Synthesis, Photophysical Characterization, and Sensor Activity of New 1,8-Naphthalimide Derivatives
Abstract
1. Introduction
2. Materials and Methods
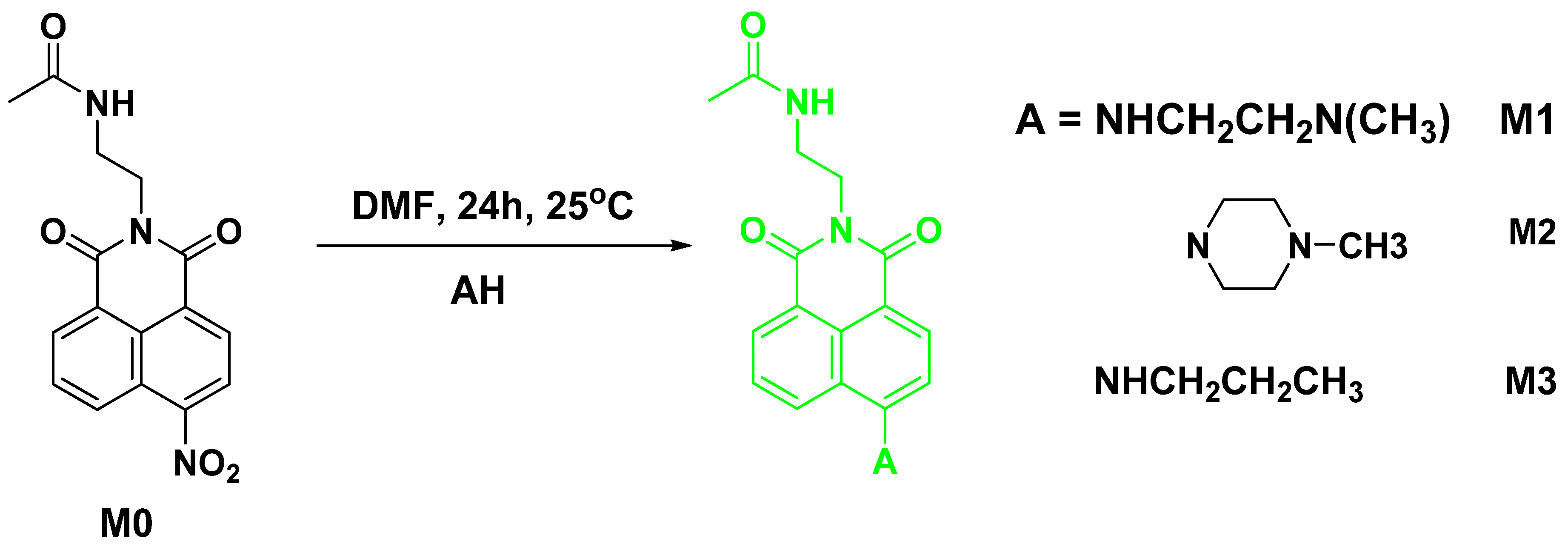
2.1. Synthesis of N-(2-(6-((2-(dimethylamino)ethyl)amino)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)acetamide M1
2.2. Synthesis of N-(2-(6-(4-methylpiperazin-1-yl)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)acetamide (M2)
2.3. Synthesis of N-(2-(1,3-dioxo-6-(propylamino)-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)acetamide (M3)
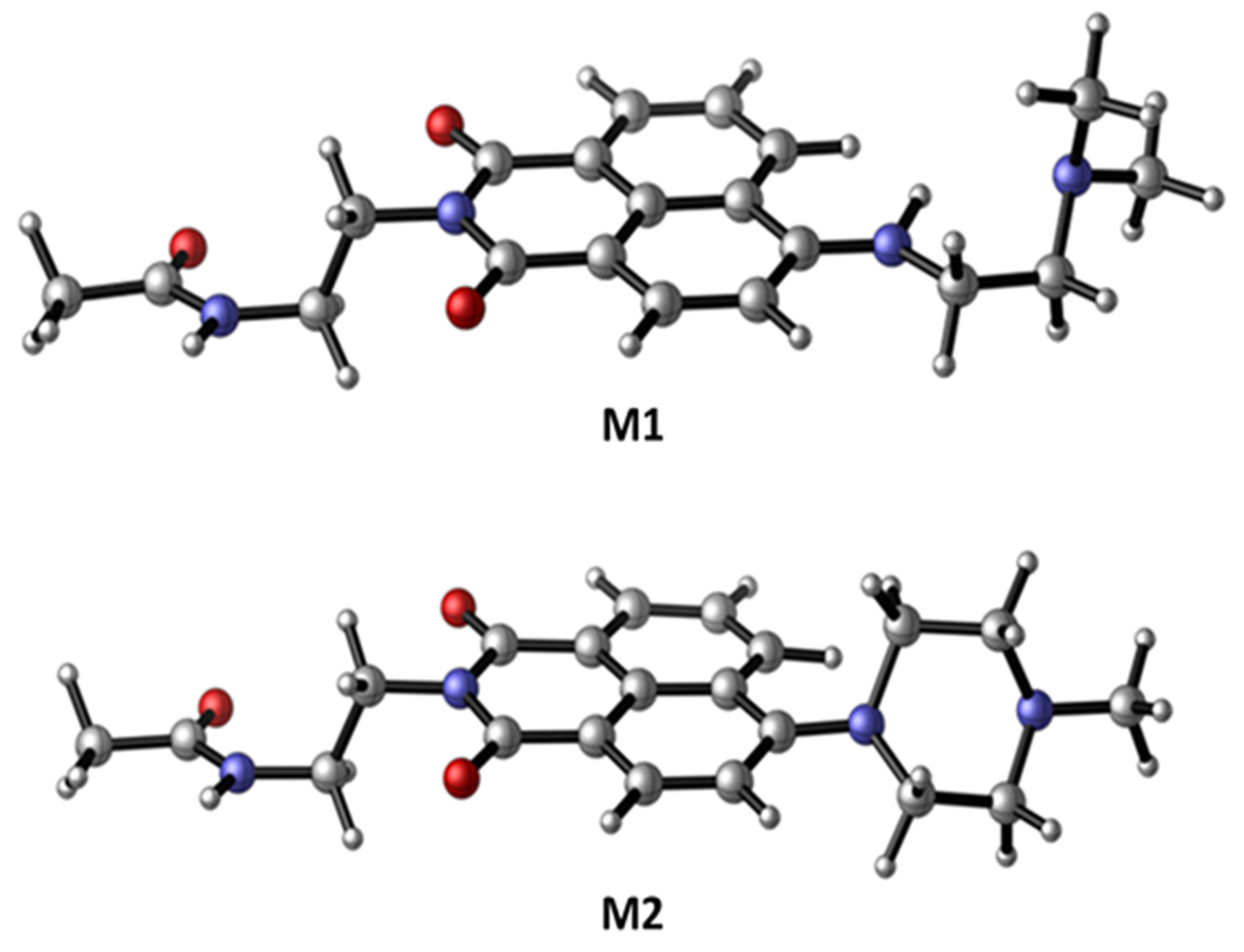
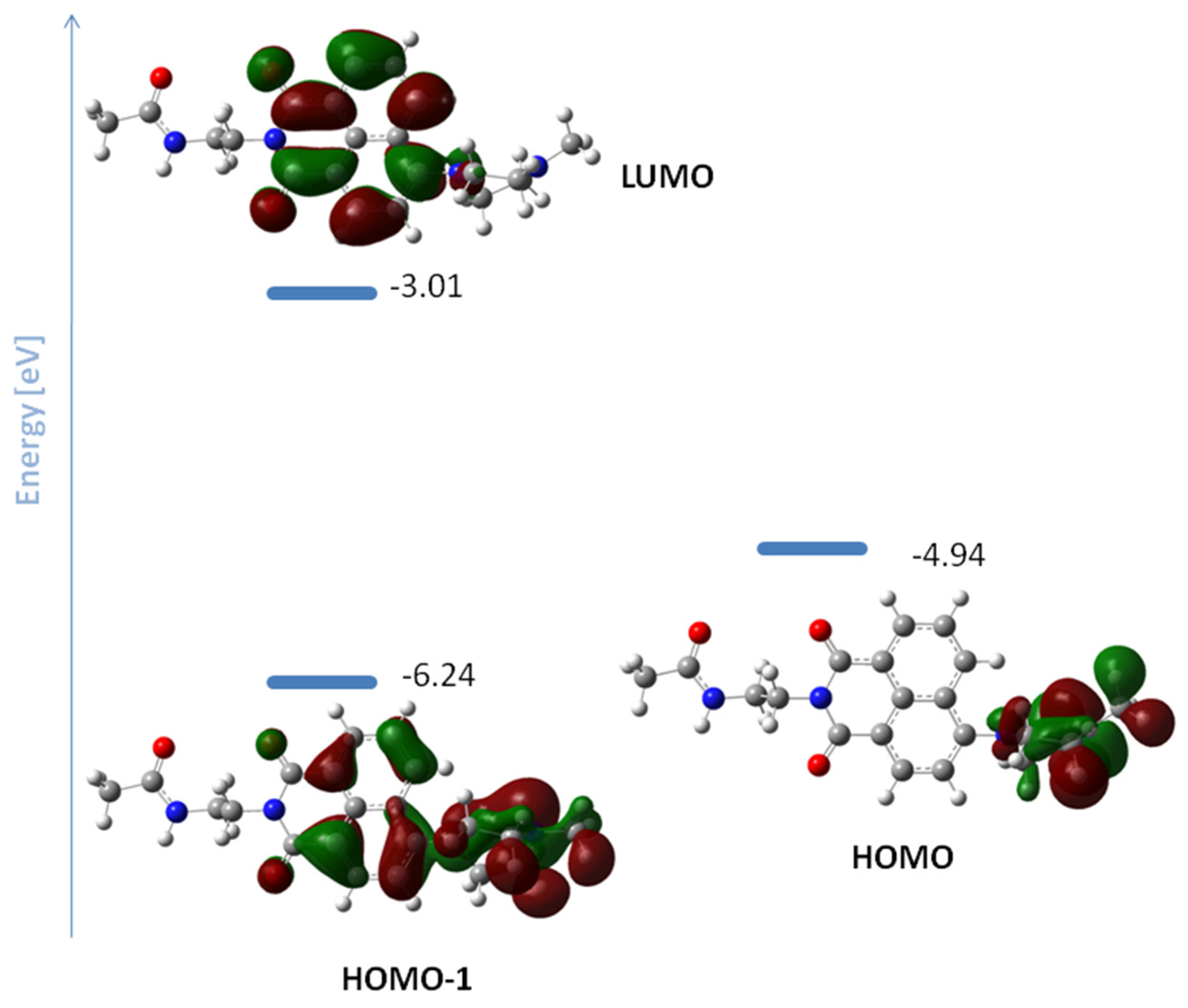
2.4. Computational Details
3. Results
3.1. Synthesis of Compounds M1–M3
3.2. Spectral Properties
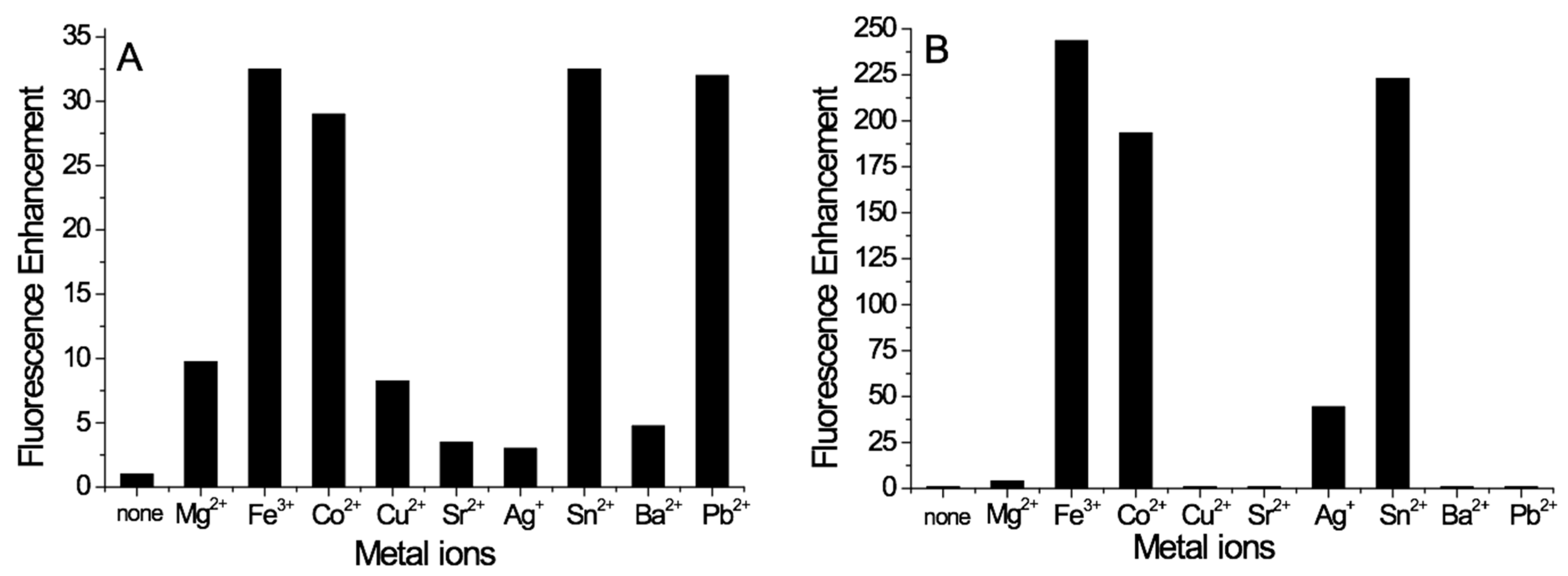
3.3. Detection Ability of Metal Cations of M1 and M2
3.4. Influence of pH on the Fluorescence Intensity of the M1 and M2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy, 3rd ed.; Springer US: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Rurack, K. Flipping the light switch ‘ON’—The design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2161–2195. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.A.; Fedorova, O.A.; Fedorov, Y.V. Fluorescent and colorimetric chemosensors for cations based on 1,8-naphthalimide derivatives: Design principles and optical signalling mechanisms. Russ. Chem. Rev. 2014, 83, 155–182. [Google Scholar] [CrossRef]
- Grabchev, I.; Qian, X.; Bojinov, V.; Xiao, Y.; Zhang, W. Synthesis and photophysical properties of 1,8-naphthalimide-labelled PAMAM as PET sensors of protons and of transition metal ions. Polymer (Guildf) 2002, 43, 5731–5736. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, M.; Qian, X.; Cui, J. A proton sponge-based fluorescent switch. Tetrahedron Lett. 2005, 46, 6289–6292. [Google Scholar] [CrossRef]
