A High-Density EEG Study Investigating VR Film Editing and Cognitive Event Segmentation Theory
Abstract
:1. Introduction
- Electroencephalograms (EEG) and visual evoked potential (VEP) analysis were performed, and the standardized low resolution brain electromagnetic tomography algorithm (sLORETA) traceability method was used to analyze the data.
- It was found that different editing techniques lead to different physiological and psychological indicators of viewers, while the physiological and psychological perception results tend to be the same.
- It is proven that the cognitive event segmentation theory also plays an instructive role in virtual reality editing. Even though VR movies are different from traditional movies in terms of presentation and viewing style to a certain extent, viewers’ perception of events in edited VR movies is similar to that of traditional photography. The experimental results will provide an experimental reference for VR movie research and necessary theoretical support for VR movie editing. Therefore, it has good academic and application value.
2. Materials and Methods
2.1. The Participants
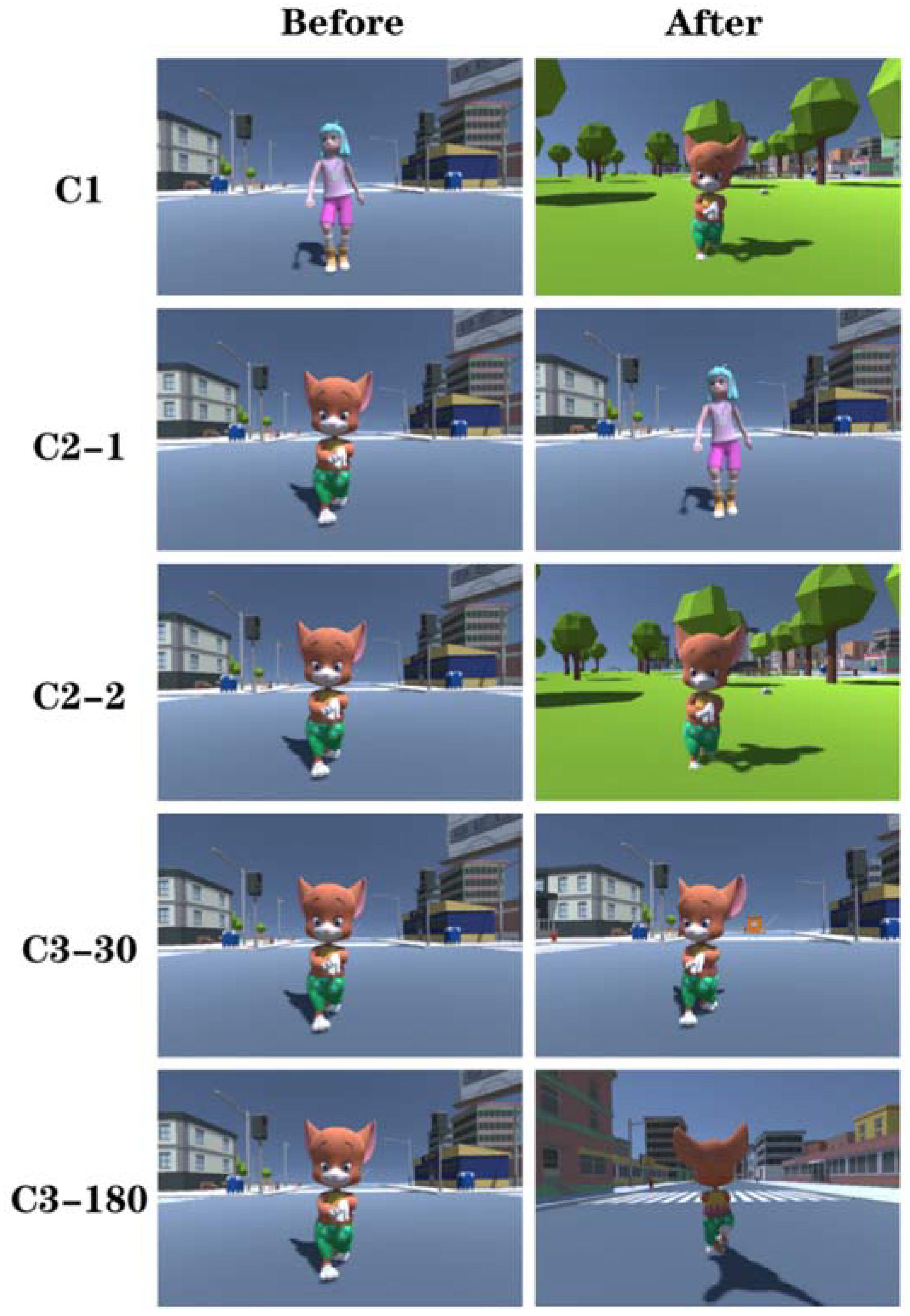
2.2. Experimental Materials and Hardware Equipment
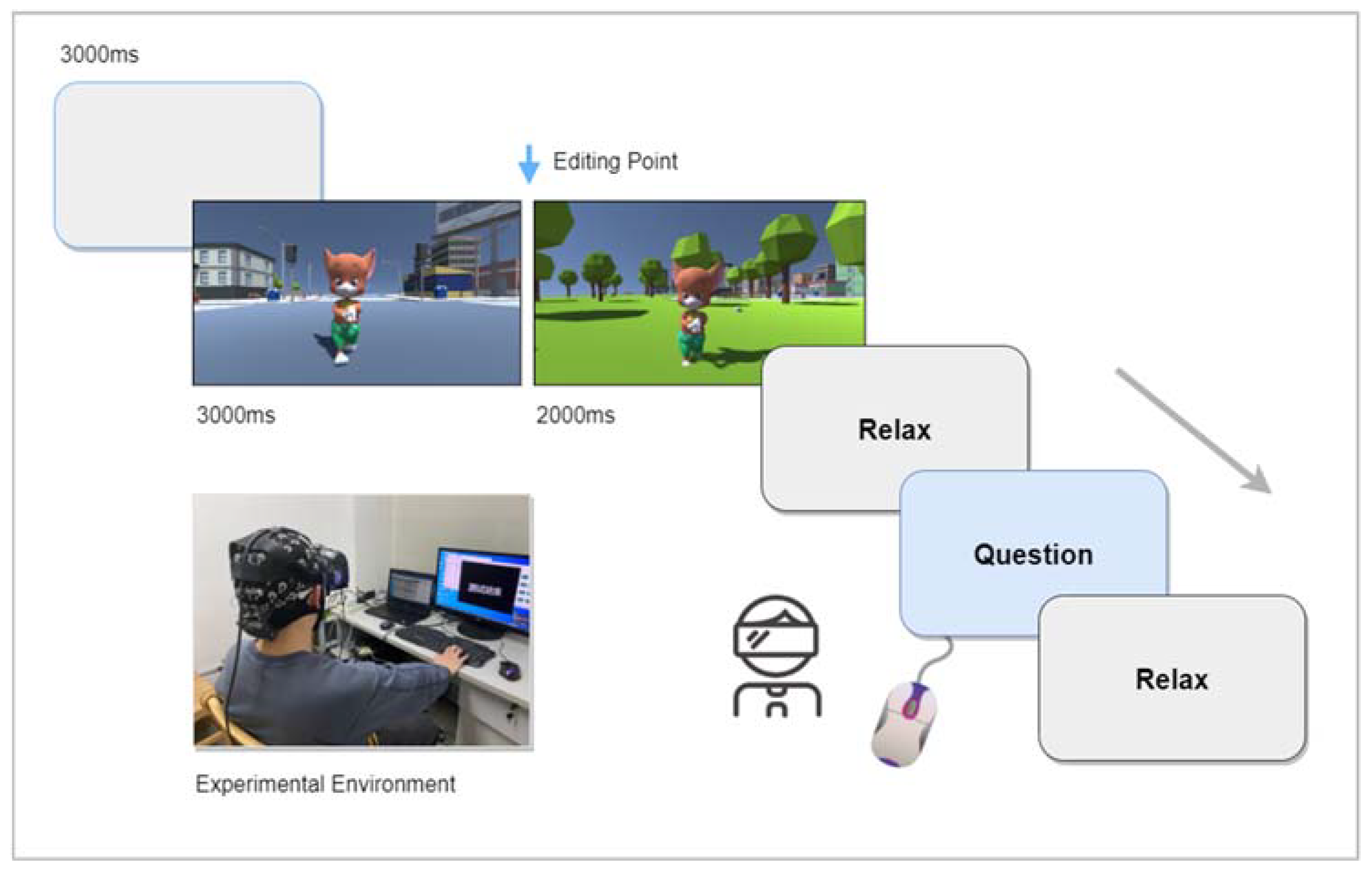
2.3. Experimental Procedure
2.4. Data Recording and Processing
2.5. Verification of Differences in VR Editing Methods Based on SVM
3. Results
3.1. Subjective Data
3.2. Classification Results
3.3. EEG Data Analysis
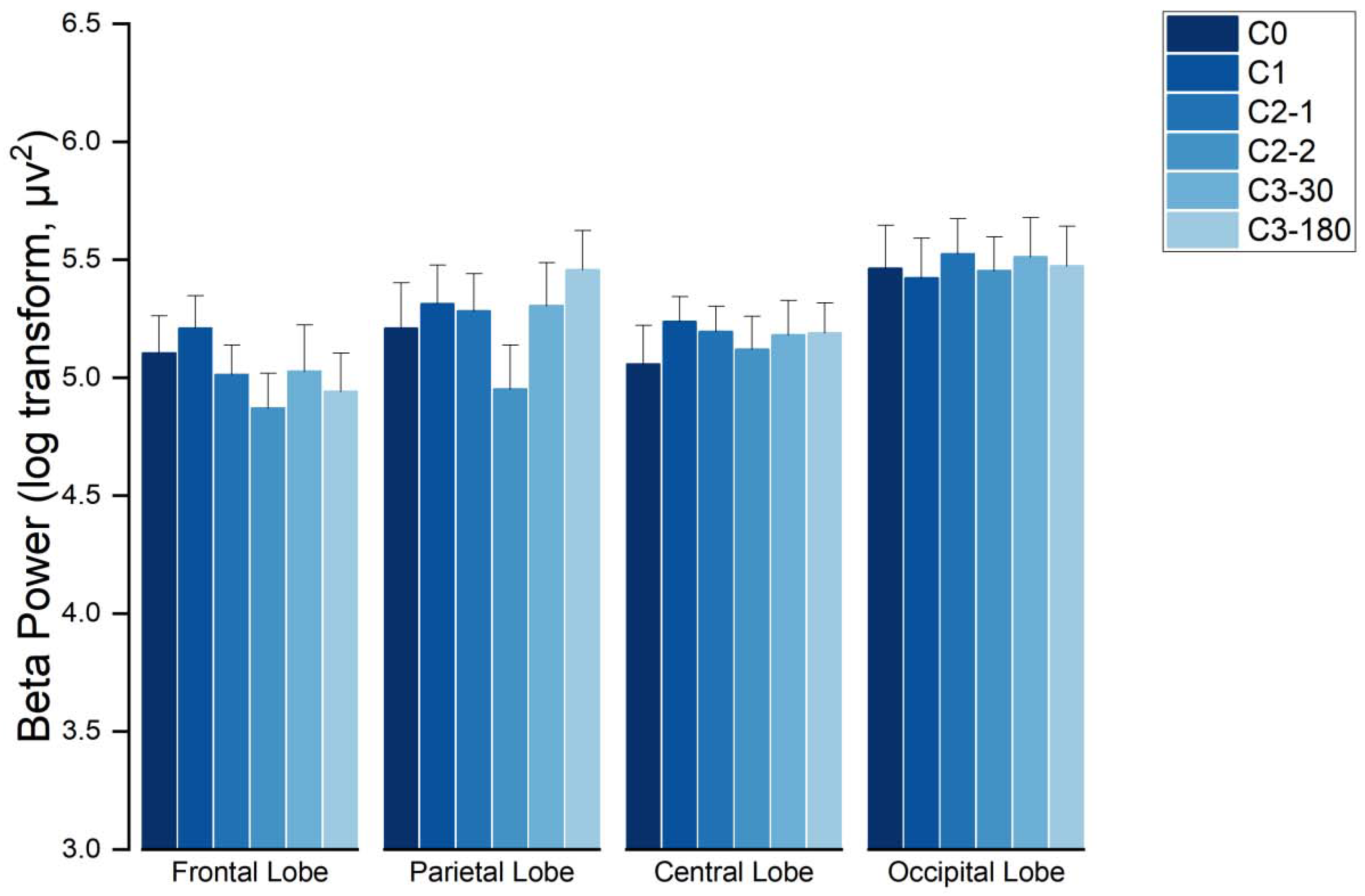
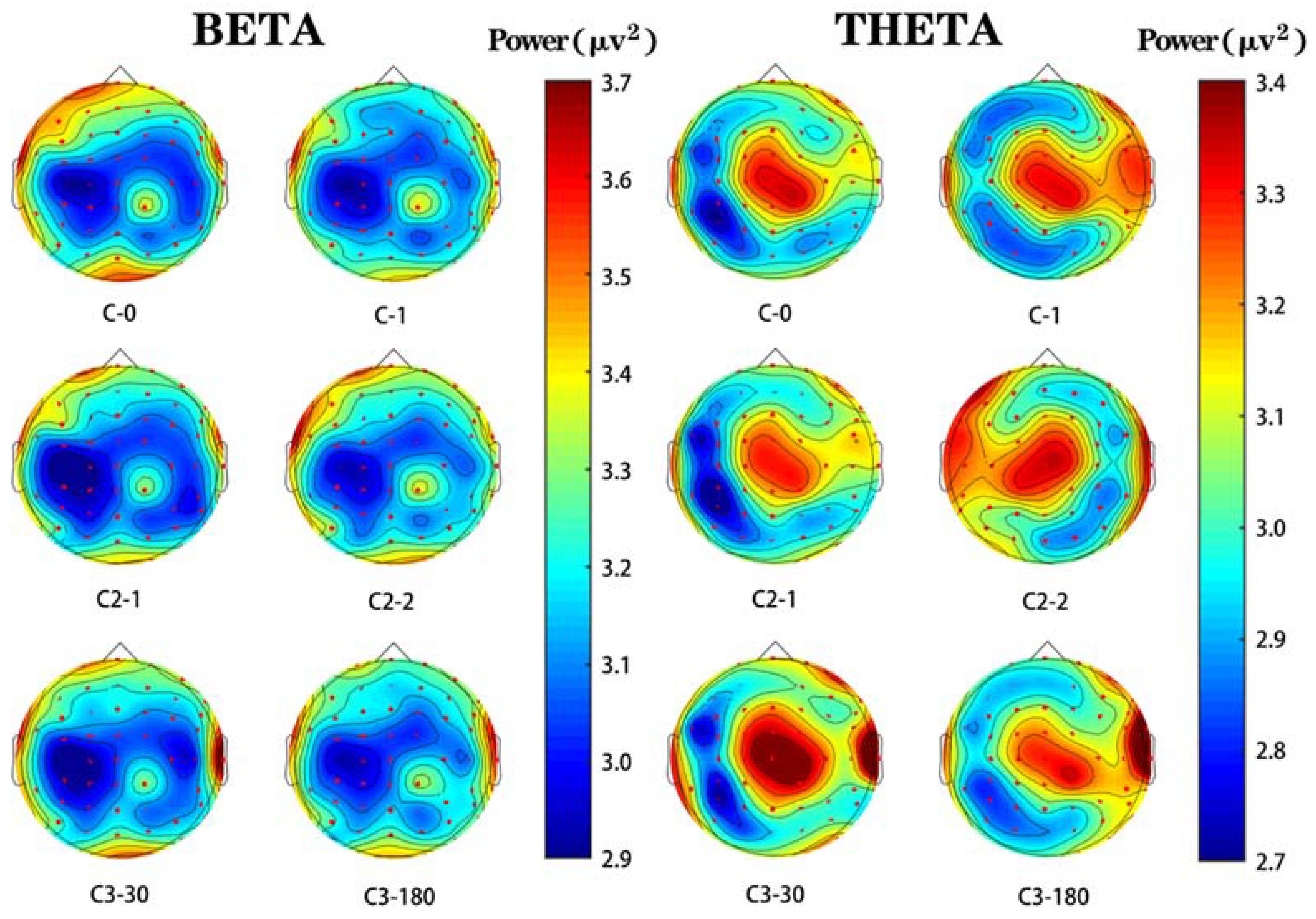
3.3.1. Characterization of EEG Spectra in the Beta Wave Frequency Band
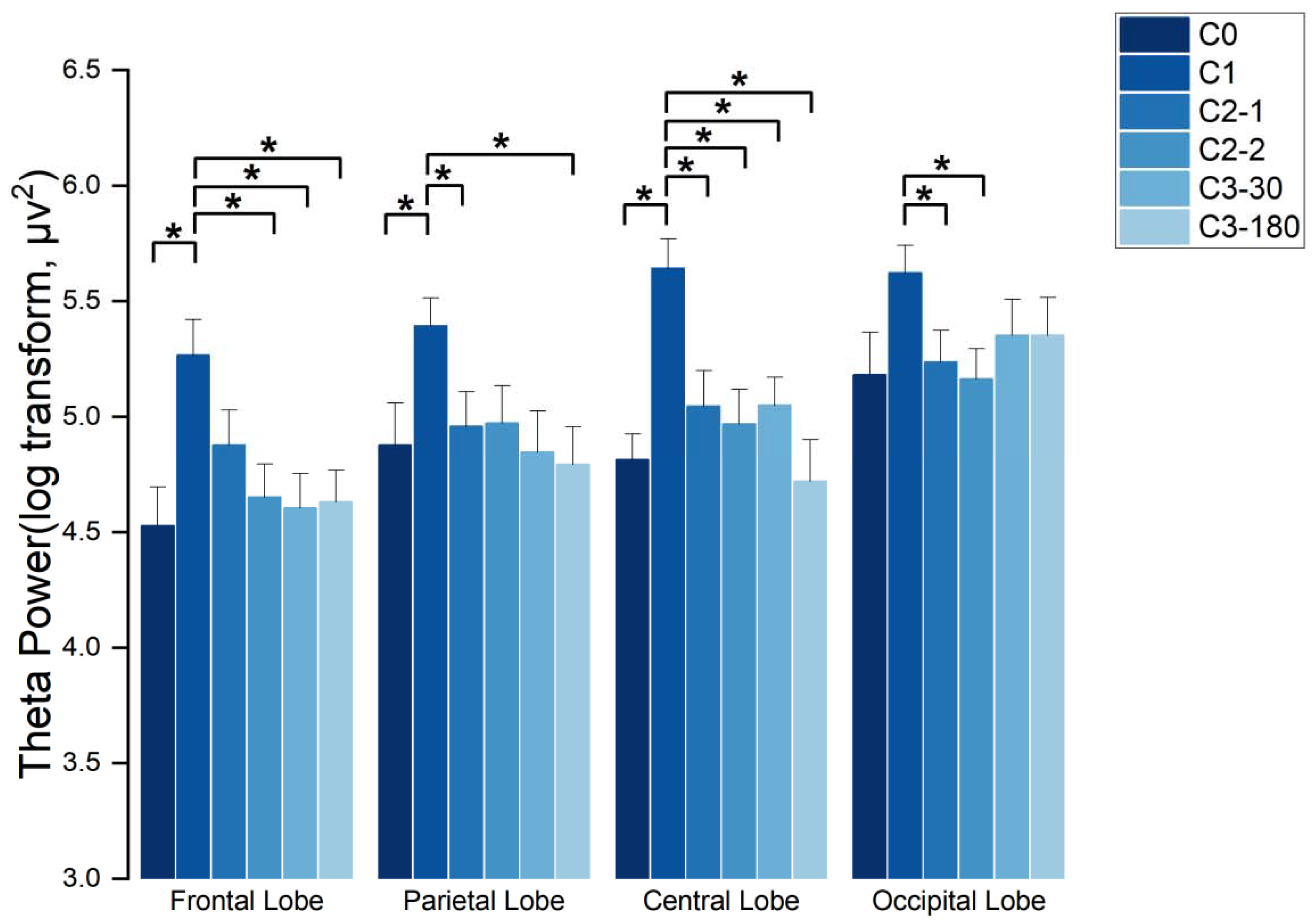
3.3.2. Characterization of EEG Spectra in the Theta Wave Frequency Band
3.3.3. Brain Topography
3.3.4. VEP Data Analysis
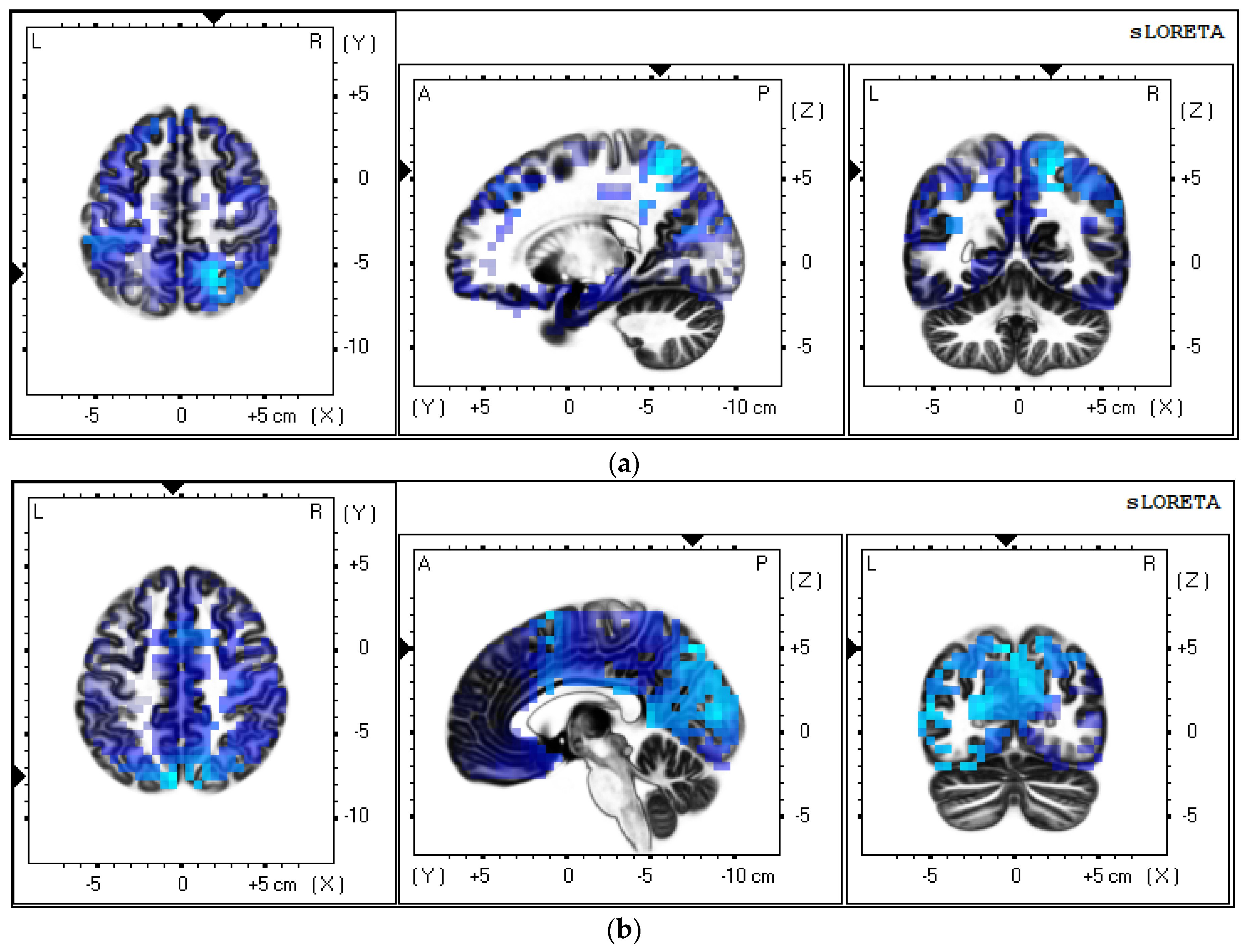
