Optical Coherence Tomography in the Differential Diagnosis of Patients with Multiple Sclerosis and Patients with MRI Nonspecific White Matter Lesions
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Data Collection
2.2.1. Magnetic Resonance Imaging
2.2.2. Optical Coherence Tomography
- -
- Quality index of the scan > 6—all scans included in the study;
- -
- Quality index of the scan < 6—only scans with a clear and complete examination area included; and
- -
- Quality index of the scan < 4—scan repeated or excluded from the study.
- -
- Average thickness in pRNFL;
- -
- pRNFL thickness in the superior, inferior, nasal, and temporal segments;
- -
- -
- Average macular RNFL thickness (mRNFL); and
- -
- Average MV.
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. OCT Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, A.; Merkel, B.; Brown, W.; Ryerson, L.Z.; Kister, I.; Malpas, C.B.; Sharmin, S.; Horakova, D.; Havrdova, E.K.; Spelman, T.; et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020, 19, 307–316. [Google Scholar] [CrossRef]
- Sotiropoulos, M.G.; Lokhande, H.; Healy, B.C.; Polgar-Turcsanyi, M.I.; Glanz, B.; Bakshi, R.; Weiner, H.L.; Chitnis, T. Relapse recovery in multiple sclerosis: Effect of treatment and contribution to long-term disability. Mult. Scler. J.—Exp. Transl. Clin. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Butzkueven, H.; Hillert, J.; Hyde, R.; Koch-Henriksen, N.; Magyari, M.; Pellegrini, F.; Spelman, T.; Sørensen, P.S.; et al. Early treatment delays long-term disability accrual in RRMS: Results from the BMSD network. Mult. Scler. J. 2021, 26, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Solomon, A.J. Diagnosis, Differential Diagnosis, and Misdiagnosis of Multiple Sclerosis. Contin. Lifelong Learn. Neurol. 2019, 25, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.J.; Naismith, R.T.; Cross, A.H. Misdiagnosis of multiple sclerosis: Impact of the 2017 McDonald criteria on clinical practice. Neurology 2019, 92, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.J.; Pettigrew, R.; Naismith, R.T.; Chahin, S.; Krieger, S.; Weinshenker, B. Challenges in multiple sclerosis diagnosis: Misunderstanding and misapplication of the McDonald criteria. Mult. Scler. 2021, 2021 27, 250–258. [Google Scholar] [CrossRef]
- Midaglia, L.; Sastre-Garriga, J.; Pappolla, A.; Quibus, L.; Carvajal, R.; Vidal-Jordana, A.; Arrambide, G.; Río, J.; Comabella, M.; Nos, C.; et al. The frequency and characteristics of MS misdiagnosis in patients referred to the multiple sclerosis centre of Catalonia. Mult. Scler. J. 2021, 27, 913–921. [Google Scholar] [CrossRef]
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult. Scler. Relat. Disord. 2019, 37, 101452. [Google Scholar] [CrossRef]
