Ratiometric Detection of Mercury (II) Ions in Living Cells Using Fluorescent Probe Based on Bis(styryl) Dye and Azadithia-15-Crown-5 Ether Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents for Spectroscopic Studies
2.2. Steady-State Optical Measurements in Solution
2.3. Determination of Complex Stability Constants
2.4. Cells and Their Treatment
2.5. Confocal Microscopy Measurements
3. Results and Discussion
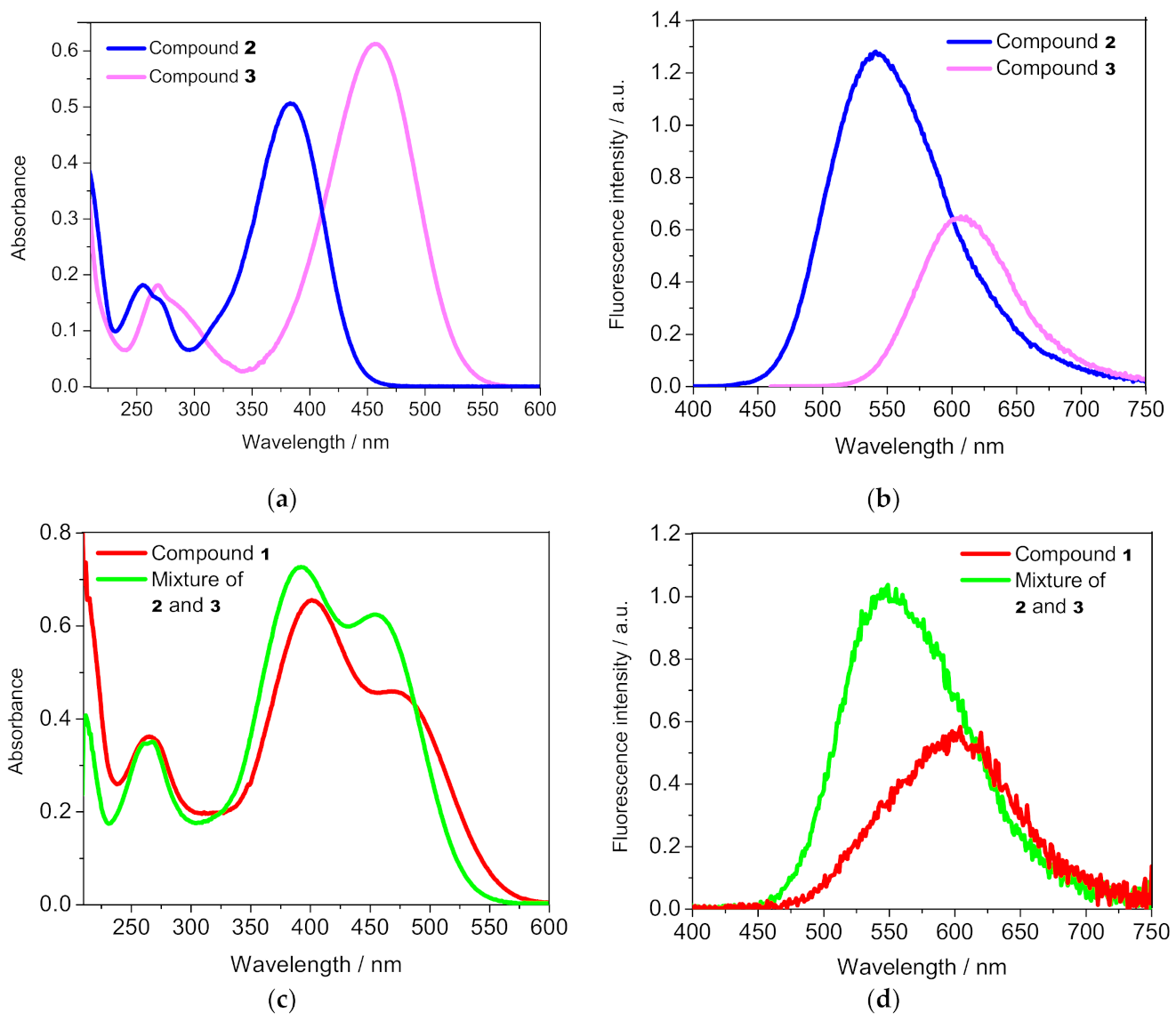
3.1. Spectral Characteristics of the Compounds
3.2. Complexation with Metal Cations in Solution
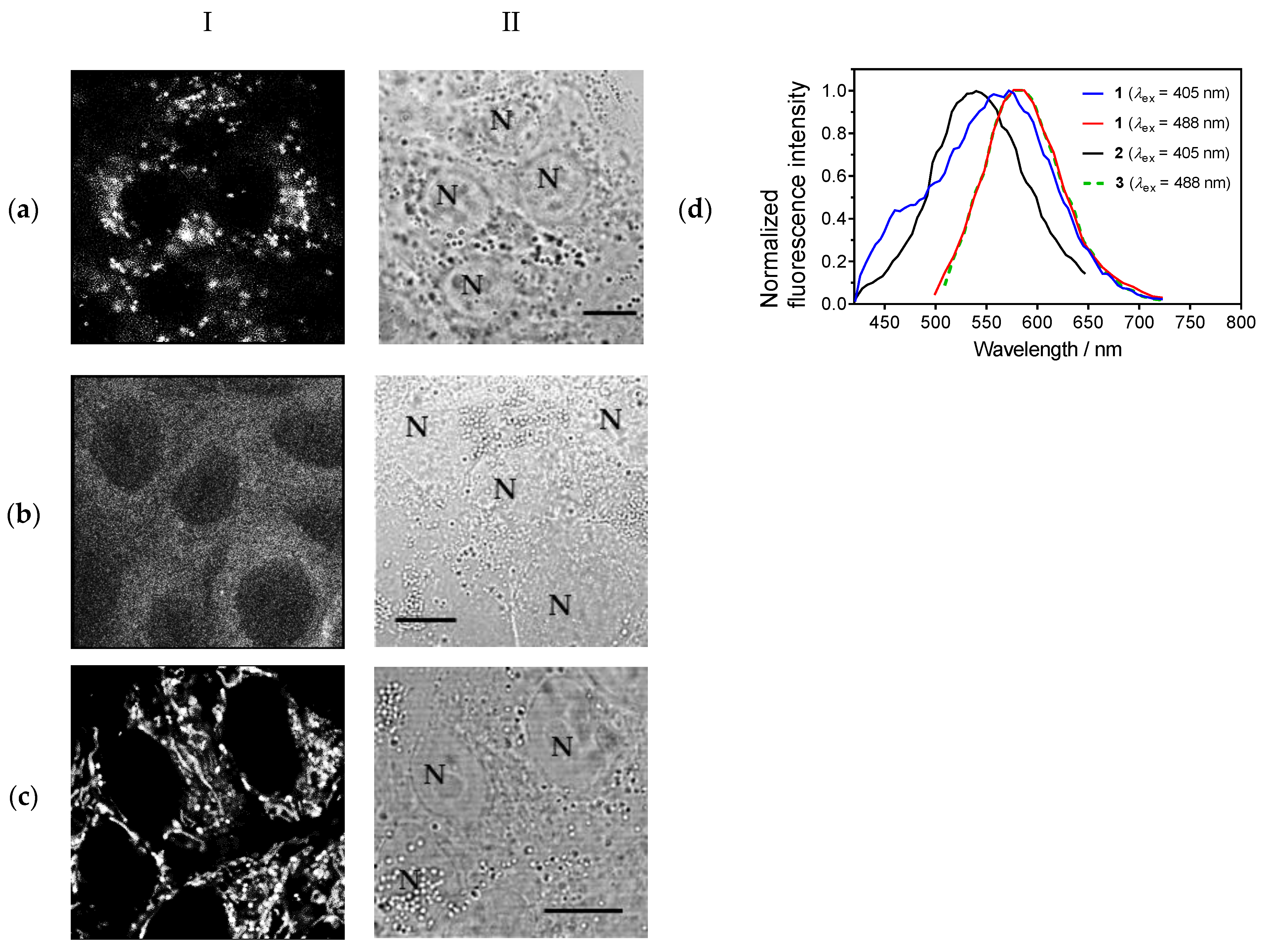
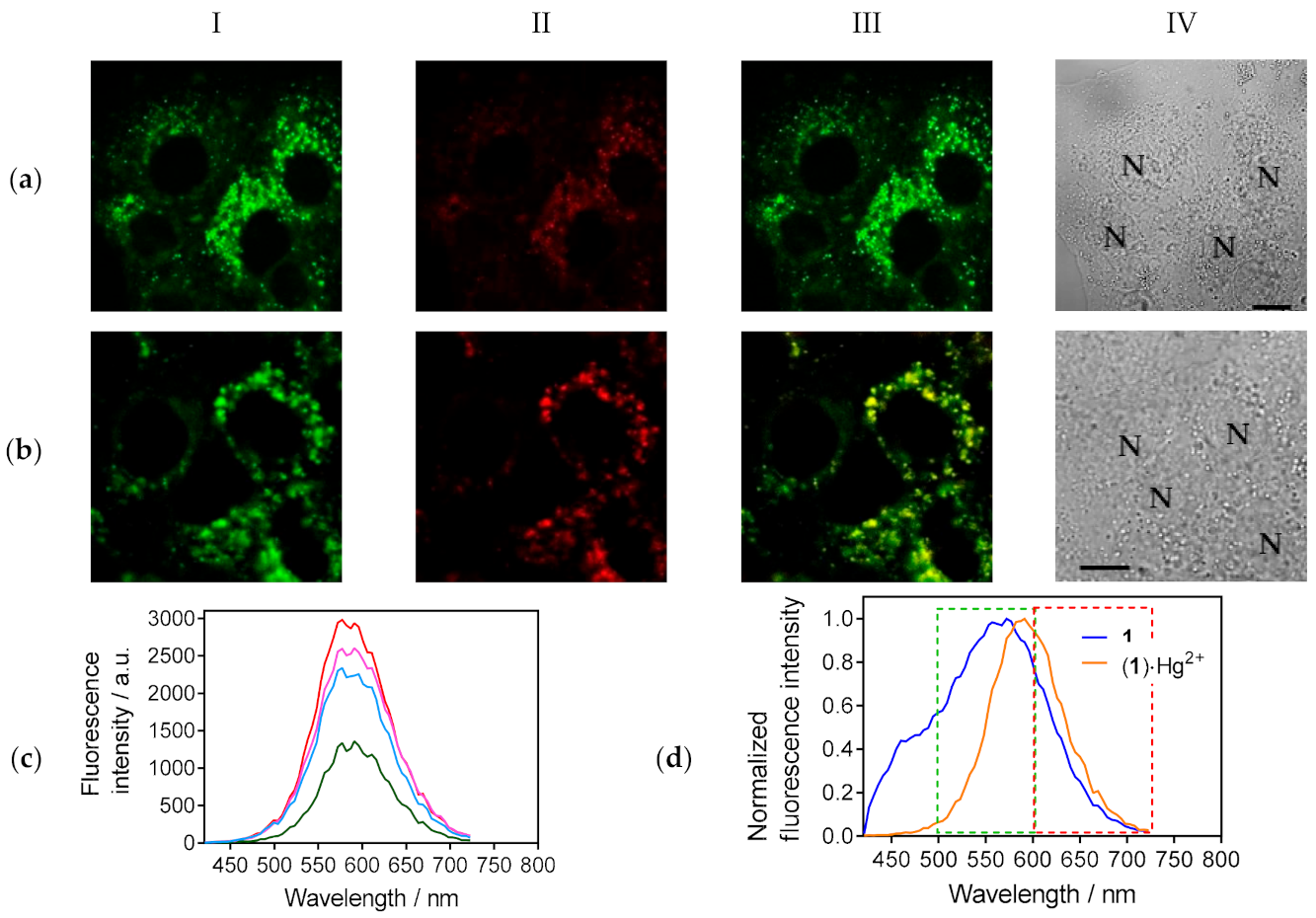
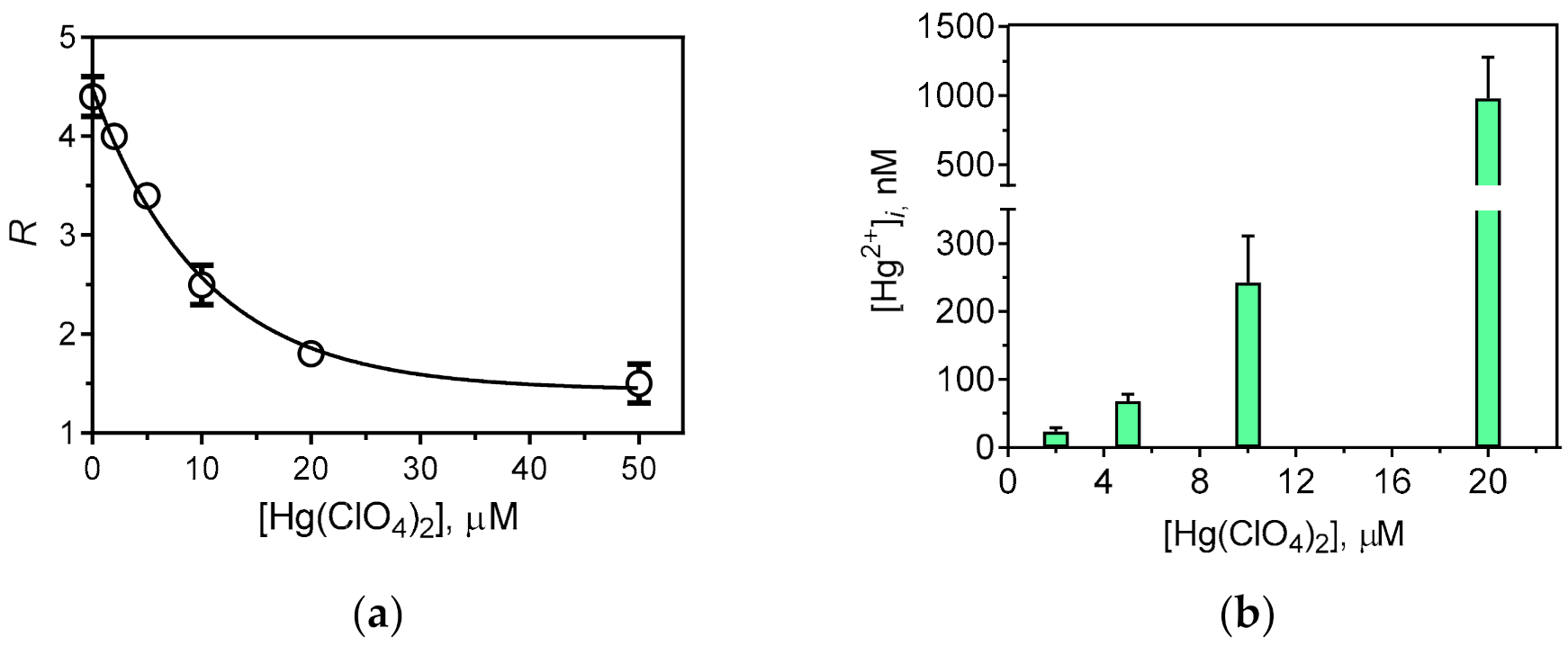
3.3. Cellular Imaging of the Studied Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Yoon, J. Recent progress on fluorescent chemosensors for metal ions. Inorganica Chim. Acta 2012, 381, 2–14. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Si, L.; Ariya, P.A. Recent Advances in Atmospheric Chemistry of Mercury. Atmosphere 2018, 9, 76. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- Nendza, M.; Herbst, T.; Kussatz, C.; Gies, A. Potential for secondary poisoning and biomagnification in marine organisims. Chemosphere 1997, 35, 1875–1885. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef]
- Magos, L.T.C. Hutchinson and K. M. Meema (Editors). Lead, Mercury, Cadmium and Arsenic in the Environment. Scope 31. John Wiley & Sons, Chichester, 1987; 360 pp, £48.00. J. Appl. Toxicol. 1988, 8, 150–151. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bok, K.H.; Kim, C. A fluorescence “turn-on” chemosensor for Hg2+ and Ag+ based on NBD (7-nitrobenzo-2-oxa-1,3-diazolyl). RSC Adv. 2017, 7, 290–299. [Google Scholar] [CrossRef]