- Staneva, D.; Betcheva, R. Synthesis and functional properties of new optical pH sensor based on benzo[de]anthracen-7-one immobilized on the viscose. Dye. Pigment. 2007, 74, 148–153. [Google Scholar] [CrossRef]
- Staneva, D.; Betcheva, R.; Chovelon, J.-M. Fluorescent benzo[de]anthracen-7-one pH-sensor in aqueous solution and immobilized on viscose fabrics. J. Photochem. Photobiol. A Chem. 2006, 183, 159–164. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I. Heterogeneous sensors for ammonia, amines and metal ions based on a dendrimer modified fluorescent viscose fabric. Dye. Pigment. 2018, 155, 164–170. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. pH sensor potential and antimicrobial activity of a new PPA dendrimer modified with benzanthrone fluorophores in solution and on viscose fabric. J. Photochem. Photobiol. A Chem. 2019, 375, 24–29. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimitrova, M.D.; Asiri, A.M.; Alamry, K.A.; Bojinov, V.B. Synthesis, sensor activity and logic behaviour of a novel bichromophoric system based on rhodamine 6G and 1,8-naphthalimide. Dye. Pigment. 2015, 115, 172–180. [Google Scholar] [CrossRef]
- Abebe, F.; Perkins, P.; Shaw, R.; Tadesse, S. A rhodamine-based fluorescent sensor for selective detection of Cu2+ in aqueous media: Synthesis and spectroscopic properties. J. Mol. Struct. 2020, 1205, 127594. [Google Scholar] [CrossRef]
