Application of a Combined Transmittance/Fluorescence Leaf Clip Sensor for the Nondestructive Determination of Nitrogen Status in White Cabbage Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. The Dualex Optical Sensor
2.2. Experimental Site and Data Acquisition
2.3. Statistical Analysis
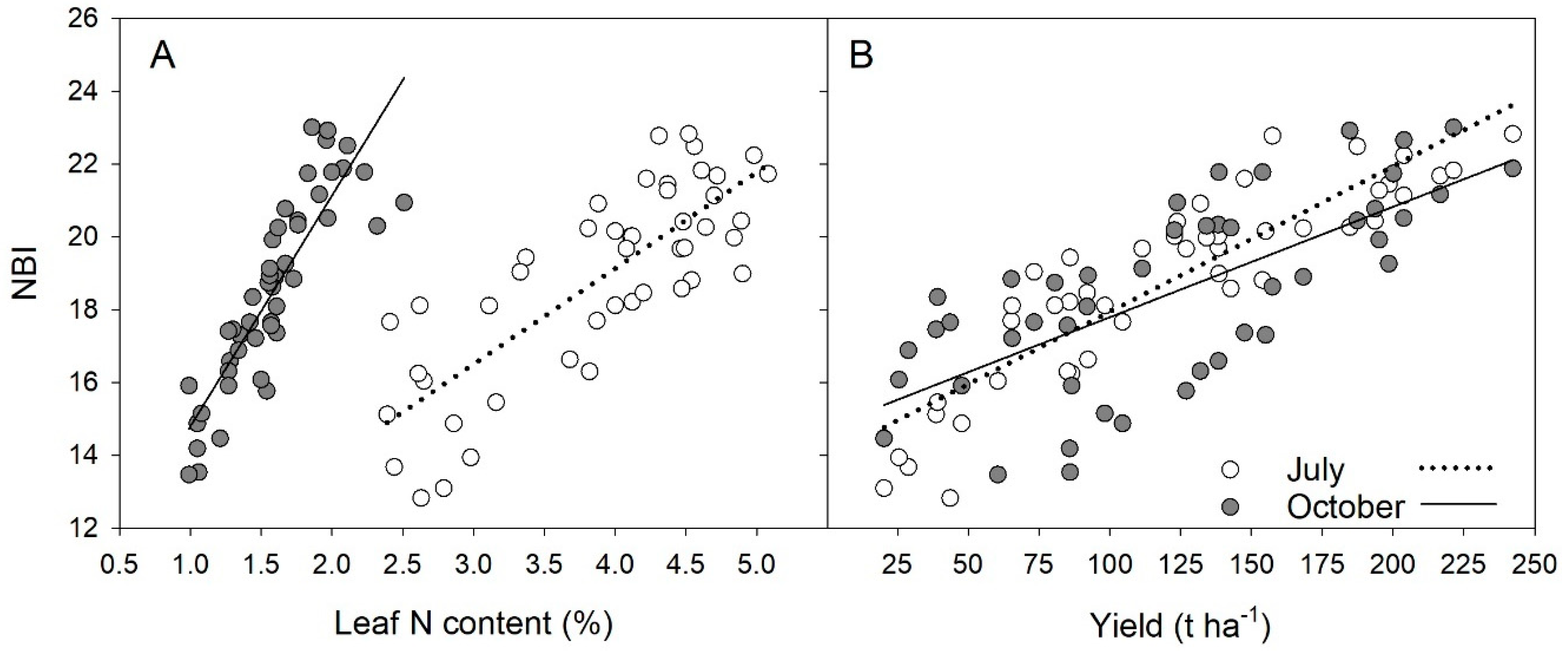
3. Results
3.1. Fertilization Effect on Leaf N and Yield
3.2. Relationship between Leaf N Content, Yield and Optical Indices
4. Discussion
4.1. Correlation between Dualex Indices and Leaf N
4.2. Correlation between NBI and Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heckman, J.R.; Morris, T.; Sims, J.T.; Sieczka, J.B.; Krogmann, U.; Nitzsche, P.; Ashley, R. Pre-sidedress Soil nitrate test is effective for fall cabbage. HortScience 2002, 37, 113–117. [Google Scholar] [CrossRef]
- Black, C.A. Soil Fertility Evaluation and Control; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Everaarts, A.P.; De Moel, C.P. The effect of nitrogen and the method of application on yield and quality of white cabbage. Eur. J. Agron. 1998, 9, 203–211. [Google Scholar] [CrossRef]
- Maršić, N.K.; Šturm, M.; Zupanc, V.; Lojen, S.; Pintar, M. Quality of white cabbage yield and potential risk of ground water nitrogen pollution, as affected by nitrogen fertilisation and irrigation practices. J. Sci. Food Agric. 2012, 92, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013. [Google Scholar]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to make use of plant sensors-based diagnostic information for nitrogen recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop sensor based non-destructive estimation of nitrogen nutritional status, yield, and grain protein content in wheat. Agronomy 2020, 10, 148. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Philippe, W.; Goffart, J.P. Comparison of optical indicators for potato crop nitrogen status assessment including novel approaches based on leaf fluorescence and flavonoid content. J. Plant Nutr. 2018, 41, 2705–2728. [Google Scholar] [CrossRef]
- Confalonieri, R.; Paleari, L.; Movedi, E.; Pagani, V.; Orlando, F.; Foi, M.; Barbieri, M.; Pesenti, M.; Cairati, O.; La Sala, M.S.; et al. Improving invivo plant nitrogen content estimates from digital images: Trueness and precision of a new approach as compared to other methods and commercial devices. Biosyst. Eng. 2015, 135, 21–30. [Google Scholar] [CrossRef]
