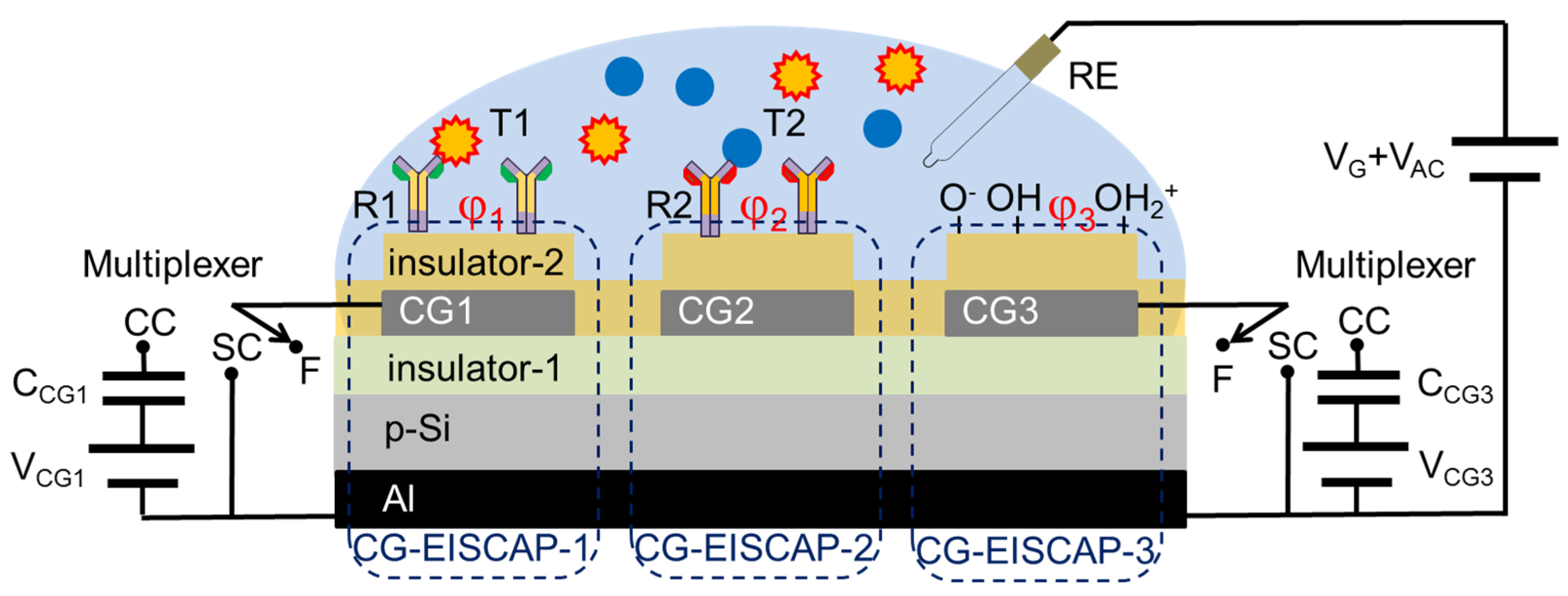
3. Modelling of On-Chip Integrated, Individually Addressable CG-EISCAPs
3.1. Setup with Floating/Short-Circuited CG
3.2. Setup with Floating/Capacitively-Coupled CGs
4. Simulation Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review—Field-effect transistor biosensing: Devices and clinical applications. ECS J. Solid State Sci. Technol. 2013, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- de Moraes, A.C.M.; Kubota, L.T. Recent trends in field-effect transistors-based immunosensors. Chemosensors 2016, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Poghossian, A.; Jablonski, M.; Molinnus, D.; Wege, C.; Schöning, M.J. Field-effect sensors for virus detection: From Ebola to SARS-CoV-2 and plant viral enhancers. Front. Plant. Sci. 2020, 11, 598103. [Google Scholar] [CrossRef]
- Poghossian, A.; Lüth, H.; Schultze, J.W.; Schöning, M.J. (Bio-)chemical and physical microsensor arrays using an identical transducer principle. Electrochim. Acta 2001, 47, 243–249. [Google Scholar] [CrossRef]
- Gao, A.; Chen, S.; Wang, Y.; Li, T. Silicon nanowire field-effect-transistor-based biosensor for biomedical applications. Sens. Mater. 2018, 30, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon nanowire field-effect transistors—A versatile class of potentiometric nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar] [CrossRef]
- Pullano, S.A.; Critello, C.D.; Mahbub, I.; Tasneem, N.T.; Shamsir, S.; Islam, S.K.; Greco, M.; Fiorillo, A.S. EGFET-based sensors for bioanalytical applications: A review. Sensors 2018, 18, 4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Poghossian, A.; Bronder, T.S.; Schöning, M.J. Sensing of double-stranded DNA molecules by their intrinsic molecular charge using the light-addressable potentiometric sensor. Sens. Actuators B 2016, 229, 506–512. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Xu, L.; Ning, Y.; Xie, S.; Zhang, G.-J. Silicon nanowire biosensor for highly sensitive and multiplexed detection of oral squamous cell carcinoma biomarkers in saliva. Anal. Sci. 2015, 31, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Label-free detection of tumor markers using field effect transistor (FET)-based biosensors for lung cancer diagnosis. Sens. Actuators B 2015, 212, 329–334. [Google Scholar] [CrossRef]
- Si, K.; Cheng, S.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Multianalyte detection of cancer biomarkers in human serum using label-free field effect transistor biosensor. Sens. Mater. 2018, 30, 991–999. [Google Scholar]
- Jia, Y.-F.; Gao, C.-Y.; He, J.; Feng, D.-F.; Xing, K.-L.; Wu, M.; Liu, Y.; Cai, W.-S.; Feng, X.-Z. Unlabeled multi tumor marker detection system based on bioinitiated light addressable potentiometric sensor. Analyst 2012, 137, 3806–3813. [Google Scholar] [CrossRef]
- Ipatov, A.; Abramova, N.; Bratov, A. Integrated multi-sensor chip with photocured polymer membranes containing copolymerised plasticizer for direct pH, potassium, sodium and chloride ions determination in blood serum. Talanta 2009, 79, 984–989. [Google Scholar]
