Abstract
Evaluation of arterial carbon dioxide pressure (PaCO2) and dead space to tidal volume ratio (VD/VT) during exercise is important for the identification of exercise limitation causes in heart failure (HF). However, repeated sampling of arterial or arterialized ear lobe capillary blood may be clumsy. The aim of our study was to estimate PaCO2 by means of a non-invasive technique, transcutaneous PCO2 (PtCO2), and to verify the correlation between PtCO2 and PaCO2 and between their derived parameters, such as VD/VT, during exercise in HF patients. 29 cardiopulmonary exercise tests (CPET) performed on a bike with a ramp protocol aimed at achieving maximal effort in ≈10 min were analyzed. PaCO2 and PtCO2 values were collected at rest and every 2 min during active pedaling. The uncertainty of PCO2 and VD/VT measurements were determined by analyzing the error between the two methods. The accuracy of PtCO2 measurements vs. PaCO2 decreases towards the end of exercise. Therefore, a correction to PtCO2 that keeps into account the time of the measurement was implemented with a multiple regression model. PtCO2 and VD/VT changes at 6, 8 and 10 min vs. 2 min data were evaluated before and after PtCO2 correction. PtCO2 overestimates PaCO2 for high timestamps (median error 2.45, IQR −0.635–5.405, at 10 min vs. 2 min, p-value = 0.011), while the error is negligible after correction (median error 0.50, IQR = −2.21–3.19, p-value > 0.05). The correction allows removing differences also in PCO2 and VD/VT changes. In HF patients PtCO2 is a reliable PaCO2 estimation at rest and at low exercise intensity. At high exercise intensity the overall response appears delayed but reproducible and the error can be overcome by mathematical modeling allowing an accurate estimation by PtCO2 of PaCO2 and VD/VT.
1. Introduction
Assessment of dead space/tidal volume ratio (VD/VT) and PaCO2 during exercise is of paramount importance for identification of exercise limitation at cardiopulmonary exercise testing (CPET) in the setting of several cardiovascular and pulmonary diseases, including heart failure (HF). VD/VT value during exercise is calculated through simultaneous measurement of PaCO2 and mean expiratory PCO2 (PECO2) [1]. VD/VT is used to assess ventilation/perfusion mismatch [2,3] and it is elevated in case of concomitant pulmonary hypertension and/or respiratory disease. PaCO2 value during the isocapnic buffering period is a recognized index of reflex ventilation regulation [4,5]. Moreover, the end tidal CO2—arterial CO2 pressure gradient (ΔPetCO2 − PaCO2) during exercise is another parameter useful to assess ventilation perfusion mismatch in the lung [6].
Repeated arterial or capillary ear lobe blood sampling, both in static conditions and during exercise, to measure/estimate PaCO2 may be uneasy on a routine basis and outside of clinical settings. The technical reasons behind this are invasiveness, size of the catheter, instability of the calibration due to clotting, possible air contamination of the arterial blood sample and lack of reusability [7,8]. Therefore, non-invasive derived PaCO2 estimation seems desirable. End-tidal PCO2 (PetCO2) has been considered as a reliable estimate of arterial PCO2, in healthy subjects and in particular conditions such as monitoring during anesthesia [9,10]. However, increase in ventilation/perfusion mismatch makes evaluation of PaCO2 by PetCO2 highly unreliable in several diseases [11,12,13,14], as well as during sleep [15]. Moreover, a lack of accuracy in the estimation of PaCO2 and VD/VT by PetCO2 has been reported during exercise making its use unreliable, at least in cardiorespiratory patients [16]. Regardless, albeit such an approximation is inaccurate, several commercial ergospirometers software report estimations of VD/VT using PetCO2 as a PaCO2 surrogate [17].
Transcutaneous PCO2 (PtCO2) devices provide another option for the continuous noninvasive estimation of PaCO2, overcoming the limitations posed by end-tidal CO2 analysis [18]. PtCO2 is measured using Severinghaus–Stow-type electrodes, i.e., with an electrochemical sensor [19], with a heating system that brings the skin temperature up to about 42 °C. Commercial devices include probes with a single PtCO2 sensor, probes with a combination of partial pressure of oxygen (PO2) and PtCO2 and probes with a combination of pulse oximetry (SpO2) and PtCO2 measurements [20]. The methodology has been constantly improved over the years, making PtCO2 systems easier to use and more reliable in clinical practice. The main characteristics of commercial sensors are small dimensions (diameter 15 mm, height 8 mm), long-time for re-membranization (every 2 weeks), calibration required twice a day, short arterialization time (3 min) and high measurement reliability thanks to the protection of the membrane. This type of measurement has shown to closely approximate PaCO2 both at rest and during symptom limited exercise in normal subjects and in patients with lung disease [16]. Whether PtCO2 can be used as a reliable surrogate of PaCO2 during a maximal effort in patients with heart failure is actually unknown. The aim of our study was therefore to verify the correlation between PtCO2 and PaCO2 and between VD/VT derived from PtCO2 and that derived from PaCO2 during a maximal exercise test in patients with stable heart failure. The novelty of this study consists in the application of such electrochemical sensors, which require stable operational conditions, in a highly dynamic situation where parameters are expected to change faster, and movement artefacts might be present. The final purpose of the study is to find an adequate protocol to use PtCO2 sensing on patients in dynamic conditions to estimate PaCO2 and derived parameters, such as VD/VT. Using such a protocol allows to study the response to exercise, i.e., to an increased metabolic demand, with a continuous sampling and a non-invasive method.