- da Silva, J.M.; Fontes, P.C.R.; do Carmo Milagres, C.; Garcia Junior, E. Application of proximal optical sensors to assess nitrogen status and yield of bell pepper grown in slab. J. Soil Sci. Plant Nutr. 2020. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Dąbrowski, P.; Cetner, M.D.; Samborska, I.A.; Łukasik, I.; Brestic, M.; Zivcak, M.; Tomasz, H.; Mojski, J.; Kociel, H.; et al. A comparison between different chlorophyll content meters under nutrient deficiency conditions. J. Plant Nutr. 2017, 40, 1024–1034. [Google Scholar] [CrossRef]
- Westerveld, S.M.; Mckeown, A.W.; Scott-dupree, C.D.; Mcdonald, M.R.; Index, A.D.; Spad, W. As field tissue and carrots. Horttechnology 2004, 14, 179–188. [Google Scholar]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [PubMed]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat. Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive diagnostic test for nitrogen nutrition of grapevine based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Belec, C. Performance of dualex in spring wheat for crop nitrogen status assessment, yield prediction and estimation of soil nitrate content. J. Plant Nutr. 2010, 33, 57–70. [Google Scholar] [CrossRef]
- Bracke, J.; Elsen, A.; Adriaenssens, S.; Vandendriessche, H.; van Labeke, M.C. Utility of proximal plant sensors to support nitrogen fertilization in Chrysanthemum. Sci. Hortic. 2019, 256. [Google Scholar] [CrossRef]
- Bracke, J.; Elsen, A.; Adriaenssens, S.; Schoeters, L.; Vandendriessche, H.; van Labeke, M.C. Application of proximal optical sensors to fine-tune nitrogen fertilization: Opportunities for woody ornamentals. Agronomy 2019, 9, 408. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Boumaza, R.; Meyer, S.; Cerovic, Z.G. Indicators of nitrogen status for ornamental woody plants based on optical measurements of leaf epidermal polyphenol and chlorophyll contents. Sci. Hortic. 2008, 115, 377–385. [Google Scholar] [CrossRef]
- Fallovo, C.; Schreiner, M.; Schwarz, D.; Colla, G.; Krumbein, A. Phytochemical changes induced by different nitrogen supply forms and radiation levels in two leafy brassica species. J. Agric. Food Chem. 2011, 59, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Nondestructive optical sensing of flavonols and chlorophyll in white head cabbage grown under different nitrogen regimens. J. Agric. Food Chem. 2016, 64, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, X.; Ma, Y.; Zhang, R.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. A comparative assessment of measures of leaf nitrogen in rice using two leaf-clip meters. Sensors 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.L.; Quemada, M.; Alonso-Ayuso, M.; Lizaso, J.I.; Martín-Lammerding, D. Predicting n status in maize with clip sensors: Choosing sensor, leaf sampling point, and timing. Sensors 2019, 19, 3881. [Google Scholar] [CrossRef]
- Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Quian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sens. 2019, 11, 2706. [Google Scholar] [CrossRef]
- Dong, R.; Miao, Y.; Wang, X.; Chen, Z.; Yuan, F.; Zhang, W.; Li, H. Estimating plant nitrogen concentration of maize using a leaf fluorescence sensor across growth stages. Remote Sens. 2020, 12, 1139. [Google Scholar] [CrossRef]
- Ji, R.; Ju, M.; Wang, Y.; Hu, C.; Zhang, H.; Shi, W. In-season yield prediction of cabbage with a hand-held active canopy sensor. Sensors 2017, 17, 2287. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Agati, G.; Bilger, W.; Cerovic, Z.G. Fluorescence tools for sensing of quality-related phytochemicals in fruits and vegetables. In Sensor-Based Quality Assessment Systems for Fruits and Vegetables; Kuswandi, B., Siddiqui, M.W., Eds.; Apple Academic Press: Boca Raton, FL, USA, 2021; pp. 79–109. [Google Scholar]
- AOAC 955.04 Nitrogen (total) in fertilizers. In Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; pp. 17–18.
- COMMISSION REGULATION (EC) No 634/2006 of 25 April 2006 laying down the marketing standard for headed cabbages and amending Regulation (EEC) No 1591/87. Off. J. Eur. Union 2006, L112, 3–8.
- Kosson, R.; Felczyński, K.; Szwejda-Grzybowska, J.; Grzegorzewska, M.; Tuccio, L.; Agati, G.; Kaniszewski, S. Nutritive value of marketable heads and outer leaves of white head cabbage cultivated at different nitrogen rates. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 524–533. [Google Scholar] [CrossRef]
- Seidel, S.J.; Werisch, S.; Schütze, N.; Laber, H. Impact of irrigation on plant growth and development of white cabbage. Agric. Water Manag. 2017, 187, 99–111. [Google Scholar] [CrossRef]
- Šturm, M.; Kacjan-Maršić, N.; Zupanc, V.; Bračič-Železnik, B.; Lojen, S.; Pintar, M. Effect of different fertilisation and irrigation practices on yield, nitrogen uptake and fertiliser use efficiency of white cabbage. Sci. Hortic. 2010, 125, 103–109. [Google Scholar] [CrossRef]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Proximal optical sensing of cucumber crop N status using chlorophyll fluorescence indices. Eur. J. Agron. 2016, 73, 83–97. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Bélec, C. Evaluation of the dualex for the assessment of corn nitrogen status. J. Plant Nutr. 2007, 30, 1355–1369. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Derivation of sufficiency values of a chlorophyll meter to estimate cucumber nitrogen status and yield. Comput. Electron. Agric. 2017, 141, 5464. [Google Scholar] [CrossRef]


| N Rate (kg ha−1) | Leaf N Content (%) | Total Yield (t ha−1) | Marketable Yield (t ha−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |||