- Moser, N.; Leong, C.L.; Hu, Y.; Cicatiello, C.; Gowers, S.; Boutelle, M.; Georgiou, P. Complementary metal-oxide-semiconductor potentiometric field-effect transistor array platform using sensor learning for multi-ion imaging. Anal. Chem. 2020, 92, 5276–5285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poghossian, A.; Schöning, M.J. Capacitive field-effect chemical sensors and biosensors: A status report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jin, Y.; Qu, X.; Jin, W.; Zhao, J. Electrochemical impedance spectroscopy study of Ta2O5 based EIOS pH sensors in acid environment. Sens. Actuators B 2014, 192, 399–405. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Meyyappan, M.; Baek, C.-K. LaF3 electrolyte-insulator-semiconductor sensor for detecting fluoride ions. Sens. Actuators B 2019, 279, 183–188. [Google Scholar] [CrossRef]
- Abouzar, M.H.; Poghossian, A.; Siqueira, J.R., Jr.; Oliveira, O.N., Jr.; Moritz, W.; Schöning, M.J. Capacitive electrolyte-insulator-semiconductor structures functionalized with a polyelectrolyte/enzyme multilayer: New strategy for enhanced field-effect biosensing. Phys. Status Solidi A 2010, 207, 884–890. [Google Scholar] [CrossRef]
- Lin, C.F.; Kao, C.H.; Lin, C.Y.; Chen, K.L.; Lin, Y.H. NH3 plasma-treated magnesium doped zinc oxide in biomedical sensors with electrolyte-insulator-semiconductor (EIS) structure for urea and glucose applications. Nanomaterials 2020, 10, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poghossian, A.; Jablonski, M.; Koch, C.; Bronder, T.S.; Rolka, D.; Wege, C.; Schöning, M.J. Field-effect biosensor using virus particles as scaffolds for enzyme immobilization. Biosens. Bioelectron. 2018, 110, 168–174. [Google Scholar] [CrossRef]
- Jablonski, M.; Münstermann, F.; Nork, J.; Molinnus, D.; Muschallik, L.; Bongaerts, J.; Wagner, T.; Keusgen, M.; Siegert, P.; Schöning, M.J. Capacitive field-effect biosensor applied for the detection of acetoin in alcoholic beverages and fermentation broths. Phys. Status Solidi A 2021, 218, 2000765. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Luo, J.-D.; Chiou, C.-C.; Yang, C.-M.; Wang, C.-Y.; Chou, C.; Lai, C.-S. Detection of KRAS mutation by combination of polymerase chain reaction (PCR) and EIS sensor with new amino group functionalization. Sens. Actuators B 2013, 186, 374–379. [Google Scholar] [CrossRef]
- Abouzar, M.H.; Poghossian, A.; Cherstvy, A.G.; Pedraza, A.M.; Ingebrandt, S.; Schöning, M.J. Label-free electrical detection of DNA by means of field-effect nanoplate capacitors: Experiments and modeling. Phys. Status Solidi A 2012, 209, 925–934. [Google Scholar] [CrossRef]
- Chand, R.; Han, D.; Neethirajan, S.; Kim, Y.-S. Detection of protein kinase using an aptamer on a microchip integrated electrolyte-insulator-semiconductor sensor. Sens. Actuators B 2017, 248, 973–979. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, S.; Kumar, J.; Panda, S. Investigation of mechanisms involved in the enhanced label free detection of prostate cancer biomarkers using field effect devices. J. Electrochem. Soc. 2017, 164, B409–B416. [Google Scholar] [CrossRef]