3.3.5. VEP Sources Analysis
4. Discussion
4.1. Subjective Rating
4.2. EEG Results
4.3. VEP Results
4.4. VEP Sources Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, N.; Zhou, W.; Fung, A.Y.H. Emotional effect of cinematic VR compared with traditional 2D film. J. Telemat. Inform. 2018, 35, 1572–1579. [Google Scholar] [CrossRef]
- Bordwell, D.; Thompson, K.J.F.A.A.I. Film Art: An Introduction; McGraw-Hill: New York, NY, USA, 2011; Volume 24, pp. 260–265. [Google Scholar]
- Kraft, R.N. The role of cutting in the evaluation and retention of film. J. Exp. Psychol. Learn. Mem. Cogn. 1986, 12, 155–163. [Google Scholar] [CrossRef]
- Serrano, A.; Sitzmann, V.; Ruiz-Borau, J.; Wetzstei, G.; Gutierrez, D.; Masia, B. Movie Editing and Cognitive Event Segmentation in Virtual Reality Video. Acm Trans. Graph. 2017, 36, 1–12. [Google Scholar] [CrossRef]
- Kachkovski, G.; Vasilyev, D.; Kuk, M.; Kingstone, A.; Street, C. Exploring the Effects of Violating the 180-Degree Rule on Film Viewing Preferences. Commun. Res. 2019, 46, 948–964. [Google Scholar] [CrossRef]
- Guiming, Z. VR film’s reconfiguration of the narrative features of images–The example of My Brother’s Keeper. J. Media 2019, 23, 44–46. [Google Scholar]
- Hasson, U.; Landesman, O.; Knappmeyer, B.; Vallines, I.; Rubin, N.; Heeger, D.J. Neurocinematics: The Neuroscience of Film. J. Proj. 2008, 2, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Gorini, A. The Role of Immersion and Narrative in Mediated Presence: The Virtual Hospital Experience. J. Cyberpsychology Behav. Soc. Netw. 2011, 14, 99–105. [Google Scholar] [CrossRef]
- Javier, M.; Carmen, L.; Jaime, G.; Mariano, A. Emotion Recognition in Immersive Virtual Reality: From Statistics to Affective Computing. Sensors 2020, 20, 5163. [Google Scholar]