| J | O | J | O | |||||
| 0 | 2.96 ± 0.66c | 1.04 ± 0.04d | 2.86 ± 0.40d | 1.34 ± 0.11b | 87 ± 14d | 33 ± 8d | 46 ± 12d | 18 ± 5d |
| 100 | 4.14 ± 0.29b | 1.44 ± 0.16c | 3.33 ± 0.60c | 1.51 ± 0.16b | 145 ± 15c | 65 ± 18c | 85 ± 6c | 42 ± 16c |
| 200 | 4.54 ± 0.23ab | 1.70 ± 0.13b | 3.97 ± 0.18b | 1.79 ± 0.46ab | 191 ± 17b | 105 ± 20b | 116 ± 10b | 67 ± 18b |
| 300 | 4.76 ± 0.22a | 1.95 ± 0.09a | 4.77 ± 0.35a | 2.13 ± 0.31a | 209 ± 16a | 141 ± 10a | 137 ± 16a | 92 ± 9a |
| Period | Chl vs. Leaf N | Flav vs. Leaf N | NBI vs. Leaf N | NBI vs. Yield |
|---|---|---|---|---|
| July 2018 | y = 2.49x + 47.9 R2 = 0.394 * | y = −0.171x + 3.6 R2 = 0.523 * | y = 1.96x + 12.3 R2 = 0.663 ** | |
| July 2019 | y = 2.06x + 43.9 R2 = 0.119 | y = −0.367x + 4.4 R2 = 0.751 ** | y = 2.66x + 7.47 R2 = 0.712 ** | |
| July 2018 + 2019 | y = 3.24x + 42.3 R2 = 0.258 * | y = −0.27x + 4.02 R2 = 0.633 ** | y = 2.64x + 8.58 R2 = 0.64 * | y = 0.04x + 13.95 R2 = 0.801 ** |
| October 2018 | y = 14.10x + 41.3 R2 = 0.889 ** | y = −0.81x + 4.75 R2 = 0.835 ** | y = 8.06x + 6.08 R2 = 0.873 ** | |
| October 2019 | y = 8.3x + 50.2 R2 = 0.705 ** | y = −0.435x + 4.18 R2 = 0.567 * | y = 4.79x + 10.72 R2 = 0.696 * | |
| October 2018 + 2019 | y = 11.1x + 45.8 R2 = 0.778 ** | y = −0.62x + 4.47 R2 = 0.693 ** | y = 6.34x + 8.45 R2 = 0.76 ** | y = 0.03x + 14.76 R2 = 0.497 ** |
| Year | Days after Transplanting | R2 |
|---|---|---|
| 2018 | 39 | 0.71 |
| 136 (harvest) | 0.88 | |
| 2019 | 49 | 0.76 |
| 126 (harvest) | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniszewski, S.; Kowalski, A.; Dysko, J.; Agati, G. Application of a Combined Transmittance/Fluorescence Leaf Clip Sensor for the Nondestructive Determination of Nitrogen Status in White Cabbage Plants. Sensors 2021, 21, 482. https://doi.org/10.3390/s21020482
Kaniszewski S, Kowalski A, Dysko J, Agati G. Application of a Combined Transmittance/Fluorescence Leaf Clip Sensor for the Nondestructive Determination of Nitrogen Status in White Cabbage Plants. Sensors. 2021; 21(2):482. https://doi.org/10.3390/s21020482
Chicago/Turabian StyleKaniszewski, Stanisław, Artur Kowalski, Jacek Dysko, and Giovanni Agati. 2021. "Application of a Combined Transmittance/Fluorescence Leaf Clip Sensor for the Nondestructive Determination of Nitrogen Status in White Cabbage Plants" Sensors 21, no. 2: 482. https://doi.org/10.3390/s21020482
APA StyleKaniszewski, S., Kowalski, A., Dysko, J., & Agati, G. (2021). Application of a Combined Transmittance/Fluorescence Leaf Clip Sensor for the Nondestructive Determination of Nitrogen Status in White Cabbage Plants. Sensors, 21(2), 482. https://doi.org/10.3390/s21020482