- Hlukhova, H.; Menger, M.; Offenhäusser, A.; Vitusevich, S. Highly sensitive aptamer-based method for the detection of cardiac biomolecules on silicon dioxide surfaces. MRS Adv. 2016, 3, 1535–1541. [Google Scholar] [CrossRef]
- Bahri, M.; Baraket, A.; Zine, N.; Ali, M.B.; Bausells, J.; Errachid, A. Capacitance electrochemical biosensor based on silicon nitride transducer for TNF-α cytokine detection in artificial human saliva: Heart failure (HF). Talanta 2020, 209, 12050. [Google Scholar] [CrossRef]
- Pan, T.-M.; Lin, T.-W.; Chen, C.-Y. Label-free detection of rheumatoid factor using YbYxOy electrolyte-insulator-semiconductor devices. Anal. Chim. Acta 2015, 891, 304–311. [Google Scholar] [CrossRef]
- Poghossian, A.; Bäcker, M.; Mayer, D.; Schöning, M.J. Gating capacitive field-effect sensors by the charge of nanoparticle/molecule hybrids. Nanoscale 2015, 7, 1023–1031. [Google Scholar] [CrossRef]
- Jablonski, M.; Poghossian, A.; Severins, R.; Keusgen, M.; Wege, C.; Schöning, M.J. Capacitive field-effect biosensor studying adsorption of tobacco mosaic virus particles. Micromachines 2021, 12, 57. [Google Scholar] [CrossRef]
- Katz, E.; Poghossian, A.; Schöning, M.J. Enzyme-based logic gates and circuits-analytical applications and interfacing with electronics. Anal. Bioanal. Chem. 2017, 409, 81–94. [Google Scholar] [CrossRef]
- Poghossian, A.; Malzahn, K.; Abouzar, M.H.; Mehndiratta, P.; Katz, E.; Schöning, M.J. Integration of biomolecular logic gates with field-effect transducers. Electrochim. Acta 2011, 56, 9661–9665. [Google Scholar] [CrossRef]
- Poghossian, A.; Katz, E.; Schöning, M.J. Enzyme logic AND-Reset and OR-Reset gates based on a field-effect electronic transducer modified with multi-enzyme membrane. Chem. Commun. 2015, 51, 6564–6567. [Google Scholar] [CrossRef]
- Taing, M.; Sweatman, D. Fabrication techniques for an arrayed EIS biosensor. In Proceedings of the Electronics Packaging Technology Conference (IEEE), Singapore, 9–11 December 2009; pp. 168–173. [Google Scholar]
- Abouzar, M.H.; Moritz, W.; Schöning, M.J.; Poghossian, A. Capacitance–voltage and impedance-spectroscopy characteristics of nanoplate EISOI capacitors. Phys. Status Solidi A 2011, 208, 1327–1332. [Google Scholar] [CrossRef]
- Dastidar, S.; Agarwal, A.; Kumar, N.; Bal, V.; Panda, S. Sensitivity enhancement of electrolyte-insulator-semiconductor sensors using mesotextured and nanotextured dielectric surfaces. IEEE Sens. J. 2015, 15, 2039–2045. [Google Scholar] [CrossRef]
- Kaisti, M.; Zhang, Q.; Prabhu, A.; Lehmusvuori, A.; Rahman, A.; Levon, K. An ion-sensitive floating gate FET model: Operating principles and electrofluidic gating. IEEE Trans. Electron Devices 2015, 62, 2628–2635. [Google Scholar] [CrossRef]
- Jayant, K.; Auluck, K.; Funke, M.; Anwar, S.; Phelps, J.B.; Gordon, P.H.; Rajwade, S.R.; Kan, E.C. Programmable ion-sensitive transistor interfaces. I. Electrochemical gating. Phys. Rev. E 2013, 88, 012801. [Google Scholar] [CrossRef] [Green Version]