- Haralur, Y.; Mechtler, L.L. Neuroimaging of Multiple Sclerosis Mimics. Neurol. Clin. 2020, 38, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Jindahra, P.; Hedges, T.R.; E Mendoza-Santiesteban, C.; Plant, G.T. Optical coherence tomography of the retina: Applications in neurology. Curr. Opin. Neurol. 2010, 23, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Pisa, M.; Ratti, F.; Vabanesi, M.; Radaelli, M.; Guerrieri, S.; Moiola, L.; Martinelli, V.; Comi, G.; Leocani, L. Subclinical neurodegeneration in multiple sclerosis and neuromyelitis optica spectrum disorder revealed by optical coherence tomography. Mult. Scler. J. 2020, 26, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Oertel, F.C.; Havla, J.; Roca-Fernández, A.; Lizak, N.; Zimmermann, H.; Motamedi, S.; Borisow, N.; White, O.B.; Bellmann-Strobl, J.; Albrecht, P.; et al. Retinal ganglion cell loss in neuromyelitis optica: A longitudinal study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Oertel, F.C.; Outteryck, O.; Knier, B.; Zimmermann, H.; Borisow, N.; Bellmann-Strobl, J.; Blaschek, A.; Jarius, S.; Reindl, M.; Ruprecht, K.; et al. Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: A longitudinal study. J. Neuroinflamm. 2019, 16, 154. [Google Scholar] [CrossRef]
- Liu, G.Y.; Utset, T.O.; Bernard, J.T. Retinal nerve fiber layer and macular thinning in systemic lupus erythematosus: An optical coherence tomography study comparing SLE and neuropsychiatric SLE. Lupus 2015, 24, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Wildner, P.; Zydorczak, E.; Oset, M.; Siger, M.; Wilczyński, M.; Stasiołek, M.; Matysiak, M. The Role of Optical Coherence Tomography in Differential Diagnosis of Multiple Sclerosis and Autoimmune Connective Tissue Diseases with CNS Involvement. J. Clin. Med. 2020, 9, 1565. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [Green Version]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knier, B.; Berthele, A.; Buck, D.; Schmidt, P.; Zimmer, C.; Mühlau, M.; Hemmer, B.; Korn, T. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult. Scler. J. 2016, 22, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Nolan-Kenney, R.C.; Liu, M.; Akhand, O.; Calabresi, P.A.; Paul, F.; Petzold, A.; Balk, L.; Brandt, A.U.; Martínez-Lapiscina, E.H.; Saidha, S.; et al. International Multiple Sclerosis Visual System Consortium. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: An international study. Ann. Neurol. 2019, 85, 618–629. [Google Scholar] [CrossRef]
- Bsteh, G.; Hegen, H.; Altmann, P.; Auer, M.; Berek, K.; Zinganell, A.; Di Pauli, F.; Rommer, P.; Deisenhammer, F.; Leutmezer, F.; et al. Validation of inter-eye difference thresholds in optical coherence tomography for identification of optic neuritis in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 02403. [Google Scholar] [CrossRef] [PubMed]
