Differences in Physiological Signals Due to Age and Exercise Habits of Subjects during Cycling Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Data Acquisition
2.2. Experimental Protocol
2.3. Data Analysis
2.3.1. ECG Analysis
2.3.2. EEG Analysis
2.3.3. EMG Analysis
2.3.4. Statistical Analysis
3. Results
3.1. Changes in Heart Rate and AMHRR with Exercise Stages
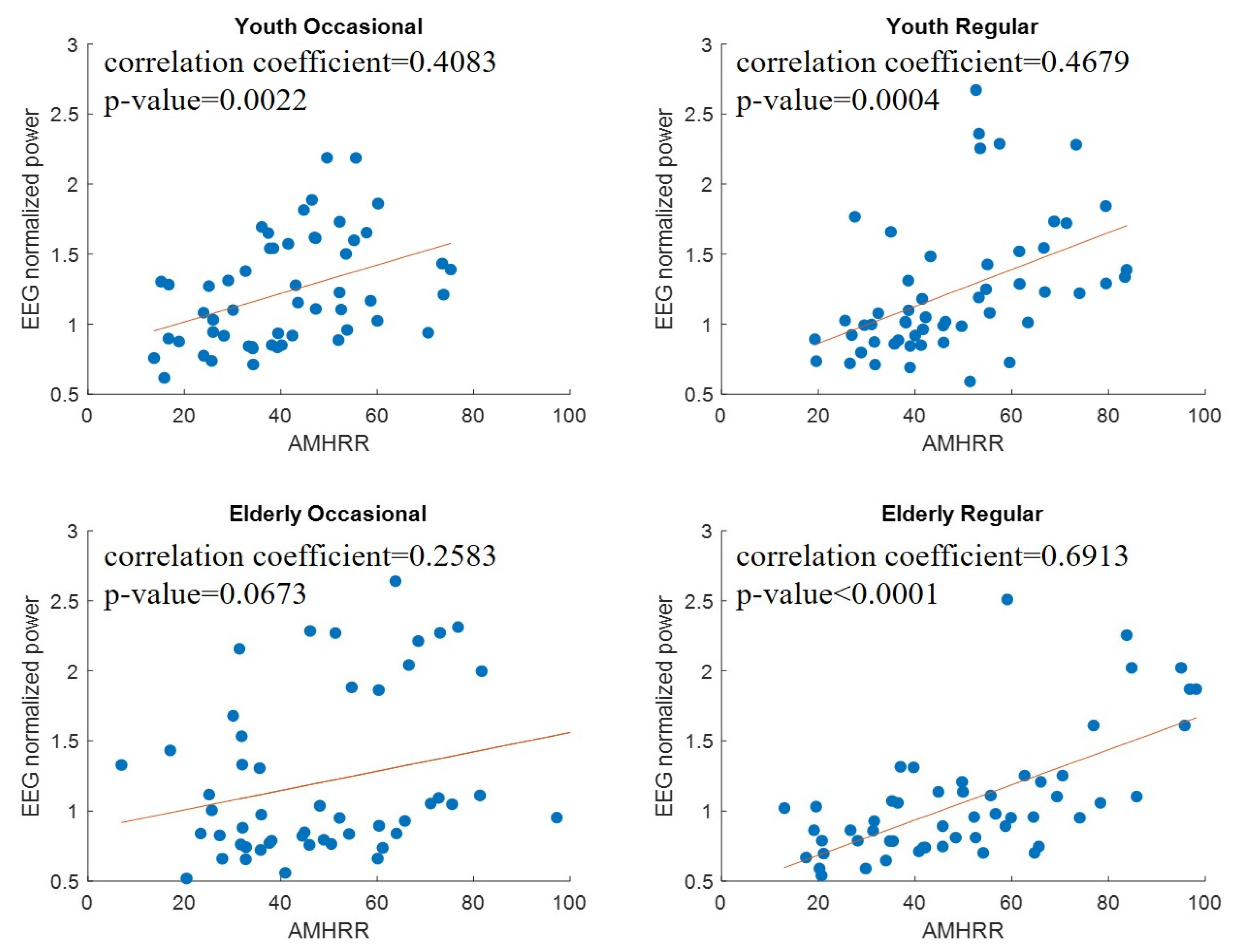
3.2. Changes in EEG during Exercise in Young Participants with and without Exercise Habits
3.3. Changes in EEG during Exercise in Elderly Participants
3.4. Paired t-Test Results Observed during and after Exercise
3.5. Relationship between EMG RMS and AMHRR for the Four Test Groups
4. Discussion
4.1. Spectral Power of EEG Increases with AMHRR during Exercise
4.2. Young People Who Exercise Regularly Have a More Coordinated Use of Their Dominant Leg
4.3. Regular Exercise in Elderly People Induces Significant Cortical Activation during Exercise
4.4. EEG Recovery after Exercise Is Slower in Elderly People
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural contributions to muscle fatigue: From the brain to the muscle and back again. Med. Sci. Sports Exerc. 2016, 48, 2294. [Google Scholar] [CrossRef] [Green Version]
- Buehler, R.; Pucher, J.; Merom, D.; Bauman, A. Active travel in Germany and the US: Contributions of daily walking and cycling to physical activity. Am. J. Prev. Med. 2011, 41, 241–250. [Google Scholar] [CrossRef]
- Bailey, S.P.; Hall, E.E.; Folger, S.E.; Miller, P.C. Changes in EEG during graded exercise on a recumbent cycle ergometer. J. Sports Sci. Med. 2008, 7, 505. [Google Scholar]
- Hottenrott, K.; Taubert, M.; Gronwald, T. Cortical brain activity is influenced by cadence in cyclists. Open Sports Sci. J. 2013, 6, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Enders, H.; Cortese, F.; Maurer, C.; Baltich, J.; Protzner, A.B.; Nigg, B.M. Changes in cortical activity measured with EEG during a high-intensity cycling exercise. J. Neurophysiol. 2016, 115, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Brümmer, V.; Schneider, S.; Strüder, H.K.; Askew, C.D. Primary motor cortex activity is elevated with incremental exercise intensity. Neuroscience 2011, 181, 150–162. [Google Scholar] [CrossRef]