2. Materials and Methods
This study was designed as a sub study of a larger trial (Ethics Committee approval number CCM966) dedicated to the analysis of exercise performance in patients with severe HF. Study inclusion criteria were stable chronic heart failure with stabilized therapy in NYHA class II or III; left ventricular ejection fraction ≤ 40%, peak oxygen uptake (VO2) ≤ 12 mL/Kg/min or E/e’ > 13 at cardiac ultrasound and ability to perform a maximal exercise test on a bike. Exclusion criteria were age < 18 years, severe primitive valvular disease, significant pericardial disease, previous pulmonary embolism, peripheral arterial disease limiting exercise capacity, effort angina, or sign of ischemia at EKG, uncontrolled arrhythmias, pregnancy, severe pulmonary disease and the presence of any counterindication to exercise testing.
2.1. Procedures
A symptoms-limited maximal CPET was performed on a bike with a personalized ramp protocol (Quark PFT, Cosmed Cart, Rome, Italy) aimed to reach the maximal effort in around 10 min. The duration of the test was chosen based on the results by Agostoni et al. [21], who demonstrated that to assess exercise performance in HF patients by cardiopulmonary exercise test the exercise protocol needs to be 10 min long. To do so, the exercise protocol needs to be performed with a personalized progressive workload which means that the protocol must be adapted to the patient’s clinical conditions. Other types of protocols such as endurance or fixed workload or step increasing protocols do not allow to identify peak VO2. CPET was performed and analyzed following standard technique [22]. Briefly, patients were encouraged to continue the test until reaching a respiratory exchange ratio (RER) of at least 1.05. VO2, CO2 production (VCO2), PetCO2, end tidal oxygen pressure (PetO2), ventilation (VE), tidal volume (VT), respiratory rate (RR) and workload were recorded breath by breath and averaged every 10 s. Several minutes before the exercise test a small catheter was inserted in the radial artery. Arterial blood samples were collected at rest, i.e., beginning of the loaded pedaling, (minute 0) and every 2 min during exercise and immediately analyzed by a blood gas analyzer (GEM Premier 4000, Werfen, Barcelona, Spain) for PaCO2 determination. Heart rate (HR), hemoglobin O2 saturation (SpO2) and PtCO2 were monitored continuously. To measure PtCO2, a commercial electrochemical sensor combined with a heating system (V-sign™ Sensor 2, Sentec AG, Therwil, Switzerland) was used. The technical characteristics of the commercial measurement system used are reported in Table 1.

Table 1.
Technical characteristics of the PtCO2 sensor as reported in the user manual.
The PCO2 measurement of the V-sign™ Sensor 2 is based on a Stow–Severinghaus type PCO2 sensor, i.e., a thin electrolyte layer is confined to the sensor surface with a hydrophobic, CO2 and O2 permeable membrane. The system was calibrated and applied over the patient’s earlobe through a clip with an adhesive layer after application of a fluid drop for optimal contact to the skin.
PtCO2 recording started after a stabilization time of around 10 min. A marker was applied at the time of arterial sample collection to synchronize PtCO2, PetCO2 and PaCO2 values. The V-sign™ Sensor 2 is known to have a response time <75 s, as reported in the user manual and in Table 1. Blood pressure was manually measured every two minutes by a sphygmomanometer. We analyzed data at rest, every two minutes of exercise and at the end of the ramp protocol. Since the loaded exercise started at 0 Watts no unloaded pedaling was done.
A total of 23 patients were enrolled in the present study, 6 of which have repeated the protocol twice, after treatment update. Overall, 29 acquisitions have been evaluated. The present study is a preliminary study, designed as a sub-study of a different research report. We were not able to define a priori the sample needed to define at each exercise step the reliability of PtCO2 measurements because no data exist to predict such a difference. For this reason, we used all measurements available from the original trial. The present report data may be used as reference for sample size determination on future studies on this topic.
2.2. Multiple Regression
As explained in detail in the following sections, it was observed (Figure 1) that the accuracy of PtCO2 measurements with respect to the reference PaCO2 values decreases at increasing timestamps, i.e., towards the end of the protocol. For this reason, the possibility of adding a correction to PtCO2 that keeps into account the time of the measurement during the protocol was exploited.
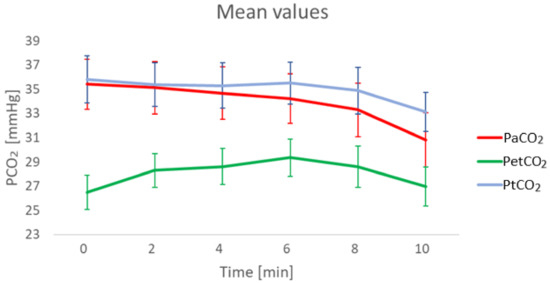
Figure 1.