- Sąsiadek, M.; Hartel, M.; Siger, M.; Katulska, K.; Majos, A.; Kluczewska, E.; Bartosik-Psujek, H.; Kułakowska, A.; Słowik, A.; Steinborn, B.; et al. Recommendations of the Polish Medical Society of Radiology and the Polish Society of Neurology for a protocol concerning routinely used magnetic resonance imaging in patients with multiple sclerosis. Neurol. i Neurochir. Polska 2020, 54, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Okuda, D.T.; Mowry, E.M.; Beheshtian, A.; Waubant, E.; Baranzini, S.E.; Goodin, D.S.; Hauser, S.L.; Pelletier, D. Incidental MRI anomalies suggestive of multiple sclerosis: The radiologically isolated syndrome. Neurology 2009, 72, 800–805. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, N.; Giorgio, A.; Tintore, M.; Amato, M.P.; Kappos, L.; Palace, J.; Yousry, T.A.; Rocca, M.A.; Ciccarelli, O.; Enzinger, C.; et al. Radiologically isolated syndrome or subclinical multiple sclerosis: MAGNIMS consensus recommendations. Mult. Scler. J. 2018, 24, 214–221. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerma, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, H.; Stein, D.M.; Wollstein, G.; Beaton, S.; Fujimoto, J.G.; Schuman, J. Macular Segmentation with Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2005, 46, 2012–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, S.D.; Ishikawa, H.; Galetta, K.M.; Sakai, R.E.; Feller, D.; Henderson, S.B.; Wilson, J.A.; Maguire, M.G.; Galetta, S.L.; Frohman, E.; et al. Ganglion Cell Loss in Relation to Visual Disability in Multiple Sclerosis. Ophthalmology 2012, 119, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Schippling, S.; Balk, L.J.; Costello, F.; Albrecht, P.; Balcer, L.; Calabresi, P.; Frederiksen, J.L.; Frohman, E.; Green, A.J.; Klistorner, A.; et al. Quality control for retinal OCT in multiple sclerosis: Validation of the OSCAR-IB criteria. Mult. Scler. J. 2015, 21, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytulun, A.; Cruz-Herranz, A.; Aktas, O.; Balcer, L.J.; Balk, L.; Barboni, P.; Blanco, A.A.; Calabresi, P.A.; Costello, F.; Sanchez-Dalmau, B.; et al. APOSTEL 2.0 Recommendations for Reporting Quantitative Optical Coherence Tomography Studies. Neurology 2021, 97, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Banwell, B.L.; Barkhof, F.; Ciccarelli, O.; De Stefano, N.; Geurts, J.J.G.; Paul, F.; Reich, D.S.; Toosy, A.T.; et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: Practical guidelines. Brain 2019, 142, 1858–1875. [Google Scholar] [CrossRef] [Green Version]
- Lambe, J.; Saidha, S.; Bermel, R. Optical coherence tomography and multiple sclerosis: Update on clinical application and role in clinical trials. Mult. Scler. J. 2020, 26, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Hegen, H.; Teuchner, B.; Berek, K.; Wurth, S.; Auer, M.; Di Pauli, F.; Deisenhammer, F.; Berger, T. Peripapillary retinal nerve fibre layer thinning rate as a biomarker discriminating stable and progressing relapsing–remitting multiple sclerosis. Eur. J. Neurol. 