- Mehta, R.; Luxami, V. Rhodamine-anthraquinone based dyad for rapid and selective sensing of Al3+ with potential application for real-time sampling and molecular logic circuits. Inorg. Chem. Commun. 2020, 115, 107863. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Polyakova, A.S.; Fedorov, Y.V.; Fedorova, O.A. Chemoselective detection of Ag + in purely aqueous solution using fluorescence ‘turn-on’ probe based on crown-containing 4-methoxy-1,8-naphthalimide. Mendeleev Commun. 2019, 29, 155–157. [Google Scholar] [CrossRef]
- Fernández-Alonso, S.; Corrales, T.; Pablos, J.L.; Catalina, F. Surface modification of poly(ethylene-butyl acrylate) copolymers by microwave methodology and functionalization with 4-dimethylamino- N -(2-hydroxyethyl)-1,8-naphthalimide for acidity sensing. React. Funct. Polym. 2016, 107, 78–86. [Google Scholar] [CrossRef]
- Mohr, G.J. Synthesis of naphthalimide-based indicator dyes with a 2-hydroxyethylsulfonyl function for covalent immobilisation to cellulose. Sens. Actuators B Chem. 2018, 275, 439–445. [Google Scholar] [CrossRef]
- Marinova, N.V.; Georgiev, N.I.; Bojinov, V.B. Facile synthesis, sensor activity and logic behaviour of 4-aryloxy substituted 1,8-naphthalimide. J. Photochem. Photobiol. A Chem. 2013, 254, 54–61. [Google Scholar] [CrossRef]
- Aderinto, S.O.; Imhanria, S. Fluorescent and colourimetric 1, 8-naphthalimide-appended chemosensors for the tracking of metal ions: Selected examples from the year 2010 to 2017. Chem. Pap. 2018, 72, 1823–1851. [Google Scholar] [CrossRef]
- Sareen, D.; Kaur, P.; Singh, K. Strategies in detection of metal ions using dyes. Coord. Chem. Rev. 2014, 265, 125–154. [Google Scholar] [CrossRef]