- Huck, C.; Poghossian, A.; Bäcker, M.; Chaudhuri, S.; Zander, W.; Schubert, J.; Begoyan, V.K.; Buniatyan, V.V.; Wagner, P.; Schöning, M.J. Capacitively coupled electrolyte-conductivity sensor based on high-k material of barium strontium titanate. Sens. Actuators B 2014, 198, 102–109. [Google Scholar] [CrossRef]
- Fabry, P.; Laurent-Yvonnou, L. The C-V method for characterizing ISFET or EOS device with ion-sensitive membranes. J. Electroanal. Chem. 1990, 286, 23–40. [Google Scholar] [CrossRef]
- Siu, W.M.; Cobbold, R.S.C. Basic properties of the electrolyte-SiO2-Si system: Physical and theoretical aspects. IEEE Trans. ED 1979, 26, 1805–1815. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. CMOS-based programmable gate ISFET. Electron. Lett. 2008, 44, 1289–1291. [Google Scholar] [CrossRef]
- Shepherd, L.; Toumazou, C. Weak inversion ISFETs for ultra-low power biochemical sensing and real-time analysis. Sens. Actuators B 2005, 107, 468–473. [Google Scholar] [CrossRef]
- del Cueto, M.E.; Altuzarra, A.C. On the analysis of C-V curves for high resistivity substrates. Solid-State Electron. 1996, 39, 1519–1521. [Google Scholar] [CrossRef]
- Prasad, B.; Lal, R. A capacitive immunosensor measurement system with a lock-in amplifier and potentiostatic control by software. Meas. Sci. Technol. 1999, 10, 1097–1104. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bergveld, P. A critical evaluation of direct electrical protein detection methods. Biosens. Bioelectron. 1991, 6, 55–72. [Google Scholar] [CrossRef] [Green Version]
- van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators B 1995, 24–25, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Winter, R.; Ahn, J.; McIntyre, P.C.; Eizenberg, M. New method for determining flat-band voltage in high mobility semiconductors. J. Vac. Sci. Technol. B 2013, 31, 030604. [Google Scholar] [CrossRef] [Green Version]
- Poghossian, A.; Werner, C.F.; Buniatyan, V.V.; Wagner, T.; Miamoto, K.; Yoshinobu, T.; Schöning, M.J. Towards addressability of light-addressable potentiometric sensors: Shunting effect of non-illuminated region and cross-talk. Sens. Actuators B 2017, 244, 1071–1079. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poghossian, A.; Welden, R.; Buniatyan, V.V.; Schöning, M.J. An Array of On-Chip Integrated, Individually Addressable Capacitive Field-Effect Sensors with Control Gate: Design and Modelling. Sensors 2021, 21, 6161. https://doi.org/10.3390/s21186161
Poghossian A, Welden R, Buniatyan VV, Schöning MJ. An Array of On-Chip Integrated, Individually Addressable Capacitive Field-Effect Sensors with Control Gate: Design and Modelling. Sensors. 2021; 21(18):6161. https://doi.org/10.3390/s21186161
Chicago/Turabian StylePoghossian, Arshak, Rene Welden, Vahe V. Buniatyan, and Michael J. Schöning. 2021. "An Array of On-Chip Integrated, Individually Addressable Capacitive Field-Effect Sensors with Control Gate: Design and Modelling" Sensors 21, no. 18: 6161. https://doi.org/10.3390/s21186161
APA StylePoghossian, A., Welden, R., Buniatyan, V. V., & Schöning, M. J. (2021). An Array of On-Chip Integrated, Individually Addressable Capacitive Field-Effect Sensors with Control Gate: Design and Modelling. Sensors, 21(18), 6161. https://doi.org/10.3390/s21186161