2019, 26, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Eslami, F.; Ghiasian, M.; Khanlarzade, E.; Moradi, E. Retinal Nerve Fiber Layer Thickness and Total Macular Volume in Multiple Sclerosis Subtypes and Their Relationship with Severity of Disease, a Cross-Sectional Study. Eye Brain 2020, 12, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Delgado, S.; Wang, J. Advances in ophthalmic structural and functional measures in multiple sclerosis: Do the potential ocular biomarkers meet the unmet needs? Curr. Opin. Neurol. 2021, 34, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Arnow, S.A.; Wilson, J.; Saidha, S.; Preiningerova, J.L.; Oberwahrenbrock, T.; Brandt, A.U.; E Pablo, L.; Guerrieri, S.; González-Suárez, I.; et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol. 2016, 15, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Bsteh, G.; Hegen, H.; Teuchner, B.; Amprosi, M.; Berek, K.; Ladstätter, F.; Wurth, S.; Auer, M.; Di Pauli, F.; Deisenhammer, F.; et al. Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult. Scler. 2019, 25, 196–203. [Google Scholar] [CrossRef]
- Bsteh, G.; Hegen, H.; Altmann, P.; Auer, M.; Berek, K.; Di Pauli, F.; Wurth, S.; Zinganell, A.; Rommer, P.; Deisenhammer, F.; et al. Retinal layer thinning is reflecting disability progression independent of relapse activity in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 29, 6–2020. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.A.; Calabresi, P.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol 2017, 16, 797–812. [Google Scholar] [CrossRef] [Green Version]
- Balk, L.J.; Petzold, A. Current and future potential of retinal optical coherence tomography in multiple sclerosis with and without optic neuritis. Neurodegener. Dis. Manag. 2014, 4, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Siger, M.; Dzięgielewski, K.; Jasek, L.; Bieniek, M.; Nicpan, A.; Nawrocki, J.; Selmaj, K. Optical coherence tomography in multiple sclerosis: Thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J. Neurol. 2008, 255, 1555–1560. [Google Scholar] [CrossRef]
- Abalo-Lojo, J.M.; Limeres, C.C.; Gómez, M.A.; Baleato-González, S.; Cadarso-Suárez, C.; Capeáns-Tomé, C.; Gonzalez, F. Retinal nerve fiber layer thickness, brain atrophy and disability in multiple sclerosis patients. J. Neuroophthalmol. 2014, 34, 23–28. [Google Scholar] [CrossRef]
- Caldito, N.G.; Saidha, S.; Sotirchos, E.S.; Dewey, B.E.; Cowley, N.J.; Glaister, J.; Fitzgerald, K.C.; Al-Louzi, O.; Nguyen, J.; Rothman, A.; et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: A longitudinal study. Brain 2018, 141, 3115–3129. [Google Scholar] [CrossRef]
- Vidal-Jordana, A.; Pareto, D.; Cabello, S.; Alberich, M.; Rio, J.; Tintore, M.; Auger, C.; Montalban, X.; Rovira, A.; Sastre-Garriga, J. Optical coherence tomography measures correlate with brain and spinal cord atrophy and multiple sclerosis disease-related disability. Eur. J. Neurol. 2020, 27, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Gabilondo, I.; Martínez-Lapiscina, E.H.; Bo, E.F.; Ortiz-Perez, S.; Torres-Torres, R.; Andorra, M.; Llufriu, S.; Zubizarreta, I.; Saiz, A.; Sanchez-Dalmau, B.; et al. Dynamics of retinal injury after acute optic neuritis. Ann. Neurol. 