- Shen, K.; Mao, S.; Shi, X.; Aderinto, S.O.; Xu, Y.; Wu, H. Development of a New 4-Amino-1,8-Naphthalimide Derivative as a Fluorescent Probe for Monitoring the Divalent Copper Ion. J. Appl. Spectrosc. 2018, 85, 665–672. [Google Scholar] [CrossRef]
- Oshchepkov, A.S.; Mittapalli, R.R.; Fedorova, O.A.; Kataev, E.A. Naphthalimide-Based Polyammonium Chemosensors for Anions: Study of Binding Properties and Sensing Mechanisms. Chem. Eur. J. 2017, 23, 9657–9665. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Krasteva, P.V.; Bojinov, V.B. A ratiometric 4-amido-1,8-naphthalimide fluorescent probe based on excimer-monomer emission for determination of pH and water content in organic solvents. J. Lumin. 2019, 212, 271–278. [Google Scholar] [CrossRef]
- Spiteri, J.C.; Johnson, A.D.; Denisov, S.A.; Jonusauskas, G.; McClenaghan, N.D.; Magri, D.C. A fluorescent AND logic gate based on a ferrocene-naphthalimide-piperazine format responsive to acidity and oxidizability. Dye. Pigment. 2018, 157, 278–283. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Betcheva, R. Sensor potential of 1,8-naphthalimide and its dyeing ability of cotton fabric. Dye. Pigment. 2013, 98, 64–70. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Betcheva, R. Fluorescent Dendrimers As Sensors for Biologically Important Metal Cations. Curr. Med. Chem. 2012, 19, 4976–4983. [Google Scholar] [CrossRef]
- Soršak, E.; Valh, J.V.; Urek, Š.K.; Lobnik, A. Application of PAMAM dendrimers in optical sensing. Analyst 2015, 140, 976–989. [Google Scholar] [CrossRef]