- Zhou, B.; Qin, S.; Chen, B.; Han, Y. A new BODIPY-based fluorescent “turn-on” probe for highly selective and rapid detection of mercury ions. Tetrahedron Lett. 2018, 59, 4359–4363. [Google Scholar] [CrossRef]
- Lu, H.L.; Wang, W.K.; Tan, X.X.; Luo, X.F.; Zhang, M.L.; Zhang, M.; Zang, S.Q. A new quinoline-based fluorescent probe for Cd2+ and Hg2+ with an opposite response in a 100% aqueous environment and live cell imaging. Dalt. Trans. 2016, 45, 8174–8181. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Gong, R.; Ma, Q.; Sun, Y.; Fu, E. A novel colorimetric and fluorescent chemosensor: Synthesis and selective detection for Cu2+ and Hg2+. Tetrahedron Lett. 2007, 48, 5525–5529. [Google Scholar] [CrossRef]
- Demchenko, A.P. The concept of λ-ratiometry in fluorescence sensing and imaging. J. Fluoresc. 2010, 20, 1099–1128. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.Y.; Xie, Y.Z.; Wang, C.J.; Fang, S.Q.; Cao, L.N.; Wang, L.L.; Jin, J.Y. A Quninolylthiazole Derivatives as an ICT-Based Fluorescent Probe of Hg(II) and its Application in Ratiometric Imaging in Live HeLa Cells. J. Fluoresc. 2018, 28, 795–800. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, Y.M.; Chao, J.B.; Wang, H.; Wang, Y.; Shuang, S.M. A simple but efficient fluorescent sensor for ratiometric sensing of Cd2+ and bio-imaging studies. Sensors Actuators, B Chem. 2020, 303, 127216. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, M.; Yang, H.; Huang, K.; Li, F.; Yi, T.; Huang, C. FRET-based sensor for imaging chromium(III) in living cells. Chem. Commun. 