2015, 77, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Kupersmith, M.J.; Garvin, M.K.; Wang, J.-K.; Durbin, M.; Kardon, R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult. Scler. J. 2016, 22, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, D.; Cheng, H.; Bonem, K.N.; Saenz, R.R.; Tang, R.A.; Frishman, L.J. Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult. Scler. J. 2014, 20, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Huang-Link, Y.-M.; Al-Hawasi, A.; Lindehammar, H. Acute optic neuritis: Retinal ganglion cell loss precedes retinal nerve fiber thinning. Neurol. Sci. 2015, 36, 617–620. [Google Scholar] [CrossRef]
- Bsteh, G.; Berek, K.; Hegen, H.; Altmann, P.; Wurth, S.; Auer, M.; Zinganell, A.; Di Pauli, F.; Rommer, P.; Leutmezer, F.; et al. Macular ganglion cell–inner plexiform layer thinning as a biomarker of disability progression in relapsing multiple sclerosis. Mult. Scler. J. 2021, 27, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, B.M.; Osborne, B.; Loguidice, M.J.; Bisker, E.; Frohman, T.C.; Conger, A.; Ratchford, J.N.; Warner, C.; Markowitz, C.E.; Jacobs, D.A.; et al. Macular Volume Determined by Optical Coherence Tomography as a Measure of Neuronal Loss in Multiple Sclerosis. Arch. Neurol. 2009, 66, 1366–1372. [Google Scholar] [CrossRef]
- Ucar, D.; Uygunoglu, U.; Dikkaya, F.; Yıldırım, Y.; Yuksel-Elgin, C.; Saip, S.; Siva, A.; Ozyazgan, Y. Retinal nerve fiber layer structure abnormalities in patients with Neuro-Behcet’s disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, K.H.; Kwon, J.W.; Jeoung, J.W.; Kim, T.-W.; Kim, D.M. Retinal Nerve Fiber Layer Defect and Cerebral Small Vessel Disease. Investig. Opthalmol. Vis. Sci. 2011, 52, 6882–6886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, M.; Kwapong, W.R.; Peng, C.; Cao, Y.; Lu, F.; Shen, M.; Han, Z. Retinal sublayer defect is independently associated with the severity of hypertensive white matter hyperintensity. Brain Behav. 2020, 10, e01521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Kwapong, W.R.; Xu, S.; Muse, F.M.; Yan, J.; Qu, M.; Cao, Y.; Miao, H.; Zhen, Z.; Wu, B.; et al. Structural and Microvascular Changes in the Macular Are Associated with Severity of White Matter Lesions. Front. Neurol. 2020, 11, 521. [Google Scholar] [CrossRef]
- Reich, D.S.; Smith, S.A.; Gordon-Lipkin, E.; Ozturk, A.; Caffo, B.S.; Balcer, L.J.; Calabresi, P.A. Damage to the Optic Radiation in Multiple Sclerosis Is Associated with Retinal Injury and Visual Disability. Arch. Neurol. 2009, 66, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Costello, F. The Afferent Visual Pathway: Designing a Structural-Functional Paradigm of Multiple Sclerosis. ISRN Neurol. 