- Grabchev, I.; Dumas, S.; Chovelon, J.-M.; Nedelcheva, A. First generation poly(propyleneimine) dendrimers functionalised with 1,8-naphthalimide units as fluorescence sensors for metal cations and protons. Tetrahedron 2008, 64, 2113–2119. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Bosch, P.; Grabchev, I. A new green fluorescent tripod based on 1,8-naphthalimide. Detection ability for metal cations and protons and antimicrobial activity. J. Photochem. Photobiol. A Chem. 2017, 344, 143–148. [Google Scholar] [CrossRef]
- Medel, S.; Bosch, P.; Grabchev, I.; de la Torre, M.C.; Ramírez, P. Click chemistry to fluorescent hyperbranched polymeric sensors. 2. Synthesis, spectroscopic and cation-sensing properties of new green fluorescent 1,8-naphthalimides. Eur. Polym. J. 2016, 74, 241–255. [Google Scholar] [CrossRef]
- Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Staneva, D.; Bosch, P.; Medel, S.; Grabchev, I. Evaluation of antimicrobial, biofilm inhibitory and cytotoxic activities of a new hiperbranched polymer modified with 1,8-naphthalimide units. Biointerface Res. Appl. Chem. 2018, 8, 3053–3059. [Google Scholar]
- Wenxun, G.; Yaqing, Z.; Botian, L.; Liming, T. Fluorescent sensor of copper(II) ions based on PMBA microtubes with 4-methoxy-1,8-naphthalimide groups. React. Funct. Polym. 2020, 146, 104400. [Google Scholar] [CrossRef]
- Grabchev, I.; Sali, S.; Betcheva, R.; Gregoriou, V. New green fluorescent polymer sensors for metal cations and protons. Eur. Polym. J. 2007, 43, 4297–4305. [Google Scholar] [CrossRef]
- Sali, S.; Guittonneau, S.; Grabchev, I. A novel blue fluorescent chemosensor for metal cations and protons, based on 1,8-naphthalimide and its copolymer with styrene. Polym. Adv. Technol. 2006, 17, 180–185. [Google Scholar] [CrossRef]