- Katrin, H.; Alessandra, U.M.; Michele, G.; Vittorio, G. Moving mirrors: A high-density EEG study investigating the effect of camera movements on motor cortex activation during action observation. J. Cogn. Neurosci. 2014, 26, 2087–2101. [Google Scholar]
- Yao, D. A method to standardize a reference of scalp EEG recordings to a point at infinity. J. Physiol. Meas. 2001, 22, 693. [Google Scholar] [CrossRef]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Lang, A. Involuntary Attention and Physiological Arousal Evoked by Structural Features and Emotional Content in TV Commercials. J. Commun. Res. 1990, 17, 275–299. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharm. 2002, 24 (Suppl. D), 5–12. [Google Scholar]
- Lorenzo-Lopez, L.; Amenedo, E.; Pascual-Marqui, R.D.; Cadaveira, F. Neural correlates of age-related visual search decline: A combined ERP and sLORETA study. Neuroimage 2008, 41, 511–524. [Google Scholar] [CrossRef]
- Lang, A.; Bolls, P.; Potter, R.F.; Kawahara, K. The effects of production pacing and arousing content on the information processing of television messages. J. Broadcasting Electron. Media 1999, 43, 451–475. [Google Scholar] [CrossRef]
- Anderson, D.R.; Fite, K.V.; Petrovich, N.; Hirsch, J. Cortical Activation While Watching Video Montage: An fMRI Study. J. Media Psychol. 2006, 8, 7–24. [Google Scholar] [CrossRef]
- Kweon, S.H.; Kweon, H.J.; Kim, S.-j.; Li, X.; Liu, X.; Kweon, H.L. A Brain Wave Research on VR (Virtual Reality) Usage: Comparison Between VR and 2D Video in EEG Measurement. In Advances in Human Factors and Systems Interaction, Advances in Intelligent Systems and Computing, Proceedings of the AHFE 2017 International Conference on Human Factors and Systems Interaction, Los Angeles, CA, USA, 17−21 July 2017; Springer: Berlin/Heidelberg, Germany, 2018; Volume 592, pp. 194–203. [Google Scholar]