2008, 3387–3389. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, M.; Jiang, D.; Zhang, H.; Li, L.; Zhang, G.; Wang, Y.; Feng, C.; Zhao, H. A FRET-based colorimetric and ratiometric fluorescent probe for the detection of Cu2+ with a new trimethylindolin fluorophore. New J. Chem. 2019, 43, 10176–10182. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, J.; Zhang, D.; Zhu, M.; Gong, L.; Su, C.; Lu, W.; Bian, Y.; Jiang, J. A phthalocyanine-porphyrin triad for ratiometric fluorescent detection of Lead(II) ions. Dye. Pigment. 2020, 173, 107941. [Google Scholar] [CrossRef]
- Prabhu, J.; Velmurugan, K.; Raman, A.; Duraipandy, N.; Kiran, M.S.; Easwaramoorthi, S.; Tang, L.; Nandhakumar, R. Pyrene-phenylglycinol linked reversible ratiometric fluorescent chemosensor for the detection of aluminium in nanomolar range and its bio-imaging. Anal. Chim. Acta 2019, 1090, 114–124. [Google Scholar] [CrossRef]
- He, X.; Xiong, W.; Lilei, Z.; Xu, C.; Fan, J.; Qian, Y.; Wen, J.; Ding, F.; Shen, J. ESIPT-based ratiometric fluorescent probe for highly selective and sensitive sensing and bioimaging of group IIIA ions in living cancer cells and zebrafish. Dye. Pigment. 2020, 174, 108059. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Qian, X. A ratiometric fluorescent probe based on FRET for imaging Hg2+ ions in living cells. Angew. Chem. Int. Ed. 2008, 47, 8025–8029. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Reja, S.I.; Bhalla, V.; Kaur, D.; Kaur, P.; Arora, S.; Kumar, M. Hexaphenylbenzene appended AIEE active FRET based fluorescent probe for selective imaging of Hg2+ ions in MCF-7 cell lines. Sens. Actuators B Chem. 2017, 249, 311–320. [Google Scholar] [CrossRef]