- Brümmer, V.; Schneider, S.; Abel, T.; Vogt, T.; Strueder, H.K. Brain cortical activity is influenced by exercise mode and intensity. Med. Sci. Sports Exerc. 2011, 43, 1863–1872. [Google Scholar] [CrossRef]
- Karvonen, J.; Vuorimaa, T. Heart rate and exercise intensity during sports activities. Sports Med. 1988, 5, 303–311. [Google Scholar] [CrossRef]
- Santos, M.A.A.; Sousa, A.C.S.; Reis, F.P.; Santos, T.R.; Lima, S.O.; Barreto-Filho, J.A. Does the aging process significantly modify the Mean Heart Rate? Arq. Bras. Cardiol. 2013, 101, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Camarda, S.R.D.A.; Tebexreni, A.S.; Páfaro, C.N.; Sasai, F.B.; Tambeiro, V.L.; Juliano, Y.; Barros Neto, T.L.D. Comparison of maximal heart rate using the prediction equations proposed by Karvonen and Tanaka. Arq. Bras. Cardiol. 2008, 91, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Karvonen, J.J.; Kentala, E.; Mustala, O. The Effects of Training on Heart Rate: A “Longitudinal” Study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Homan, R.W.; Herman, J.; Purdy, P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 376–382. [Google Scholar] [CrossRef]
- Goldberg, L.; Elliot, D.L.; Kuehl, K.S. Assessment of exercise intensity formulas by use of ventilatory threshold. Chest 1988, 94, 95–98. [Google Scholar] [CrossRef]
- Daubechies, I. The Wavelet Transform, Time-Frequency Localization and Signal Analysis; Princeton University Press: Princeton, NJ, USA, 2009; pp. 442–486. [Google Scholar]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—A review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Schillings, M.L.; Kalkman, J.S.; Van Der Werf, S.P.; Bleijenberg, G.; van Engelen, B.G.M.; Zwarts, M.J. Central adaptations during repetitive contractions assessed by the readiness potential. Eur. J. Appl. Physiol. 2006, 97, 521–526. [Google Scholar] [CrossRef]
- Crabbe, J.B.; Dishman, R.K. Brain electrocortical activity during and after exercise: A quantitative synthesis. Psychophysiology 2004, 41, 563–574. [Google Scholar] [CrossRef]
- Kamijo, K.; Nishihira, Y.; Hatta, A.; Kaneda, T.; Kida, T.; Higashiura, T.; Kuroiwa, K. Changes in arousal level by differential exercise intensity. Clin. Neurophysiol. 2004, 115, 2693–2698. [Google Scholar] [CrossRef]