Mean values of PaCO2, PtCO2 and end-tidal CO2 partial pressure (PetCO2) at different timestamps. PetCO2 is shown for completeness of CO2 measurement parameters. 29 sets of data were available at minute 0, 2 and 4, 28 at min 6, 26 at min 8 and 22 at min 10.
This was implemented by means of a multiple regression model. Multiple regression is an extension of linear regression and is used to predict the value of a variable based on two or more inputs [23]; the general formula describing multiple regression is (1):
where m is the number of input variables, is the predicted output, are the coefficients of the model ( is the value when all input variables are zero) and are the input variables.
In this case, the value to be predicted is PaCO2 and the two variables that are used are PtCO2 and the timestamp (0 min, 2 min, 4 min, 6 min, 8 min, and 10 min). A corrected PtCO2 value is obtained afterwards, and this new value is compared to the previous results; the used formulation is given by (2):
In (2), is a continuous variable expressed in min and is the beginning of the loaded pedaling in the ramp protocol. The function f is given by the multiple regression model, which was developed with a Python software based on the library scikit-learn [24].
2.3. Uncertainty of PtCO2 and VD/VT Measurements
A first analysis consisted in estimating the uncertainty of PtCO2 and VD/VT measurements obtained at different measuring times (0 min, 2 min, 4 min, 6 min, 8 min and 10 min) by analyzing the error between the two measurement methods: PaCO2 vs. PtCO2 and VD/VT obtained from PaCO2 versus VD/VT obtained from PtCO2.
Then, a multiple regression was applied to PtCO2 values at different timestamps to improve the measurements; the obtained values are referred to as corrected PtCO2. The formulas to compute the error of PtCO2 and corrected PtCO2 are, respectively, (3) and (4):
where is a continuous variable expressed in minutes, is the beginning of the loaded pedaling in the ramp protocol and their difference is the timestamp at which the measurement is performed.
Bland–Altman plots [25,26], also known as Tukey mean-difference plots [27] in fields other than medicine and biosciences, were used to assess the agreement between the two measurement methods before and after the correction by multiple regression was applied.
In the case of VD/VT, the estimation of the parameters is analyzed when each PCO2 value (arterial, non-corrected transcutaneous or corrected transcutaneous) is used, and the error is computed with the same method. In the present work, estimations of VD/VT are performed with the Enghoff equation, i.e., using PaCO2, instead of the Bohr equation because the latter uses alveolar CO2 (PaCO2) [28]. is the mean expiratory partial pressure of CO2 and was obtained from cardiopulmonary exercise testing (CPET). The formulas to obtain VD/VT in the different cases are (5)–(7):
2.4. Analysis of the Deltas
Another characteristic that is important to evaluate is the ability of the transcutaneous measurement system to follow differential variations of the value during the protocol. For this reason, variations of the values at 6, 8, and 10 min with respect to 2 min have been evaluated before and after the correction of PtCO2 is applied. This was applied both in the case of PCO2 and VD/VT extracted from transcutaneous measurements. The 2-min blood sample was chosen due to its lower variability.
The distributions of the deltas of PtCO2 and corrected PtCO2 are compared pairwise with the deltas of PaCO2; in the case of VD/VT, the deltas of the estimations obtained with PtCO2 and corrected PtCO2 are compared pairwise with the deltas of the estimations obtained from PaCO2. PCO2 deltas are expressed in mmHg, while VD/VT deltas are adimensional.
2.5. Statistical Analysis
The distributions of the errors at different timestamps were analyzed with One-way repeated measurement ANOVA or an equivalent method for non-normal distributions. This method was chosen due to the repeated and correlated nature of the measurements. The comparison was performed considering minute 0 as the reference distribution in the case of PtCO2 and the derived VD/VT.
The pairings of the deltas were first tested for normality with the Kolmogorov-Smirnov test [29]: for normal distributions, the paired t-test was used; for non-normal distributions, the Wilcoxon signed rank test was chosen instead.
3. Results
29 sets of data were available at minute 0, 2 and 4, 28 at minute 6, 26 at minute 8 and 22 at minute 10. Main clinical characteristics of the patients and main CPET results are reported in Table 2.

Table 2.
Heart failure patient characteristics. Six subjects were tested twice. CPET data refer to 29 measurements. BMI = body mass index; CPET = cardiopulmonary exercise testing; VO2 = oxygen uptake; VE = ventilation; VCO2 = carbon dioxide production; RER = respiratory exchange ratio.
3.1. Uncertainty of PtCO2 Measurements
In Figure 2, the boxplots of the errors in PaCO2 estimation before (left) and after PtCO2 is corrected (right) are reported. PtCO2 without correction tends to overestimate PaCO2 for high timestamps, while the error is stably centered around 0 after correction is applied.
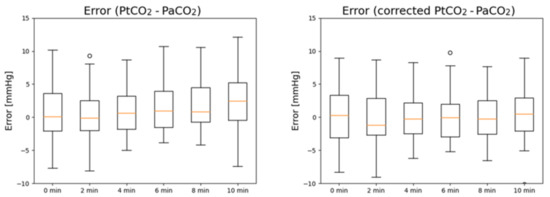
Figure 2.