- Li, Y.; Qi, S.; Xia, C.; Xu, Y.; Duan, G.; Ge, Y. A FRET ratiometric fluorescent probe for detection of Hg2+ based on an imidazo[1,2-a]pyridine-rhodamine system. Anal. Chim. Acta 2019, 1077, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Berdnikova, D.V.; Fedorov, Y.V.; Fedorova, О.A.; Jonusauskas, G. Supramolecular tuning of energy transfer efficiency and direction in a bis(styryl) dye–crown ether conjugate. Dye. Pigment. 2018, 151, 227–232. [Google Scholar] [CrossRef]
- Ustimova, M.A.; Lebedeva, A.Y.; Fedorov, Y.V.; Berdnikova, D.V.; Fedorova, O.A. FRET-based metal ion sensing by a crown-containing bisstyryl dye. New J. Chem. 2018, 42, 7908–7913. [Google Scholar] [CrossRef]
- Tulyakova, E.V.; Fedorova, O.A.; Fedorov, Y.V.; Jonusauskas, G.; Anisimov, A.V. Effect of arrangement of the styryl fragment on the optical properties and complexation of mono-and bis(styryl)-substituted N-methylpyridinium perchlorates containing benzo-15-crown-5 ether moieties. Russ. Chem. Bull. 2007, 56, 2166–2174. [Google Scholar] [CrossRef]
- Tulyakova, E.V.; Fedorova, O.A.; Fedorov, Y.V.; Jonusauskas, G.; Anisimov, A.V. Spectroscopic study of mono- and bis(styryl) dyes of the pyridinium series containing azathiacrown ether residue. J. Phys. Org. Chem. 2008, 21, 372–380. [Google Scholar] [CrossRef]
- Nad, S.; Kumbhakar, M.; Pal, H. Photophysical properties of coumarin-152 and coumarin-481 dyes: Unusual behavior in nonpolar and in higher polarity solvents. J. Phys. Chem. A 2003, 107, 4808–4816. [Google Scholar] [CrossRef]