- Norrgrann, A.M. Analysis of event related potentials in a word recognition experiment with fluent and dysfluent readers. J. Contact Dermat. 2014, 49, 124–132. [Google Scholar]
- Chang, W. Virtual Reality Filmmaking Methodology (Animation Producing). TECHART J. Arts Imaging Sci. 2016, 3, 23. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Kim, J.; Shin, Y.-S.; Jang, D.-P.; Kim, S.-P. Coherent neural responses of human populations during watching movie. In 2014 14th International Conference on Control, Automation and Systems (ICCAS 2014), Proceedings of the 14th International Conference on Control, Automation and Systems (ICCAS 2014), Gyeonggi-do, Korea, 22–25 October 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 272–274. [Google Scholar]
- Feng, T.; Shuaifan, D.; Yan, Z. Model construction of VR image depth jumping and viewers’ perceived reaction time. J. Shanghai Univ. (Nat. Sci. Ed.) 2020, 26, 702–714. [Google Scholar]
- Lee, J.; Kim, B.; Kim, K.; Kim, Y.; Noh, J. Rich360: Optimized Spherical Representation from Structured Panoramic Camera Arrays. ACM Trans. Graph. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Celine, T. Virtual Reality Filmmaking: Techniques & Best Practices for VR Filmmakers; Taylor and Francis: Abingdon, UK, 2017. [Google Scholar]
- Nielsen, L.T.; Møller, M.B.; Hartmeyer, S.D.; Ljung, T.C.M.; Nilsson, N.C.; Nordahl, R.; Serafifin, S. Missing the point: An exploration of how to guide users’ attention during cinematic virtual reality. In Proceedings of the 22nd ACM Conference on Virtual Reality Software and Technology, Munich, Germany, 2–4 November 2016. [Google Scholar]