Boxplots of the measurement errors of PtCO2 (left) and corrected PtCO2 (right) at different timestamps with respect to PaCO2.
The Bland–Altman analysis before and after correction is reported in Figure 3 and Figure 4, respectively. In both cases, the difference is computer as (non-corrected or corrected) PtCO2 minus PaCO2.
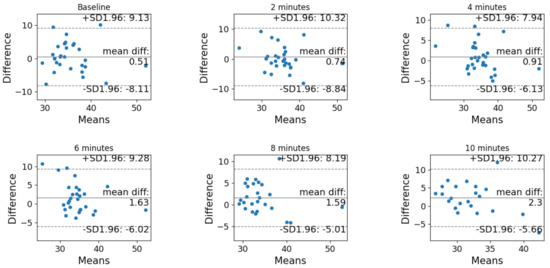
Figure 3.
Bland–Altman analysis of the agreement between PtCO2 and PaCO2 before the correction at each timestamp (baseline, after 2 min, after 4 min, after 6 min, after 8 min and after 10 min).
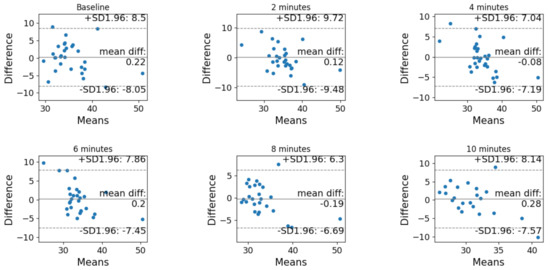
Figure 4.
Bland–Altman analysis of the agreement between PtCO2 and PaCO2 after the correction at each timestamp (baseline, after 2 min, after 4 min, after 6 min, after 8 min and after 10 min).
The results of the One-way repeated measurements ANOVA or equivalent test for non-normal distributions are different before and after the correction with multiple regressions is performed.
Before the correction, the distributions are not normal. According to the Bonferroni t-tests (multiple comparisons vs. a control group, in this case the baseline), the distribution of the error after 10 min is significantly different from the minute 0 distribution (p = 0.011), while the distributions at other timestamps (2, 4, 6 and 8 min) have a p-value > 0.05.
After the correction, the distributions are normal. The differences in the mean values among the groups are not great enough to exclude the possibility that the difference is due to random sampling variability; there is not a statistically significant difference (p = 0.978).
3.2. Uncertainty of VD/VT Measurements Derived from PtCO2
In Figure 5, the boxplots of the errors in VD/VT estimation from PtCO2 before (left) and after PtCO2 is corrected (right) are reported. Similar to PCO2, VD/VT calculated with PtCO2 without correction tends to overestimate VD/VT calculated with PaCO2 for high timestamps, while the error is stably centered around 0 after correction is applied.
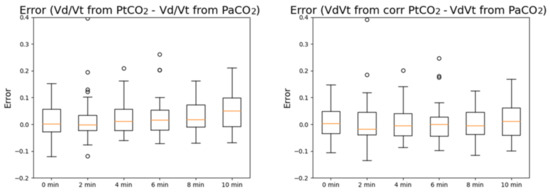
Figure 5.
Boxplots of the measurement errors of VD/VT computed with PtCO2 (left) and VD/VT computed with the corrected PtCO2 (right) at different timestamps with respect to VD/VT computed with PaCO2. The errors are expressed in mmHg.
Additionally in this case, the results of the One-way repeated measurements ANOVA or equivalent test for non-normal distributions are different before and after the correction of PtCO2 with multiple regressions is performed.
Before the correction, the distributions are not normal. According to the Bonferroni t-tests (multiple comparisons versus a control group, in this case the baseline), the distribution of the error after 10 min is significantly different from the minute 0 distribution (p < 0.001), while the distributions at other timestamps (2, 4, 6 and 8 min) have a p-value > 0.05.
After the correction, the distributions are still not normal. The differences in the mean values among the groups are not great enough to exclude the possibility that the difference is due to random sampling variability; there is not a statistically significant difference (p = 0.864).
3.3. Analysis of the Deltas
In Figure 6, the deltas of PaCO2, PtCO2 and corrected PtCO2 at different timestamps with respect to the values measured after 2 min are reported.
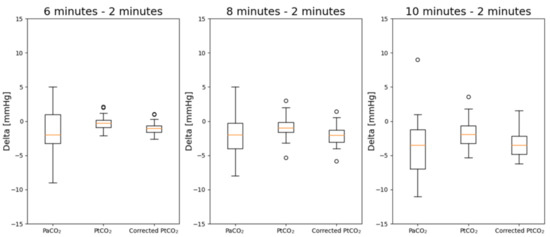
Figure 6.
Boxplots of the deltas of PaCO2, PtCO2 and corrected PtCO2. The deltas are evaluated at 6, 8 and 10 min with respect to the values measured after 2 min.