- Connors, K.A. Binding constants: The measurement of molecular complex stability. Eur. J. Med. Chem. 1987, 22, 373. [Google Scholar] [CrossRef]
- Beck, M.T.; Nagypál, I. Chemistry of Complex Equilibria; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Hou, H.; Zhao, Y.; Li, C.; Wang, M.; Xu, X.; Jin, Y. Single-cell pH imaging and detection for pH profiling and label-free rapid identification of cancer-cells. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Selektor, S.L.; Bogdanova, L.B.; Shokurov, A.V.; Panchenko, P.A.; Fedorova, O.A.; Arslanov, V.V. Intra- and interlayer energy transfer in planar systems based on amphiphilic naphthalimide derivatives. Macroheterocycles 2014, 7, 311–320. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorov, Y.V.; Fedorova, O.A. Selective fluorometric sensing of Hg2+ in aqueous solution by the inhibition of PET from dithia-15-crown-5 ether receptor conjugated to 4-amino-1,8-naphthalimide fluorophore. J. Photochem. Photobiol. A Chem. 2018, 364, 124–129. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science + Business Media, LLC: New York, NY, USA, 2006; ISBN 0387312781. [Google Scholar]
- Efremenko, A.V.; Ignatova, A.A.; Grin, M.A.; Sivaev, I.B.; Mironov, A.F.; Bregadze, V.I.; Feofanov, A.V. Chlorin e6 fused with a cobalt-bis(dicarbollide) nanoparticle provides efficient boron delivery and photoinduced cytotoxicity in cancer cells. Photochem. Photobiol. Sci. 2014, 13, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.A.; Grin, M.A.; Fedorova, O.A.; Zakharko, M.A.; Pritmov, D.A.; Mironov, A.F.; Arkhipova, A.N.; Fedorov, Y.V.; Jonusauskas, G.; Yakubovskaya, R.I.; et