2013, 2013, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, A.; Catrinescu, M.M.; Belisle, J.M.; Costantino, S.; Levin, L.A. Retrograde and Wallerian axonal degeneration occur synchronously after retinal ganglion cell axotomy. Am. J. Pathol. 2012, 181, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uggetti, C.; Egitto, M.G.; Fazzi, E.; Bianchi, P.; Zappoli, F.; Martelli, A.; Lanzi, G. Transsynaptic degeneration of lateral geniculate bodies in blind children: In vivo MR demonstration. Am. J. Neuroradiol. 1997, 18, 233–238. [Google Scholar] [PubMed]
- Keller, J.; Sánchez-Dalmau, B.F.; Villoslada, P. Lesions in the Posterior Visual Pathway Promote Trans-Synaptic Degeneration of Retinal Ganglion Cells. PLoS ONE 2014, 9, e97444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, F.; Burton, J.M. Retinal imaging with optical coherence tomography: A biomarker in multiple sclerosis? Eye Brain 2018, 10, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, U.; Bonnemaijer, P.W.; Ikram, M.A.; Colijn, J.M.; Cremers, L.G.; Buitendijk, G.H.; Vingerling, J.R.; Niessen, W.J.; Vernooij, M.W.; Klaver, C. Retinal neurodegeneration and brain MRI markers: The Rotterdam Study. Neurobiol. Aging 2017, 60, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Britze, J.; Frederiksen, J.L. Optical coherence tomography in multiple sclerosis. Eye 2018, 32, 884–888. [Google Scholar] [CrossRef] [Green Version]
- Costello, F. Optical Coherence Tomography in Neuro-ophthalmology. Neurol. Clin. 2017, 35, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, N.M.; Alasbali, T.; Khandekar, R. Macular ganglion cell complex parameters by optical coherence tomography in cases of multiple sclerosis without optic neuritis compared to healthy eyes. Indian J Ophthalmol. 2019, 67, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Oberwahrenbrock, T.; Ringelstein, M.; Jentschke, S.; Deuschle, K.; Klumbies, K.; Bellmann-Strobl, J.; Harmel, J.; Ruprecht, K.; Schippling, S.; Hartung, H.-P.; et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult. Scler. J. 2013, 19, 1887–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britze, J.; Pihl-Jensen, G.; Frederiksen, J.L. Retinal ganglion cell analysis in multiple sclerosis and optic neuritis: A systematic review and meta-analysis. J. Neurol. 2017, 264, 1837–1853. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; Dell’Arti, L.; Caprioli, M.; Scarioni, M.; Carandini, T.; Arighi, A.; Ghezzi, L.; Fumagalli, G.G.; A De Riz, M.; Basilico, P.; et al. The loss of macular ganglion cells begins from the early stages of disease and correlates with brain atrophy in multiple sclerosis patients. Mult Scler. 2019, 25, 31–38. [Google Scholar] [CrossRef]
- Saidha, S.; Syc, S.B.; Durbin, M.K.; Eckstein, C.; Oakley, J.D.; Meyer, S.A.; Conger, A.; Frohman, T.C.; Newsome, S.; Ratchford, J.N.; et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult. Scler. 2011, 17, 1449–1463. [Google Scholar] [CrossRef]