The results of the statistical analysis are reported in Table 3. Deltas with respect to 2 min have been considered. The deltas are evaluated at 6, 8 and 10 min with respect to the values measured after 2 min. 28 pairs of data were available at minute 6, 26 at minute 8 and 22 at minute 10. The values in italic are those where the distribution is not normal. The values in bold are those with a p-value < 0.05 which highlights a statistically significant difference.

Table 3.
p-values after the pairwise comparisons of deltas of PtCO2 and corrected PtCO2 with respect to deltas of PaCO2.
Without the correction by means of multiple regressions, there are statistically significant differences between the deltas at 8 and 10 min with respect to the measurements obtained after 2 min. The correction allows to remove such difference. Furthermore, the distributions after the correction are normal.
The same analysis was repeated for VD/VT. In Figure 7, the deltas of VD/VT estimated with PaCO2, PtCO2 and corrected PtCO2 at different timestamps with respect to the values measured after 2 min are reported while the overall results of the statistical analysis are reported in Table 4. The deltas are evaluated at 6, 8 and 10 min with respect to the values measured after 2 min. The values in bold are those with a p-value < 0.05 which highlights a statistically significant difference.
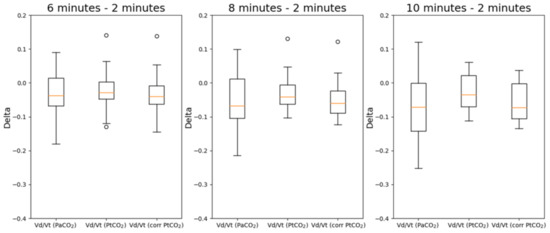
Figure 7.
Boxplots of the deltas of VD/VT estimated with PaCO2, PtCO2 and corrected PtCO2. The deltas are evaluated at 6, 8 and 10 min with respect to the values measured after 2 min.

Table 4.
p-values after the pairwise comparisons of deltas obtained with VD/VT estimated with PtCO2 and corrected PtCO2 with respect to deltas of VD/VT estimated with PaCO2.
Deltas with respect to the value measured 2 min after the beginning of the protocol have been considered. In this case, all distributions are normal, so the only used test was the paired t-test. Without the correction by means of multiple regressions, there are statistically significant differences between the deltas at 8 and 10 min with respect to the measurements obtained after 2 min. The correction allows to remove such difference in all cases.
4. Discussion
It is known from the literature that physiological parameters change with different levels of activity both inside and outside clinical settings [30,31] and that, at the same time, the performance of sensors and instrumentation in detecting these changes decreases with increasing levels of activity [32]. For this reason, it is relevant to study the performance of different types of instrumentation also during experimental protocols with highly dynamic activities and maximal exercise. The present study shows that in patients with severe but stable heart failure PtCO2 without any further correction is a reliable estimation of PaCO2 at rest and during a progressive workload exercise only at low exercise intensity. Indeed, when exercise effort increases and PaCO2 reduces the PCO2 value derived by transcutaneous sensors show a delayed response. This delayed response is partly due to the delay of the sensor system, which is known to be <75 s; however, it must be noted that even if the PtCO2 were partially shifted to take into account the delay, this correction would not be sufficient. Nevertheless, the overall response appears reproducible and therefore predictable, and the overall error can be overcome by mathematical modeling so that PtCO2 allow a precise estimation of PaCO2 and of PaCO2 derived data such as VD/VT. In brief our model allows to accurately estimate PaCO2 from PtCO2 during a maximal effort exercise and notably it allows to analyze exercise induced PaCO2 changes.
In terms of mathematical method, the presented article implemented a correction based on multiple regression. The implemented correction was performed by minimizing the error with a fitting on the existing samples, but if piecewise linearity is assumed the correction remains valid at any time sample between the beginning of the loaded pedaling of the ramp protocol and 10 min afterwards. The validity of the applied correction is therefore limited to this 10 min ramp protocol.
This correction method can be further improved by acquiring more data samples with the same technique and retraining the regression model accordingly. Another strategy could be in changing the timestamps for some subjects, for instance in sampling after 1, 3, 5, 7 and 9 min, to make the model more robust. Models that consider the correlated nature of such measurements could be also exploited, such as mixed-effects models. With an increasing number of subjects, however, it will be possible to use artificial intelligence techniques, such as strategies based on machine learning [33] and deep learning. In terms of time series forecasting, deep learning techniques are highly performing [34] and continuously improving; examples of possible methods include convolutional neural networks (CNNs), multilayer perceptions (MLPs) and long-short term memory (LSTM) networks. Accordingly, the present results, this study can be considered as a preliminary and feasibility analysis.