al. A novel bacteriochlorin-styrylnaphthalimide conjugate for simultaneous photodynamic therapy and fluorescence imaging. Phys. Chem. Chem. Phys. 2017, 19, 30195–30206. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejewska, B.; Kabatc, J.; Paczkowski, J. 1,3-Bis[4-(p-aminostyryl)-pyridinyl]-propane dibromide derivatives: Synthesis and spectroscopic investigation. Dyes Pigments 2007, 73, 361–367. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Arkhipova, A.N.; Fedorova, O.A.; Fedorov, Y.V.; Zakharko, M.A.; Arkhipov, D.E.; Jonusauskas, G. Controlling photophysics of styrylnaphthalimides through TICT, fluorescence and: E, Z -photoisomerization interplay. Phys. Chem. Chem. Phys. 2017, 19, 1244–1256. [Google Scholar] [CrossRef]
- Beltrán, A.; Burguete, M.I.; Luis, S.V.; Galindo, F. Styrylpyrylium Dyes as Solvent-Sensitive Molecules Displaying Dual Fluorescence. Eur. J. Org. Chem. 2017, 2017, 4864–4870. [Google Scholar] [CrossRef]
- Nolan, D.P.; Voorheis, H.P. Hydrogen ion gradients across the mitochondrial, endosomal and plasma membranes in bloodstream forms of Trypanosoma brucei. Eur. J. Biochem. 2000, 267, 4601–4614. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, J.Y.; Kang, E.J.; Lee, J.E.; Lee, S.S. A highly selective fluorescent chemosensor for silver(I) in water/ethanol mixture. Tetrahedron Lett. 2009, 50, 671–675. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Polyakova, A.S.; Fedorov, Y.V.; Fedorova, O.A. Chemoselective detection of Ag + in purely aqueous solution using fluorescence ‘turn-on’ probe based on crown-containing 4-methoxy-1,8-naphthalimide. Mendeleev Commun. 2019, 29, 155–157. [Google Scholar] [CrossRef]