- Petzold, A.; Chua, S.Y.L.; Khawaja, A.P.; A Keane, P.; Khaw, P.T.; Reisman, C.; Dhillon, B.; Strouthidis, N.G.; Foster, P.J.; Patel, P.J.; et al. Retinal asymmetry in multiple sclerosis. Brain 2021, 144, 224–235. [Google Scholar] [CrossRef]
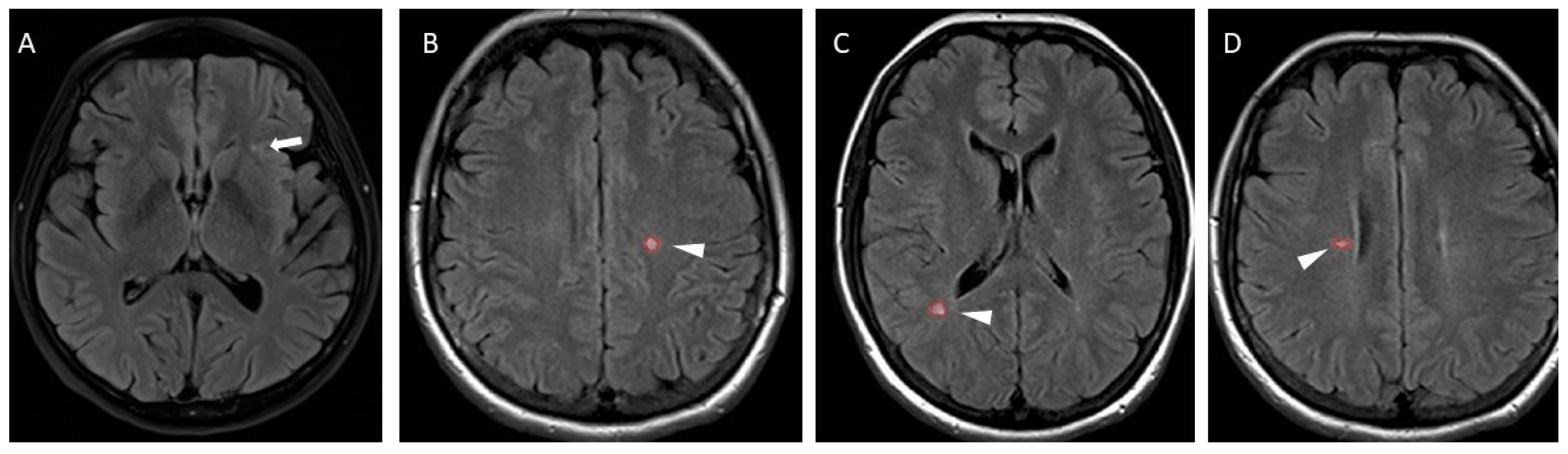


| . | MS | NSWMLs | p-Value |
|---|---|---|---|
| Subjects, n | 41 | 19 | |
| M/F | 10/31 | 5/14 | >0.999 |
| Number of eyes | 82 | 38 | |
| Age, years, mean (±SD) | 41.9 (±13.3) | 43.8 (±12.0) | 0.418 |
| Disease duration, years, median (Q1; Q3) | 3.0 (1.0; 7.0) | - | - |
| EDSS score, median (Q1; Q3) | 1.0 (0.0; 3.0) | - | - |
| Characteristic | MS (n = 41) | NSWMLs (n = 19) | p-Value |
|---|---|---|---|
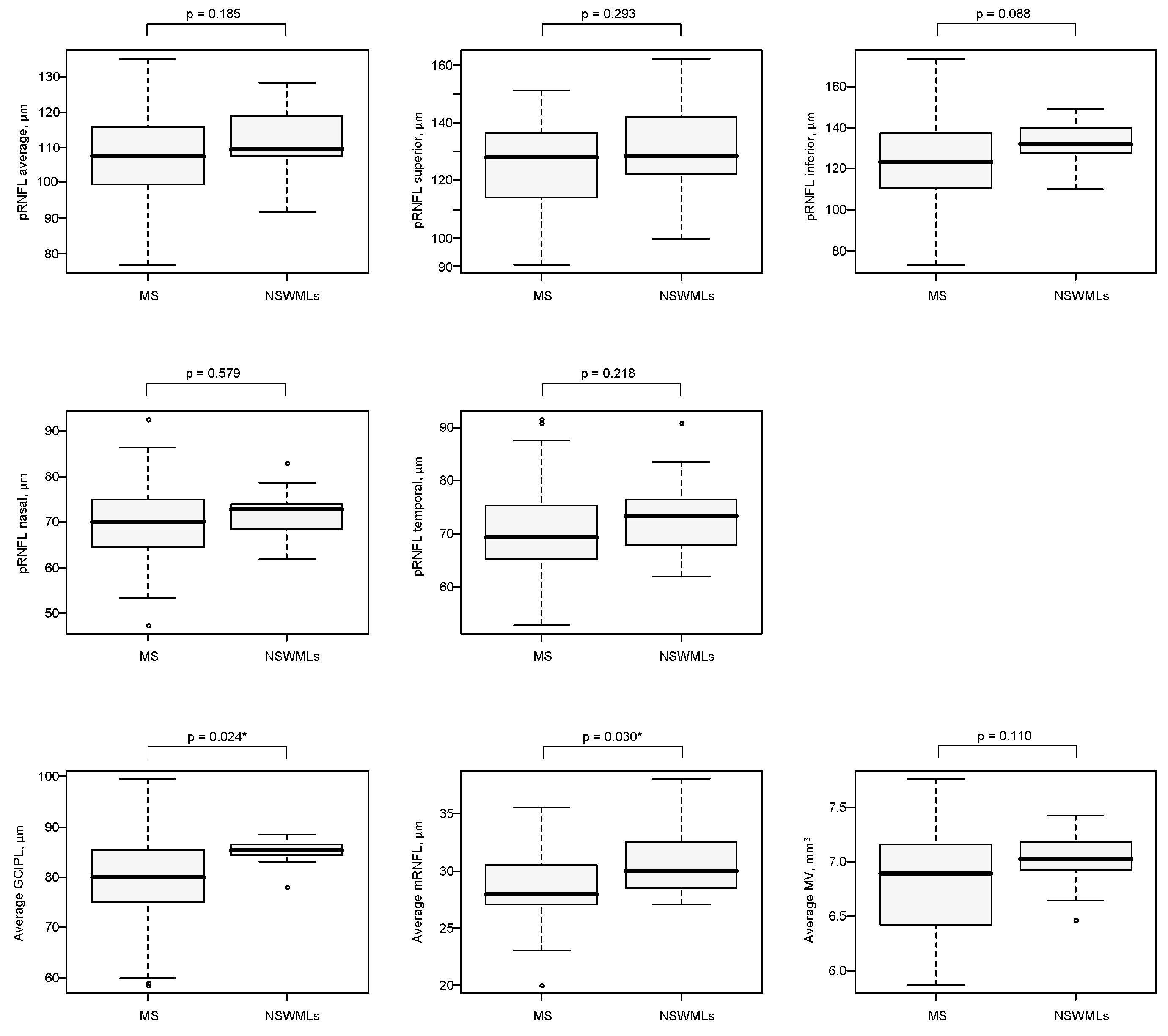
| pRNFL average, µm | 107.5 (99.5; 116.0) | 109.5 (107.5; 119.0) | 0.185 |
| pRNFL superior, µm | 128.0 (114.0; 136.5) | 128.5 (122.0; 142.0) | 0.293 |
| pRNFL inferior, µm | 123.0 (110.5; 137.0) | 131.5 (127.5; 140.0) | 0.088 |
| pRNFL nasal, µm | 70.0 (64.5; 75.0) | 73.0 (68.5; 74.0) | 0.579 |
| pRNFL temporal, µm | 69.5 (65.5; 75.5) | 73.5 (68.0; 76.5) | 0.218 |
| Average GCIPL, µm | 80.0 (75.0; 85.5) | 85.5 (84.5; 86.5) | 0.024 * |
| Average mRNFL, µm | 28.0 (27.0; 30.5) | 30.0 (28.5; 32.5) | 0.030 * |
| Average MV, mm3 | 6.90 (6.43; 7.17) | 7.03 (6.93; 7.19) | 0.110 |
| Characteristic | Simple Regression | Stepwise Regression | ||||
|---|---|---|---|---|---|---|
| Coeff. (SE) | p | OR (95% CI) | Coeff. (SE) | p | OR (95% CI) | |
| Average GCIPL, µm | −0.11 (0.05) | 0.024 | 0.894 (0.798; 0.974) | −0.11 (0.05) | 0.024 | 0.894 (0.798; 0.974) |
| Average mRNFL, µm | −0.24 (0.11) | 0.028 | 0.785 (0.616; 0.957) | - | - | - |
| Constant | 11.68 (4.73) | 0.014 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siger, M.; Owidzka, M.; Świderek-Matysiak, M.; Omulecki, W.; Stasiołek, M. Optical Coherence Tomography in the Differential Diagnosis of Patients with Multiple Sclerosis and Patients with MRI Nonspecific White Matter Lesions. Sensors 2021, 21, 7127. https://doi.org/10.3390/s21217127
Siger M, Owidzka M, Świderek-Matysiak M, Omulecki W, Stasiołek M. Optical Coherence Tomography in the Differential Diagnosis of Patients with Multiple Sclerosis and Patients with MRI Nonspecific White Matter Lesions. Sensors. 2021; 21(21):7127. https://doi.org/10.3390/s21217127
Chicago/Turabian StyleSiger, Małgorzata, Marta Owidzka, Mariola Świderek-Matysiak, Wojciech Omulecki, and Mariusz Stasiołek. 2021. "Optical Coherence Tomography in the Differential Diagnosis of Patients with Multiple Sclerosis and Patients with MRI Nonspecific White Matter Lesions" Sensors 21, no. 21: 7127. https://doi.org/10.3390/s21217127
APA StyleSiger, M., Owidzka, M., Świderek-Matysiak, M., Omulecki, W., & Stasiołek, M. (2021). Optical Coherence Tomography in the Differential Diagnosis of Patients with Multiple Sclerosis and Patients with MRI Nonspecific White Matter Lesions. Sensors, 21(21), 7127. https://doi.org/10.3390/s21217127