The electrochemical sensor we tested for PtCO2 measurements needs heating of the earlobe skin up to a temperature of 42 °C. Consequently, it can only be applied for a relatively short time and not to prolonged measurements. No safety issue related to heating arose during the study protocol. However, in the literature, other strategies have been studied to measure transcutaneous blood gases and overcome the limitations of electrochemical sensors. As regards CO2 there have been recent projects attempting to measure PtCO2 with optical sensors, with a technology which is conceptually similar to pulse oximetry. These attempts include using an optical CO2 NDIR (non-dispersive infrared) sensor. Since CO2 gas reacts to 4.3 µm wavelength, this wavelength is selected using an optical filter before the sensor, so that only the presence of CO2 is detected [35]. With this technique, it might become possible to have more responsive sensors compared to electrochemical ones, thus better performances in continuous measurements with changes in parameters. This type of sensors could also be embedded in wearable devices, as it has been recently published by Tipparaju et al. [36], or garments [37] and integrated in telemedicine platforms [38], thus overcoming the limitations of measurements that are obtained in laboratory settings under the supervision of the clinician. Finally, optical sensors do not require any change of membrane, thus reducing the costs and making it possible to obtain also unsupervised measurements [7,20].
The present findings are a relevant step forward toward an extension in clinical practice of integrated exercise analysis which is needed for a better comprehension of exercise abnormalities [22]. Indeed, it allows to know PaCO2 dynamics during exercise without an arterial catheter otherwise needed for multiple sampling. At present, in the clinical field, direct or ear lobe PaCO2 are omitted or measured only immediately after the end of exercise limiting the observation of data only to peak exercise. Vice versa, PtCO2 continuous analysis allows to reliably estimate PaCO2 changes during exercise and precisely to obtain data at the anaerobic threshold, during the isocapnic buffering period as well as at the respiratory compensation point also known as the second ventilatory threshold. Indeed, PaCO2 data collected at these exercise steps provide relevant information about the chemoreflex regulation of ventilation and the causes of exercise induced hyperventilation which can be associated to VD/VT as well as to reflex regulation [39]. As regards heart failure the information obtainable by PtCO2, and therefore PaCO2, VD/VT and ΔPetCO2-PaCO2 allow to evaluate the possible presence of a concomitant lung disease, the reflex regulation of ventilation as well as the development during exercise of ventilation perfusion mismatch in the lung [6]. Moreover, the knowledge of PaCO2 values and derived data during exercise will allow a more personalized and efficacious heart failure therapy for examples as regards the choice of the of the most efficacious β-blocker in a specific patient [40].
Moreover, PtCO2 analysis seems to us a promising technique for future studies providing continuous information on CO2 changes and therefore to assess, much better than with repeated arterial blood sampling, CO2 dynamics, ventilation/perfusion mismatch, blood flow, through alveolar to earlobe transit time, and chemoreflex, through the CO2 value during the isocapnic buffering period.
However, at present, a few limitations to the widespread use of the present technique must be acknowledge, such as the cost of the instrumentation, the time needed to heat the system before its use and the need of studies about its reliability and reproducibility in larger populations of patients with different heart failure etiologies and severity, as well as in patients with different diseases. Finally, our modeling has been built in ramp exercise protocol with expected exercise duration of approximately 10 min. We do not know whether it works in longer or shorter exercise or with different exercise protocols. A personalized exercise protocol with a progressively increasing workload built to achieve peak exercise in 10 min is considered the more physiologically correct and should be chosen in most cases [21,41]. Of note our model cannot be applied to other transcutaneous PCO2 transducers. To assess whether this method has clinical applicability, an external validation is required, such as a replication in a greater, different cohort. Furthermore, we studied a mainly male HF population. Accordingly, our results should be applied with caution in female patients at least before a dedicated study is done on female HF patients. Finally, as PtCO2 is largely affected by age, skin thickness, local temperature, usage of vasoactive drugs, poor tissue perfusion, and acidosis, the correlation between PtCO2 and PaCO2 might vary when these conditions exist: for this reason, our results should be applied only during exercise and in HF patients.
5. Conclusions
In heart failure patients PtCO2 is a reliable PaCO2 estimation at rest and at low exercise intensity. At high exercise intensity the overall response appears delayed but reproducible and the error can be overcome by mathematical modeling. In conclusion, during exercise PaCO2 and VD/VT can be estimated from PtCO2 at rest and during a maximal workload exercise provided that a correction of a time delay is applied. A widespread use of this technique will likely enhance our knowledge in exercise physiology and allow a more personalized and efficacious patients assessment and, hopefully, treatment.