- Walcarius, A.; Etienne, M.; Delacote, C. Uptake of inorganic HgII by organically modified silicates: Influence of pH and chloride concentration on the binding pathways and electrochemical monitoring of the processes. Anal. Chim. Acta 2004, 508, 87–98. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Ohkuma, S.; Poole, B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. PNAS 1978, 75, 3327–3331. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Thi Van Anh, H.; Yamamoto, A.; Koriyama, C.; Ishibashi, Y.; Tabata, M.; Nakano, A.; Yamamoto, M. Simple analysis of total mercury and methylmercury in seafood using heating vaporization atomic absorption spectrometry. J. Toxicol. Sci. 2016, 41, 489–500. [Google Scholar] [CrossRef]
- Loock, H.P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Zhao, M.; Shao, G.-K.; Guo, Y.-S.; Tang, Y.-L.; Liu, J.-B.; Guo, D.-S. A reaction-type receptor for the multi-feature detection of Hg2+ in water and living cells. New J. Chem. 2020, 44, 12538–12545. [Google Scholar] [CrossRef]
- Hoyle, I.; Handy, R.D. Dose-dependent inorganic mercury absorption by isolated perfused intestine of rainbow trout, Oncorhynchus mykiss, involves both amiloride-sensitive and energy-dependent pathways. Aquat. Toxicol. 2005, 72, 147–159. [Google Scholar] [CrossRef]
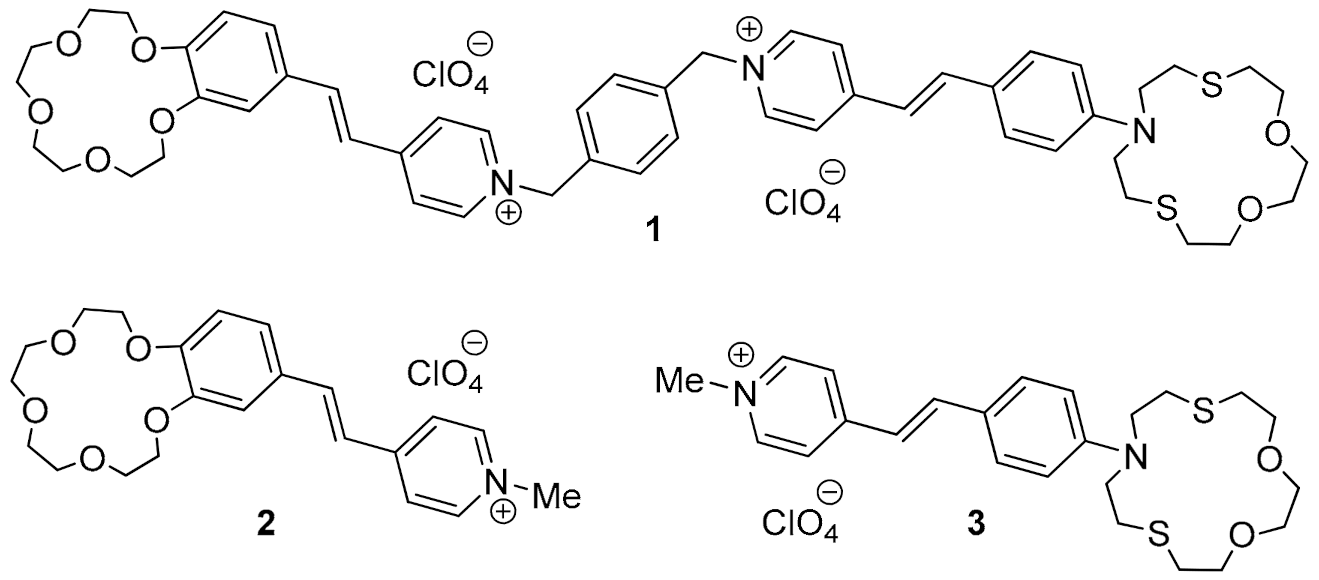

| Species | ||||||
|---|---|---|---|---|---|---|
| Spectrophotometric Titration | Spectrofluorometric Titration | |||||