- Syrett, H.; Calvi, L.; van Gisbergen, M. The Oculus Rift Film Experience: A Case Study on Understanding Films in a Head Mounted Display. In Intelligent Technologies for Interactive Entertainment, Intetain 2016; Poppe, R., Meyer, J.J., Veltkamp, R., Dastani, M., Eds.; Lecture Notes of the Institute for Computer Sciences Social Informatics and Telecommunications Engineering; Springer: Berlin/Heidelberg, Germany, 2017; Volume 178, pp. 197–208. [Google Scholar]
- Rothe, S.; Buschek, D.; Hussmann, H. Guidance in Cinematic Virtual Reality-Taxonomy, Research Status and Challenges. Multimodal Technol. Interact. 2019, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Heimann, K.S.; Uithol, S.; Calbi, M.; Umilta, M.A.; Guerra, M.; Gallese, V. “Cuts in Action”: A High-Density EEG Study Investigating the Neural Correlates of Different Editing Techniques in Film. Cogn. Sci. 2017, 41, 1555–1588. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. In Advances in Psychology; Hancock, P.A., Meshkati, N., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 52, pp. 139–183. [Google Scholar]
- Schubert, T.; Friedmann, F.; Regenbrecht, H. The Experience of Presence: Factor Analytic Insights. J. Presence: Teleoperators Virtual Environ. 2001, 10, 266–281. [Google Scholar] [CrossRef]
- Tian, F.; Hua, M.; Zhang, W.; Li, Y.; Yang, X.J.P.O. Emotional arousal in 2D versus 3D virtual reality environments. PLoS ONE 2021, 16, e0256211. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.; Couceiro, R.; Duarte, G.; Durães, J.; Castelhano, J.; Duarte, C.; Castelo-Branco, M.; Madeira, H.; de Carvalho, P.; Teixeira, C. Can EEG Be Adopted as a Neuroscience Reference for Assessing Software Programmers’ Cognitive Load? Sensors 2021, 21, 2338. [Google Scholar] [CrossRef]
- James, C.J.; Hesse, C.W.J.P.M. Independent component analysis for biomedical signals. Physiol. Meas. 2005, 26, R15. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Z.G.; Yang, Y.F. (Eds.) EEG Signal Processing and Feature Extraction; Chinese Academy of Sciences University Graduate Textbook Series; Science Press: Beijing, China, 2020. [Google Scholar]