Author Contributions
Conceptualization, M.C., A.A. (Alessandra Angelucci), A.A. (Andrea Aliverti) and P.A.; methodology, M.C., A.A. (Alessandra Angelucci), A.A. (Andrea Aliverti), P.G., G.B. and B.P.; software, A.A. (Alessandra Angelucci); validation, M.C., A.A. (Alessandra Angelucci), A.A. (Andrea Aliverti) and P.G.; formal analysis, C.C.T.; investigation, M.C., P.G., G.B. and B.P.; resources, M.C., P.G., G.B. and B.P.; data curation, M.C., P.G., B.P., G.B. and S.R.; writing—original draft preparation, M.C., A.A. (Alessandra Angelucci), A.A. (Andrea Aliverti) and P.A.; writing—review and editing, B.P., G.B., S.R. and P.A.; visualization, A.A. (Alessandra Angelucci) and C.C.T.; supervision, P.A.; project administration, M.C. and P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of IRCCS Centro Cardiologico Monzino (protocol code CCM966 and 30 January 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wasserman, K.; Hansen, J.; Sue, D.; Stringer, W.; Whipp, B. Calculations, formulas, and examples. In Principles of Exercise Testing and Interpretation, 4th ed.; Lippincott Williams Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Agostoni, P.; Smith, D.D.; Schoene, R.B.; Robertson, H.T.; Butler, J. Evaluation of breathlessness in asbestos workers: Results of exercise testing. Am. Rev. Respir. Dis. 1987, 135, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Marenzi, G.; Assanelli, E.; Perego, G.B.; Cattadori, G.; Doria, E.; Agostoni, P.G. Evaluation of the dead space/tidal volume ratio in patients with chronic congestive heart failure. J. Card. Fail. 1995, 1, 401–408. [Google Scholar] [CrossRef]
- Agostoni, P.; Apostolo, A.; Cattadori, G.; Salvioni, E.; Berna, G.; Antonioli, L.; Vignati, C.; Schina, M.; Sciomer, S.; Bussotti, M. Effects of β-blockers on ventilation efficiency in heart failure. Am. Heart J. 2010, 159, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Apostolo, A.; Cattadori, G.; Paolillo, S.; Iorio, A.; Bertella, E.; Salvioni, E.; Alimento, M.; Farina, S.; Palermo, P. Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure: The CARNEBI trial. Int. J. Cardiol. 2013, 168, 2134–2140. [Google Scholar] [CrossRef]
- Wasserman, K.; Zhang, Y.-Y.; Gitt, A.; Belardinelli, R.; Koike, A.; Lubarsky, L.; Agostoni, P.G. Lung function and exercise gas exchange in chronic heart failure. Circulation 1997, 96, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, P. The design, use, and results of transcutaneous carbon dioxide analysis: Current and future directions. Anesth. Analg. 2007, 105, 48–52. [Google Scholar] [CrossRef]
- Venkatesh, B. Continuous intra-arterial blood gas monitoring. Crit. Care Resusc. 1999, 1, 140. [Google Scholar]
- Weinger, M.B.; Brimm, J.E. End-tidal carbon dioxide as a measure of arterial carbon dioxide during intermittent mandatory ventilation. J. Clin. Monit. 1987, 3, 73–79. [Google Scholar] [CrossRef]
- Casati, A.; Gallioli, G.; Scandroglio, M.; Passaretta, R.; Borghi, B.; Torri, G. Accuracy of end-tidal carbon dioxide monitoring using the NBP-75 microstream capnometer. A study in intubated ventilated and spontaneously breathing nonintubated patients. Eur. J. Anaesthesiol. 2000, 17, 622–626. [Google Scholar] [CrossRef]
- Hinkelbein, J.; Floss, F.; Denz, C.; Krieter, H. Accuracy and precision of three different methods to determine PCO2 (PaCO2 vs. PetCO2 vs. PtcCO2) during interhospital ground transport of critically ill and ventilated adults. J. Trauma 2008, 65, 10–18. [Google Scholar] [CrossRef]
- Belpomme, V.; Ricard-Hibon, A.; Devoir, C.; Dileseigres, S.; Devaud, M.-L.; Chollet, C.; Marty, J. Correlation of arterial PCO2 and PETCO2 in prehospital controlled ventilation. Am. J. Emerg. Med. 2005, 23, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Russo, P.; Russo, J.; Tobias, J.D. Noninvasive monitoring of carbon dioxide in infants and children with congenital heart disease: End-tidal versus transcutaneous techniques. J. Intensive Care Med. 2005, 20, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Morley, T.F.; Giaimo, J.; Maroszan, E.; Bermingham, J.; Gordon, R.; Griesback, R.; Zappasodi, S.J.; Giudice, J.C. Use of capnography for assessment of the adequacy of alveolar ventilation during weaning from mechanical ventilation. Am. Rev. Respir. Dis. 1993, 148, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.H.; Kern, N.B.; Costantino, J.P.; Stiller, R.A.; Strollo, P.J.J.; Studnicki, K.A.; Coates, J.A.; Richards, T.J. Accuracy of end-tidal and transcutaneous PCO2 monitoring during sleep. Chest 1994, 106, 472–483. [Google Scholar] [CrossRef]
- Stege, G.; van den Elshout, F.J.J.; Heijdra, Y.F.; van de Ven, M.J.T.; Dekhuijzen, P.N.R.; Vos, P.J.E. Accuracy of transcutaneous carbon dioxide tension measurements during cardiopulmonary exercise testing. Respiration 2009, 78, 147–153. [Google Scholar] [CrossRef]