| 1 | 402; 469 | 32,700; 22,950 | 603 (380) | 0.015 | – | – |
| (1)·Hg2+ | 395 | 40,970 | 581 (380) | 0.041 | 6.27 ± 0.12 | 6.24 ± 0.11 |
| 2 | 384 | 25,300 | 541 (380) | 0.029 | – | – |
| 3 | 457 | 30,650 | 607 (405) | 0.012 | – | – |
| (3)·Hg2+ | 380 | 18,390 | 571 (405) | 0.052 | 6.03 ± 0.06 | 6.05 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, P.A.; Efremenko, A.V.; Feofanov, A.V.; Ustimova, M.A.; Fedorov, Y.V.; Fedorova, O.A. Ratiometric Detection of Mercury (II) Ions in Living Cells Using Fluorescent Probe Based on Bis(styryl) Dye and Azadithia-15-Crown-5 Ether Receptor. Sensors 2021, 21, 470. https://doi.org/10.3390/s21020470
Panchenko PA, Efremenko AV, Feofanov AV, Ustimova MA, Fedorov YV, Fedorova OA. Ratiometric Detection of Mercury (II) Ions in Living Cells Using Fluorescent Probe Based on Bis(styryl) Dye and Azadithia-15-Crown-5 Ether Receptor. Sensors. 2021; 21(2):470. https://doi.org/10.3390/s21020470
Chicago/Turabian StylePanchenko, Pavel A., Anastasija V. Efremenko, Alexey V. Feofanov, Mariya A. Ustimova, Yuri V. Fedorov, and Olga A. Fedorova. 2021. "Ratiometric Detection of Mercury (II) Ions in Living Cells Using Fluorescent Probe Based on Bis(styryl) Dye and Azadithia-15-Crown-5 Ether Receptor" Sensors 21, no. 2: 470. https://doi.org/10.3390/s21020470
APA StylePanchenko, P. A., Efremenko, A. V., Feofanov, A. V., Ustimova, M. A., Fedorov, Y. V., & Fedorova, O. A. (2021). Ratiometric Detection of Mercury (II) Ions in Living Cells Using Fluorescent Probe Based on Bis(styryl) Dye and Azadithia-15-Crown-5 Ether Receptor. Sensors, 21(2), 470. https://doi.org/10.3390/s21020470