- Chella, F.; Pizzella, V.; Zappasodi, F.; Marzetti, L. Impact of the reference choice on scalp EEG connectivity estimation. J. Neural Eng. 2016, 13, 036016. [Google Scholar] [CrossRef]
- Berta, R.; Bellotti, F.; De Gloria, A.; Pranantha, D.; Schatten, C. Electroencephalogram and Physiological Signal Analysis for Assessing Flow in Games. IEEE Trans. Comput. Intell. Ai Games 2013, 5, 164–175. [Google Scholar] [CrossRef]
- Sun, H.; Yang, X.; Sun, Y.; Zhu, H.; Zeng, J. On-line EEG classification for brain-computer interface based on CSP and SVM. In 2010 3rd International Congress on Image and Signal Processing, Proceedings of the 3rd International Congress on Image and Signal Processing, Yantai, China, 16–18 October 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Hui, W.; Lei, Z.; Longxu, Y. Application of genetic algorithm based support vector machine in selection of new EEG rhythms for drowsiness detection. J. Expert Syst. Appl. 2021, 171, 114634. [Google Scholar]
- Lee, S.H.; Matteson, A.; Hooshyar, D.; Kim, S.H.; Lim, H.S. Comparing Programming Language Comprehension between Novice and Expert Programmers Using EEG Analysis. In 2016 IEEE 16th International Conference on Bioinformatics and Bioengineering (BIBE), Proceedings of the IEEE International Conference on Bioinformatics & Bioengineering, 31 October–2 November 2016, Taichung, Taiwan; IEEE: Piscataway, NJ, USA, 2016. [Google Scholar]
- Jensen, E. Brain-Based Learning–The New Science of Teaching and Training (Revised Edition); Corwin Press: Thousand Oaks, CA, USA, 2000. [Google Scholar]