- Nielsen, B.; Hyldig, T.; Bidstrup, F.; Gonzalez-Alonso, J.; Christoffersen, G.R.J. Brain activity and fatigue during prolonged exercise in the heat. Pflügers Arch. 2001, 442, 41–48. [Google Scholar] [CrossRef]
- Nybo, L.; Nielsen, B. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J. Appl. Physiol. 2001, 91, 2017–2023. [Google Scholar] [CrossRef]
- Lin, M.A.; Meng, L.F.; Ouyang, Y.; Chan, H.L.; Chang, Y.J.; Chen, S.W.; Liaw, J.W. Resistance-induced brain activity changes during cycle ergometer exercises. BMC Sports Sci. Med. Rehabil. 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Velotta, J.; Weyer, J.; Ramirez, A.; Winstead, J.; Bahamonde, R. Relationship between leg dominance tests and type of task. In Proceedings of the ISBS-Conference Proceedings Archive, Porto, Portugal, 27 June–1 July 2011. [Google Scholar]
- Bhise, S.A.; Patil, N.K. Dominant and Non dominant Leg Activities in Young Adults. Int. J. Ther. 2016, 5, 257–264. [Google Scholar] [CrossRef]
- Carpes, F.; Rossato, M.; Faria, I.; Mota, C.B. During a simulated 40-km cycling time-trial. J. Sports Med. Phys. Fit. 2007, 47, 51–57. [Google Scholar]
- Lepers, R.O.; Hausswirth, C.H.; Maffiuletti, N.I.; Brisswalter, J.E.; Van Hoecke, J. Evidence of neuromuscular fatigue after prolonged cycling exercise. Med. Sci. Sports Exerc. 2000, 32, 1880–1886. [Google Scholar] [CrossRef]
- Iannetta, D.; Passfield, L.; Qahtani, A.; MacInnis, M.J.; Murias, J.M. Interlimb differences in parameters of aerobic function and local profiles of deoxygenation during double-leg and counterweighted single-leg cycling. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2019, 317, R840–R851. [Google Scholar] [CrossRef]
- Nybo, L.; Secher, N.H. Cerebral perturbations provoked by prolonged exercise. Prog. Neurobiol. 2004, 72, 223–261. [Google Scholar] [CrossRef]
- Fell, J.; Williams, A.D. The effect of aging on skeletal-muscle recovery from exercise: Possible implications for aging athletes. J. Aging Phys. Act. 2008, 16, 97–115. [Google Scholar] [CrossRef] [Green Version]




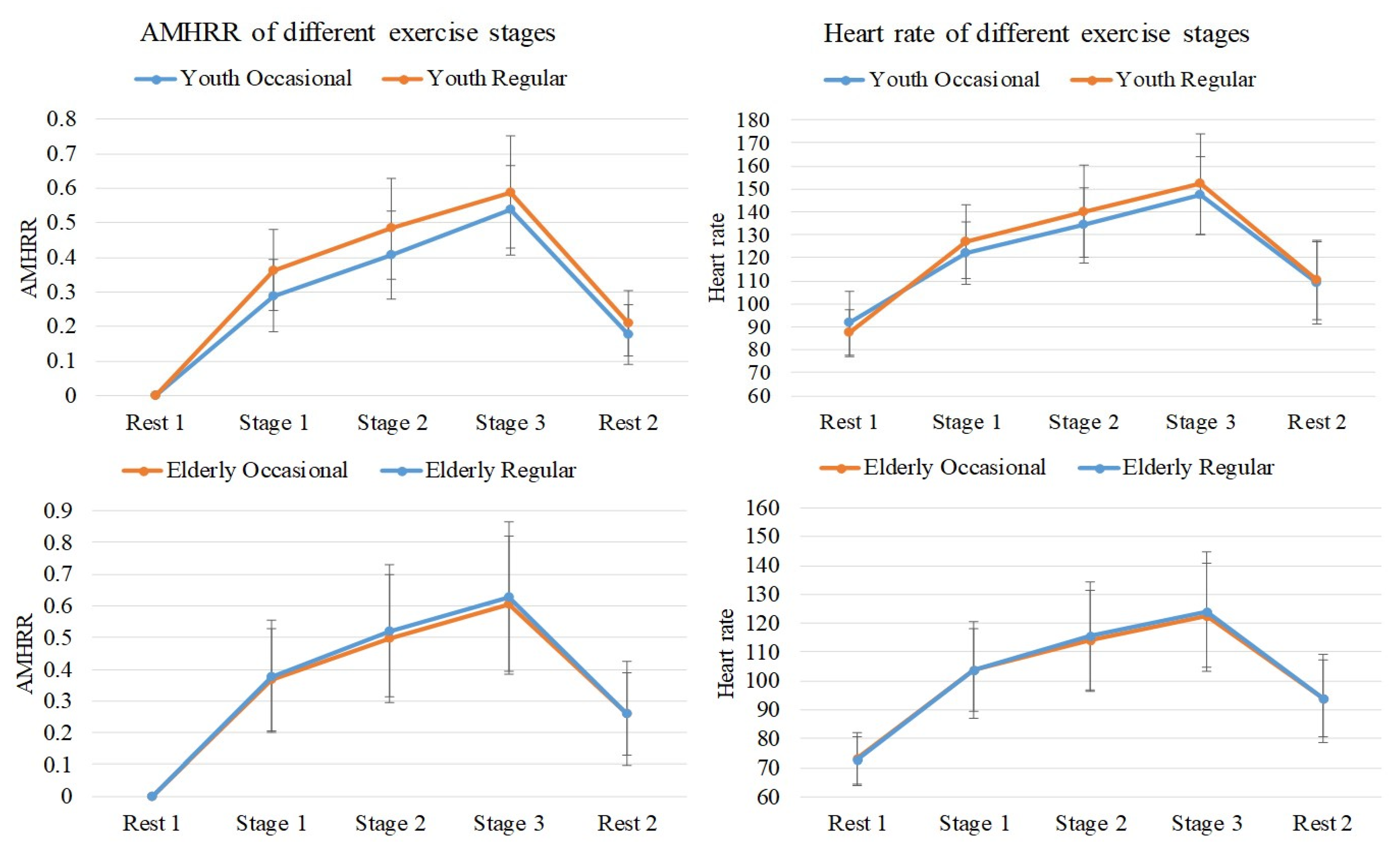


| Heart Rate (BPm) | |||||
|---|---|---|---|---|---|
| Mean | SD | F | Post Hoc Test (p < 0.05) | ||