- Zhao, P.; Jiang, J.; Leng, B.; Tian, H. Polymer Fluoride Sensors Synthesized by RAFT Polymerization. Macromol. Rapid Commun. 2009, 30, 1715–1718. [Google Scholar] [CrossRef]
- Grabchev, I.; Bojinov, V.; Chovelon, J.M. Synthesis, photophysical and photochemical properties of fluorescent poly(amidoamine) dendrimers. Polymer (Guildf) 2003, 44, 4421–4428. [Google Scholar] [CrossRef]
- Yordanova, S.; Grabchev, I.; Stoyanov, S.; Petkov, I. New detectors for metal cations and protons based on PAMAM dendrimers modified with 1,8-naphthalimide units. J. Photochem. Photobiol. A Chem. 2014, 283, 1–7. [Google Scholar] [CrossRef]
- Salman, H.; Tal, S.; Chuvilov, Y.; Solovey, O.; Abraham, Y.; Kapon, M.; Suwinska, K.; Eichen, Y. Sensitive and selective PET-based diimidazole luminophore for Zn II ions: A structure-activity correlation. Inorg. Chem. 2006, 45, 5315–5320. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hancock, R.D.; Lee, H.-S. Role of Fluorophore–Metal Interaction in Photoinduced Electron Transfer (PET) Sensors: Time-Dependent Density Functional Theory (TDDFT) Study. J. Phys. Chem. A 2013, 117, 13345–13355. [Google Scholar] [CrossRef]
- Reynolds, G.A.; Drexhage, K.H. New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt. Commun. 1975, 13, 222–225. [Google Scholar] [CrossRef]
- Ramachandram, B.; Saroja, G.; Sankaran, B.; Samanta, A. Unusually High Fluorescence Enhancement of Some 1,8-Naphthalimide Derivatives Induced by Transition Metal Salts. J. Phys. Chem. B 2000, 104, 11824–11832. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision 16.A.03. Available online: https://gaussian.com/g09citation/. (accessed on 13 July 2020).