- Ray, W.; Cole, H.J.S. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science 1985, 228, 750–752. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Shackman, A.J. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J. Physiol. 2015, 109, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamińskia, J.; Brzezickab, A.; Golac, M.; Wróbela, A. Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012, 85, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psychophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Kiiski, H.; Bennett, M.; Rueda-Delgado, L.M.; Farina, F.R.; Knight, R.; Boyle, R.; Roddy, D.; Grogan, K.; Bramham, J.; Kelly, C.; et al. EEG spectral power, but not theta/beta ratio, is a neuromarker for adult ADHD. Eur. J. Neurosci. 2020, 51, 2095–2109. [Google Scholar] [CrossRef]
- Hasan, R.A.; Sulaiman, S.; Ashykin, N.; Abdullah, M.N.; Ali, S.J.S. Workplace Mental State Monitoring during VR-Based Training for Offshore Environment. Sensors 2021, 21, 4885. [Google Scholar] [CrossRef]
- Vukelić, M.; Gharabaghi, A. Oscillatory entrainment of the motor cortical network during motor imagery is modulated by the feedback modality. NeuroImage 2015, 111, 1–11. [Google Scholar] [CrossRef]
- Kosti, M.V.; Georgiadis, K.; Adamos, D.A.; Laskaris, N.; Spinellis, D.; Angelis, L. Towards an affordable brain computer interface for the assessment of programmers’ mental workload. Int. J. Hum. Comput. Stud. 2018, 115, 52–66. [Google Scholar] [CrossRef]
- Maffongelli, L.; Bartoli, E.; Sammler, D.; Kolsch, S.; Campus, C.; Olivier, E.; Fadiga, L.; D’Ausilio, A. Distinct brain signatures of content and structure violation during action observation. Neuropsychologia 2015, 75, 30–39. [Google Scholar] [CrossRef]
- Jansma, J.M.; Ramsey, N.F.; Coppola, R.; Kahn, R.S. Specific versus Nonspecific Brain Activity in a Parametric N-Back Task. NeuroImage 2000, 12, 688–697. [Google Scholar] [CrossRef] [Green Version]