| Rest 1 | Youth Regular | 87.26 | 10.39 | 14.97 * | Elderly Regular, Occasional |
| Youth Occasional | 91.67 | 13.86 | Elderly Regular, Occasional | ||
| Elderly Regular | 72.57 | 8.18 | Youth Regular, Occasional | ||
| Elderly Occasional | 73.07 | 9.27 | Youth Regular, Occasional | ||
| Stage 1 | Youth Regular | 127.33 | 16.11 | 11.65 * | Elderly Regular, Occasional |
| Youth Occasional | 122.19 | 13.39 | Elderly Regular, Occasional | ||
| Elderly Regular | 103.95 | 16.63 | Youth Regular, Occasional | ||
| Elderly Occasional | 103.65 | 14.23 | Youth Regular, Occasional | ||
| Stage 2 | Youth Regular | 140.34 | 19.90 | 9.35 * | Elderly Regular, Occasional |
| Youth Occasional | 134.19 | 16.38 | Elderly Regular, Occasional | ||
| Elderly Regular | 115.43 | 19.18 | Youth Regular, Occasional | ||
| Elderly Occasional | 114.12 | 17.14 | Youth Regular, Occasional | ||
| Stage 3 | Youth Regular | 152.12 | 21.66 | 10.81 * | Elderly Regular, Occasional |
| Youth Occasional | 147.16 | 17.20 | Elderly Regular, Occasional | ||
| Elderly Regular | 124.22 | 20.69 | Youth Regular, Occasional | ||
| Elderly Occasional | 122.78 | 18.06 | Youth Regular, Occasional | ||
| Rest 2 | Youth Regular | 110.19 | 16.87 | 5.65 | |
| Youth Occasional | 109.42 | 18.10 | |||
| Elderly Regular | 94.03 | 15.45 | |||
| Elderly Occasional | 94.20 | 13.32 | |||
| AMHRR (%) | |||||
|---|---|---|---|---|---|
| Mean | SD | F | Post Hoc Test (p < 0.05) | ||
| Rest 1 | Youth Regular | ||||
| Youth Occasional | |||||
| Elderly Regular | |||||
| Elderly Occasional | |||||
| Stage 1 | Youth Regular | 36.33 | 11.79 | 1.47 | |
| Youth Occasional | 28.29 | 10.36 | |||
| Elderly Regular | 37.78 | 17.69 | |||
| Elderly Occasional | 36.89 | 16.17 | |||
| Stage 2 | Youth Regular | 48.33 | 14.58 | 1.41 | |
| Youth Occasional | 40.72 | 12.84 | |||
| Elderly Regular | 51.98 | 20.87 | |||
| Elderly Occasional | 49.79 | 20.29 | |||
| Stage 3 | Youth Regular | 58.96 | 16.26 | 0.71 | |
| Youth Occasional | 53.69 | 12.83 | |||
| Elderly Regular | 62.54 | 24.02 | |||
| Elderly Occasional | 60.56 | 21.28 | |||
| Rest 2 | Youth Regular | 20.95 | 9.60 | 1.96 | |
| Youth Occasional | 17.59 | 8.66 | |||
| Elderly Regular | 26.08 | 16.53 | |||
| Elderly Occasional | 25.86 | 13.06 | |||