- Labanowski, J.K.; Andzelm, J.W. (Eds.) Density Functional Methods in Chemistry; Springer: New York, NY, USA, 1991. [Google Scholar]
- Casida, M.E.; Chong, D.P. Recent Advances in Density Functional Methods; World Scientific: Singapore, 1995; Volume 1, ISBN 978-981-02-2442-4. [Google Scholar]
- Furche, F.; Ahlrichs, R. Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 2002, 117, 7433–7447. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable adiabatic connection models free from adjustable parameters. Chem. Phys. Lett. 1997, 274, 242–250. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Bojinov, V.; Guittonneau, S. Synthesis and properties of fluorescent 1,8-naphthalimide dyes for application in liquid crystal displays. J. Mater. Chem. 2000, 10, 1291–1296. [Google Scholar] [CrossRef]
- Alexiou, M.S.; Tychopoulos, V.; Ghorbanian, S.; Tyman, J.H.P.; Brown, R.G.; Brittain, P.I. The UV-visible absorption and fluorescence of some substituted 1,8-naphthalimides and naphthalic anhydrides. J. Chem. Soc. Perkin Trans. 2 1990, 837–842. [Google Scholar] [CrossRef]
- Stoyanov, S.; Petrov, P.; Stoyanova, M.; Dangalov, M.; Shivachev, B.; Nikolova, R.; Petkov, I. 4-Amino-3-nitro naphthalimides—Structures and spectral properties. J. Photochem. Photobiol. A Chem. 2012, 250, 92–98. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview, 1.0b. Université de Sherbrooke. 2009. Available online: http://www.cylview.org (accessed on 13 July 2020).
- Guido, C.A.; Mennucci, B.; Jacquemin, D.; Adamo, C. Planar vs. twisted intramolecular charge transfer mechanism in Nile Red: New hints from theory. Phys. Chem. Chem. Phys. 2010, 12, 8016. [Google Scholar] [CrossRef]
- Jacquemin, D.; Mennucci, B.; Adamo, C. Excited-state calculations with TD-DFT: From benchmarks to simulations in complex environments. Phys. Chem. Chem. Phys. 2011, 13, 16987. [Google Scholar] [CrossRef] [PubMed]
- Briggs, E.A.; Besley, N.A. Density Functional Theory Based Analysis of Photoinduced Electron Transfer in a Triazacryptand Based K + Sensor. J. Phys. Chem. A 2015, 119, 2902–2907. [Google Scholar] [CrossRef]






| DMSO | DMF | MeCN | MeOH | EtOH | CH2Cl2 | Dioxane | EtOAc | CHCl3 | |
|---|---|---|---|---|---|---|---|---|---|
| M1 | |||||||||
| λabs (nm) | 429 | 438 | 432 | 436 | 436 | 434 | 426 | 426 | 433 |
| λflu (nm) | 516 | 523 | 509 | 520 | 522 | 509 | 508 | 510 | 505 |