| Group | Load | Immersion | ||
|---|---|---|---|---|
| C0 | 184.72 | 81.385 | 4.400 | 9.3941 |
| C1 | 195.86 | 83.635 | −3.467 | 10.8841 |
| C2-1 | 212.99 | 103.279 | −4.167 | 11.9340 |
| C2-2 | 231.94 | 102.753 | −1.800 | 11.1244 |
| C3-30 | 225.90 | 115.002 | −2.600 | 12.7863 |
| C3-180 | 236.20 | 104.177 | −6.333 | 13.6895 |
| Classifier | Frequency Band | Accuracy |
|---|---|---|
| SVM | θ | 52.191% |
| α | 19.203% | |
| β | 87.012% | |
| θ + α | 19.402% | |
| α + β | 92.590% | |
| θ + β | 22.948% |
| Talairach Coordinate(TAL) | Brodmann Area | Lobe | Structure | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| −54 | 20 | 4 | 45 | Frontal Lobe | Inferior Frontal Gyrus |
| −54 | 34 | −2 | 47 | Frontal Lobe | Inferior Frontal Gyrus |
| Talairach Coordinate(TAL) | Brodmann Area | Lobe | Structure | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| −5 | −70 | 50 | 7 | Parietal Lobe | Precuneus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Wang, H.; Cheng, W.; Zhang, W.; Li, Y. A High-Density EEG Study Investigating VR Film Editing and Cognitive Event Segmentation Theory. Sensors 2021, 21, 7176. https://doi.org/10.3390/s21217176
Tian F, Wang H, Cheng W, Zhang W, Li Y. A High-Density EEG Study Investigating VR Film Editing and Cognitive Event Segmentation Theory. Sensors. 2021; 21(21):7176. https://doi.org/10.3390/s21217176
Chicago/Turabian StyleTian, Feng, Hui Wang, Wanqiu Cheng, Wenrui Zhang, and Yingjie Li. 2021. "A High-Density EEG Study Investigating VR Film Editing and Cognitive Event Segmentation Theory" Sensors 21, no. 21: 7176. https://doi.org/10.3390/s21217176
APA StyleTian, F., Wang, H., Cheng, W., Zhang, W., & Li, Y. (2021). A High-Density EEG Study Investigating VR Film Editing and Cognitive Event Segmentation Theory. Sensors, 21(21), 7176. https://doi.org/10.3390/s21217176