- Lewis, D.A.; Sietsema, K.E.; Casaburi, R.; Sue, D.Y. Inaccuracy of noninvasive estimates of VD/VT in clinical exercise testing. Chest 1994, 106, 1476–1480. [Google Scholar] [CrossRef]
- Casati, A.; Squicciarini, G.; Malagutti, G.; Baciarello, M.; Putzu, M.; Fanelli, A. Transcutaneous monitoring of partial pressure of carbon dioxide in the elderly patient: A prospective, clinical comparison with end-tidal monitoring. J. Clin. Anesth. 2006, 18, 436–440. [Google Scholar] [CrossRef]
- Severinghaus, J.W. Methods of measurement of blood and gas carbon dioxide during anesthesia. Anesthesiol. J. Am. Soc. Anesthesiol. 1960, 21, 717–726. [Google Scholar] [CrossRef]
- Eberhard, P.; Gisiger, P.A.; Gardaz, J.P.; Spahn, D.R. Combining transcutaneous blood gas measurement and pulse oximetry. Anesth. Analg. 2002, 94, S76–S80. [Google Scholar]
- Agostoni, P.; Bianchi, M.; Moraschi, A.; Palermo, P.; Cattadori, G.; La Gioia, R.; Bussotti, M.; Wasserman, K. Work-rate affects cardiopulmonary exercise test results in heart failure. Eur. J. Heart Fail. 2005, 7, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, P.; Dumitrescu, D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 2019, 288, 107–113. [Google Scholar] [CrossRef]
- Allison, P.D. Multiple Regression: A Primer; Pine Forge Press: Thousand Oaks, CA, USA, 1999; ISBN 0761985336. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Parker, R.A.; Scott, C.; Inácio, V.; Stevens, N.T. Using multiple agreement methods for continuous repeated measures data: A tutorial for practitioners. BMC Med. Res. Methodol. 2020, 20, 154. [Google Scholar] [CrossRef]
- Kozak, M.; Wnuk, A. Including the Tukey mean-difference (Bland–Altman) plot in a statistics course. Teach. Stat. 2014, 36, 83–87. [Google Scholar] [CrossRef]
- Bourgoin, P.; Baudin, F.; Brossier, D.; Emeriaud, G.; Wysocki, M.; Jouvet, P. Assessment of Bohr and Enghoff dead space equations in mechanically ventilated children. Respir. Care 2017, 62, 468–474. [Google Scholar] [CrossRef]
- Massey, F.J., Jr. The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Angelucci, A.; Kuller, D.; Aliverti, A. A home telemedicine system for continuous respiratory monitoring. IEEE J. Biomed. Health Inform. 2020, 25, 1247–1256. [Google Scholar] [CrossRef]
- Angelucci, A.; Kuller, D.; Aliverti, A. Respiratory rate and tidal volume change with posture and activity during daily life. Eur. Respir. J. 2020, 56, 2130. [Google Scholar]
- Antonelli, A.; Guilizzoni, D.; Angelucci, A.; Melloni, G.; Mazza, F.; Stanzi, A.; Venturino, M.; Kuller, D.; Aliverti, A. Comparison between the AirgoTM Device and a Metabolic Cart during Rest and Exercise. Sensors 2020, 20, 3943. [Google Scholar] [CrossRef]
- Bontempi, G.; Taieb, S.B.; Le Borgne, Y.A. Machine learning strategies for time series forecasting. In Proceedings of the European Business Intelligence Summer School, Brussels, Belgium, 15–21 July 2012; Springer: Berlin/Heidelberg, Germany, 2012; pp. 62–77. [Google Scholar]
- Längkvist, M.; Karlsson, L.; Loutfi, A. A review of unsupervised feature learning and deep learning for time-series modeling. Pattern Recognit. Lett. 2014, 42, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kang, S.W. Noninvasive Optical Transcutaneous PCO2 Gas Sensor. Sens. Mater. 2005, 17, 249–257. [Google Scholar]
- Tipparaju, V.V.; Mora, S.J.; Yu, J.; Tsow, F.; Xian, X. Wearable Transcutaneous CO Monitor Based on Miniaturized Nondispersive Infrared Sensor. IEEE Sens. J. 2021, 21, 17327–17334. [Google Scholar] [CrossRef]
- Angelucci, A.; Cavicchioli, M.; Cintorrino, I.A.; Lauricella, G.; Rossi, C.; Strati, S.; Aliverti, A. Smart textiles and sensorized garments for physiological monitoring: A review of available solutions and techniques. Sensors 2021, 21, 814. [Google Scholar] [CrossRef]
- Angelucci, A.; Aliverti, A. Telemonitoring systems for respiratory patients: Technological aspects. Pulmonology 2020, 26, 221–232. [Google Scholar] [CrossRef]
- Farina, S.; Bruno, N.; Agalbato, C.; Contini, M.; Cassandro, R.; Elia, D.; Harari, S.; Agostoni, P. Physiological insights of exercise hyperventilation in arterial and chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. 2018, 259, 178–182. [Google Scholar] [CrossRef]
- Sinagra, G.; Corrà, U.; Contini, M.; Magrì, D.; Paolillo, S.; Filardi, P.P.; Sciomer, S.; Badagliacca, R.; Agostoni, P. Choosing among β-blockers in heart failure patients according to β-receptors’ location and functions in the cardiopulmonary system. Pharmacol. Res. 2020, 156, 104785. [Google Scholar] [CrossRef]
- Buchfuhrer, M.J.; Hansen, J.E.; Robinson, T.E.; Sue, D.Y.; Wasserman, K.; Whipp, B.J. Optimizing the exercise protocol for cardiopulmonary assessment. J. Appl. Physiol. 1983, 55, 1558–1564. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).