| ΔνSt (cm−1) | 3930 | 3711 | 3502 | 3705 | 3779 | 3395 | 3789 | 3866 | 3293 |
| ԑ 10−3 (L mol−1 cm−1) | 14.3 | 14.1 | 14.7 | 17.0 | 15.0 | 17.0 | 12.7 | 14.0 | 15.9 |
| ΦF | 0.006 | 0.009 | 0.12 | 0.019 | 0.017 | 0.358 | 0.274 | 0.22 | 0.53 |
| M2 | |||||||||
| λabs (nm) | 406 | 403 | 399 | 396 | 397 | 406 | 395 | 394 | 402 |
| λflu (nm) | 520 | 527 | 525 | 518 | 520 | 514 | 506 | 509 | 499 |
| ΔνSt (cm−1) | 5400 | 5839 | 6015 | 5948 | 5958 | 5175 | 5554 | 5734 | 4836 |
| ԑ 10−3 (L mol−1 cm−1) | 7.1 | 9.0 | 10.6 | 10.9 | 10.4 | 11.2 | 11.2 | 10.1 | 10.5 |
| ΦF | 0.007 | 0.003 | 0.002 | 0.008 | 0.007 | 0.01 | 0.054 | 0.031 | 0.064 |
| M3 | |||||||||
| λabs (nm) | 444 | 440 | 434 | 442 | 444 | 432 | 427 | 431 | 433 |
| λflu (nm) | 529 | 523 | 521 | 534 | 528 | 508 | 502 | 510 | 502 |
| ΔνSt (cm−1) | 3619 | 3607 | 3848 | 3898 | 3583 | 3463 | 3499 | 3594 | 3371 |
| ԑ 10−3 (L mol−1 cm−1) | 7.5 | 9.5 | 9.8 | 11.3 | 9.7 | 11.2 | 9.2 | 9.5 | 10.0 |
| ΦF | 0.59 | 0.424 | 0.401 | 0.259 | 0.334 | 0.533 | 0.551 | 0.467 | 0.552 |
| Dye | ||||
|---|---|---|---|---|
| Exp | B3LYP | PBE0 | M062x | |
| 6-311++G(d,p) | ||||
| M1 | 2.87 | 2.87 (0.3284) | 2.98 (0.3551) | 3.23 (0.4709) |
| M2 | 3.11 | 2.68 (0.1899) 3.10 (0.1154) | 2.81 (0.2332) 3.27 (0.0977) | 3.27 (0.4251) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanova-Tomova, S.; Cheshmedzhieva, D.; Stoyanov, S.; Dudev, T.; Grabchev, I. Synthesis, Photophysical Characterization, and Sensor Activity of New 1,8-Naphthalimide Derivatives. Sensors 2020, 20, 3892. https://doi.org/10.3390/s20143892
Yordanova-Tomova S, Cheshmedzhieva D, Stoyanov S, Dudev T, Grabchev I. Synthesis, Photophysical Characterization, and Sensor Activity of New 1,8-Naphthalimide Derivatives. Sensors. 2020; 20(14):3892. https://doi.org/10.3390/s20143892
Chicago/Turabian StyleYordanova-Tomova, Stanislava, Diana Cheshmedzhieva, Stanimir Stoyanov, Todor Dudev, and Ivo Grabchev. 2020. "Synthesis, Photophysical Characterization, and Sensor Activity of New 1,8-Naphthalimide Derivatives" Sensors 20, no. 14: 3892. https://doi.org/10.3390/s20143892
APA StyleYordanova-Tomova, S., Cheshmedzhieva, D., Stoyanov, S., Dudev, T., & Grabchev, I. (2020). Synthesis, Photophysical Characterization, and Sensor Activity of New 1,8-Naphthalimide Derivatives. Sensors, 20(14), 3892. https://doi.org/10.3390/s20143892