| Delta | Theta | L-Alpha | H-Alpha | L-Beta | H-Beta | Gamma | ||
|---|---|---|---|---|---|---|---|---|
| C3 | Youth Occasional | 0.1787 (0.1959) | 0.2137 (0.1207) | 0.4083 (0.0022) | 0.4699 (0.0003) | 0.3848 (0.0041) | 0.2407 (0.0796) | 0.1770 (0.2004) |
| Youth Regular | 0.4426 (0.0008) | 0.4215 (0.0015) | 0.4679 (0.0004) | 0.4493 (0.0007) | 0.3831 (0.0042) | 0.2130 (0.1220) | 0.1404 (0.3113) | |
| Elderly Occasional | 0.3759 (0.0066) | 0.2405 (0.0892) | 0.2583 (0.0673) | 0.2672 (0.0580) | 0.2463 (0.0815) | 0.2088 (0.1414) | 0.2381 (0.0925) | |
| Elderly Regular | 0.7037 (<0.0001) | 0.6519 (<0.0001) | 0.6913 (<0.0001) | 0.6441 (<0.0001) | 0.5516 (<0.0001) | 0.5376 (<0.0001) | 0.5284 (<0.0001) | |
| C4 | Youth Occasional | 0.2498 (0.0685) | 0.2907 (0.0329) | 0.4961 (0.0001) | 0.5641 (<0.0001) | 0.4637 (0.0004) | 0.3110 (0.0221) | 0.2288 (0.0961) |
| Youth Regular | 0.2103 (0.1268) | 0.2221 (0.1065) | 0.3645 (0.0067) | 0.3643 (0.0068) | 0.2607 (0.0569) | 0.0716 (0.6068) | −0.0280 (0.8406) | |
| Elderly Occasional | 0.3585 (0.0098) | 0.2222 (0.1170) | 0.2012 (0.1569) | 0.2087 (0.1417) | 0.2184 (0.1237) | 0.1866 (0.1899) | 0.2366 (0.0947) | |
| Elderly Regular | 0.6107 (<0.0001) | 0.6164 (<0.0001) | 0.6644 (<0.0001) | 0.6070 (<0.0001) | 0.3623 (0.0071) | 0.2948 (0.0305) | 0.2788 (0.0412) | |
| Cz | Youth Occasional | 0.1729 (0.2112) | 0.2076 (0.1319) | 0.3869 (0.0038) | 0.4639 (0.0004) | 0.3673 (0.0063) | 0.2385 (0.0824) | 0.1706 (0.2176) |
| Youth Regular | 0.2310 (0.0929) | 0.2845 (0.0371) | 0.4181 (0.0017) | 0.4113 (0.0020) | 0.3397 (0.0120) | 0.1716 (0.2146) | 0.0650 (0.6404) | |
| Elderly Occasional | 0.3904 (0.0046) | 0.2652 (0.0600) | 0.2624 (0.0629) | 0.2700 (0.0553) | 0.2668 (0.0584) | 0.2342 (0.0981) | 0.2798 (0.0468) | |
| Elderly Regular | 0.4380 (0.0009) | 0.4607 (0.0005) | 0.5498 (<0.0001) | 0.5505 (<0.0001) | 0.4959 (0.0001) | 0.5027 (0.0001) | 0.4986 (0.0001) |
| Delta | Theta | L-Alpha | H-Alpha | L-Beta | H-Beta | Gamma | ||
|---|---|---|---|---|---|---|---|---|
| C3 | Youth Occasional | p = 0.0861 | p = 0.0872 | p = 0.1032 | p = 0.0545 | p = 0.0093 | p = 0.0272 | p = 0.0064 |
| Youth Regular | p < 0.001 | p = 0.0011 | p = 0.6801 | p = 0.5305 | p = 0.0023 | p = 0.0011 | p < 0.001 | |
| Elderly Occasional | p = 0.0122 | p = 0.0621 | p = 0.9527 | p = 0.9249 | p = 0.6933 | p = 0.1791 | p = 0.0027 | |
| Elderly Regular | p = 0.1019 | p = 0.6662 | p = 0.0180 | p = 0.0324 | p = 0.1596 | p = 0.3657 | p = 0.3446 | |
| C4 | Youth Occasional | p = 0.0658 | p = 0.0499 | p = 0.0738 | p = 0.0348 | p = 0.0049 | p = 0.0151 | p = 0.0034 |
| Youth Regular | p < 0.001 | p = 0.0022 | p = 0.4626 | p = 0.4761 | p = 0.0021 | p < 0.001 | p < 0.001 | |
| Elderly Occasional | p = 0.0240 | p = 0.0543 | p = 0.7284 | p = 0.8185 | p = 0.5308 | p = 0.1379 | p = 0.0017 | |
| Elderly Regular | p = 0.0122 | p = 0.4295 | p = 0.0197 | p = 0.0569 | p = 0.5484 | p = 0.9590 | p = 0.1634 | |
| Cz | Youth Occasional | p = 0.0808 | p = 0.0902 | p = 0.1752 | p = 0.0640 | p = 0.0106 | p = 0.0252 | p = 0.0045 |
| Youth Regular | p = 0.0015 | p = 0.0211 | p = 0.9572 | p = 0.8219 | p = 0.0109 | p = 0.0037 | p < 0.001 | |
| Elderly Occasional | p = 0.0254 | p = 0.0952 | p = 0.8433 | p = 0.8706 | p = 0.9117 | p = 0.4567 | p = 0.0040 | |
| Elderly Regular | p = 0.1706 | p = 0.3402 | p = 0.6988 | p = 0.6412 | p = 0.7912 | p = 0.6926 | p = 0.0321 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-Y.; Jao, C.-W.; Wang, P.-S.; Liou, M.; Wu, J.-L.; Chun, H.; Tseng, C.-T.; Wu, Y.-T. Differences in Physiological Signals Due to Age and Exercise Habits of Subjects during Cycling Exercise. Sensors 2021, 21, 7220. https://doi.org/10.3390/s21217220
Lin S-Y, Jao C-W, Wang P-S, Liou M, Wu J-L, Chun H, Tseng C-T, Wu Y-T. Differences in Physiological Signals Due to Age and Exercise Habits of Subjects during Cycling Exercise. Sensors. 2021; 21(21):7220. https://doi.org/10.3390/s21217220
Chicago/Turabian StyleLin, Szu-Yu, Chi-Wen Jao, Po-Shan Wang, Michelle Liou, Jun-Liang Wu, Hsiao Chun, Ching-Ting Tseng, and Yu-Te Wu. 2021. "Differences in Physiological Signals Due to Age and Exercise Habits of Subjects during Cycling Exercise" Sensors 21, no. 21: 7220. https://doi.org/10.3390/s21217220
APA StyleLin, S.-Y., Jao, C.-W., Wang, P.-S., Liou, M., Wu, J.-L., Chun, H., Tseng, C.-T., & Wu, Y.-T. (2021). Differences in Physiological Signals Due to Age and Exercise Habits of Subjects during Cycling Exercise. Sensors, 21(21), 7220. https://doi.org/10.3390/s21217220






