On Measuring Implant Fixation Stability in ACL Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surgical Procedure
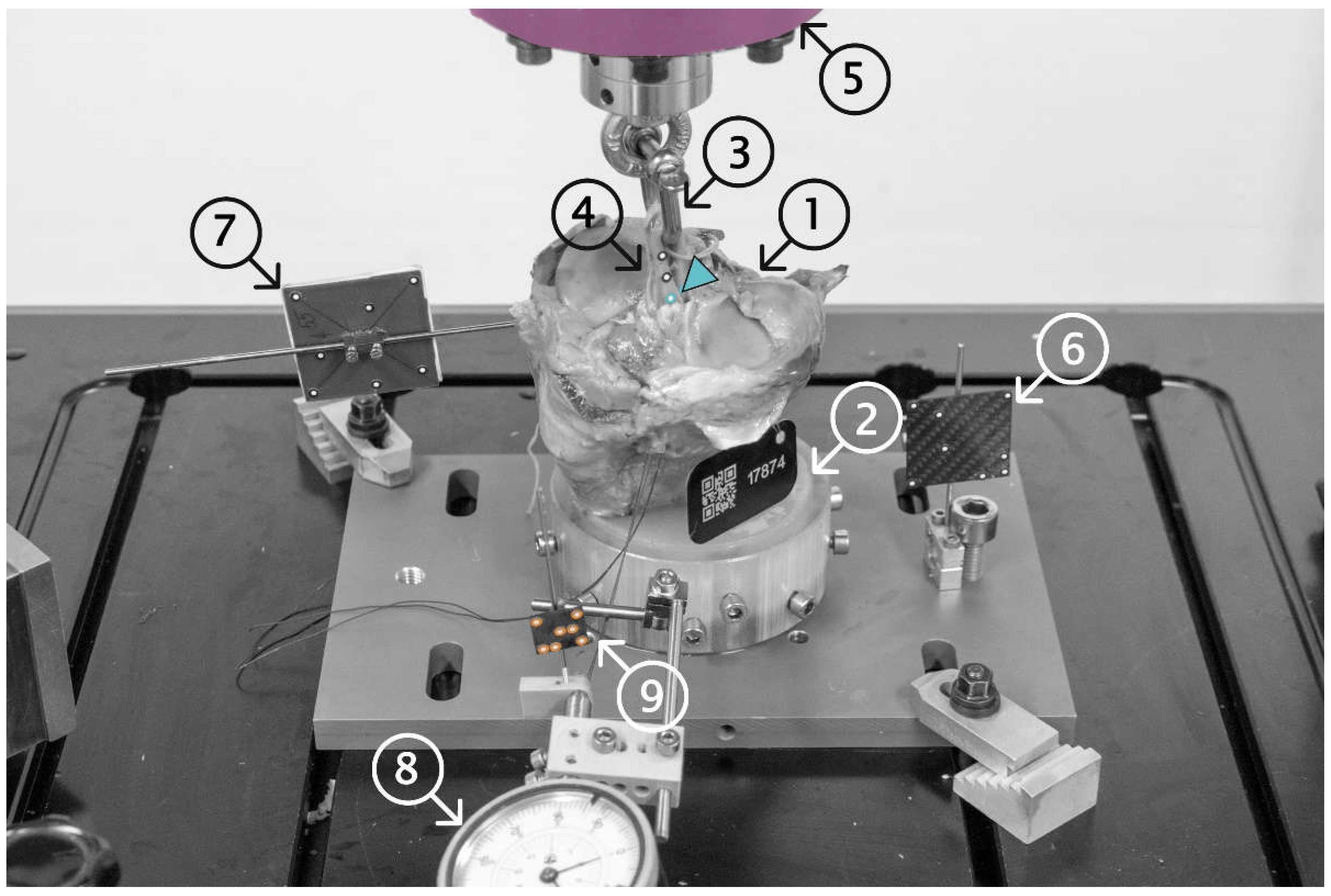
2.3. Biomechanical Testing
2.4. Statistical Analysis
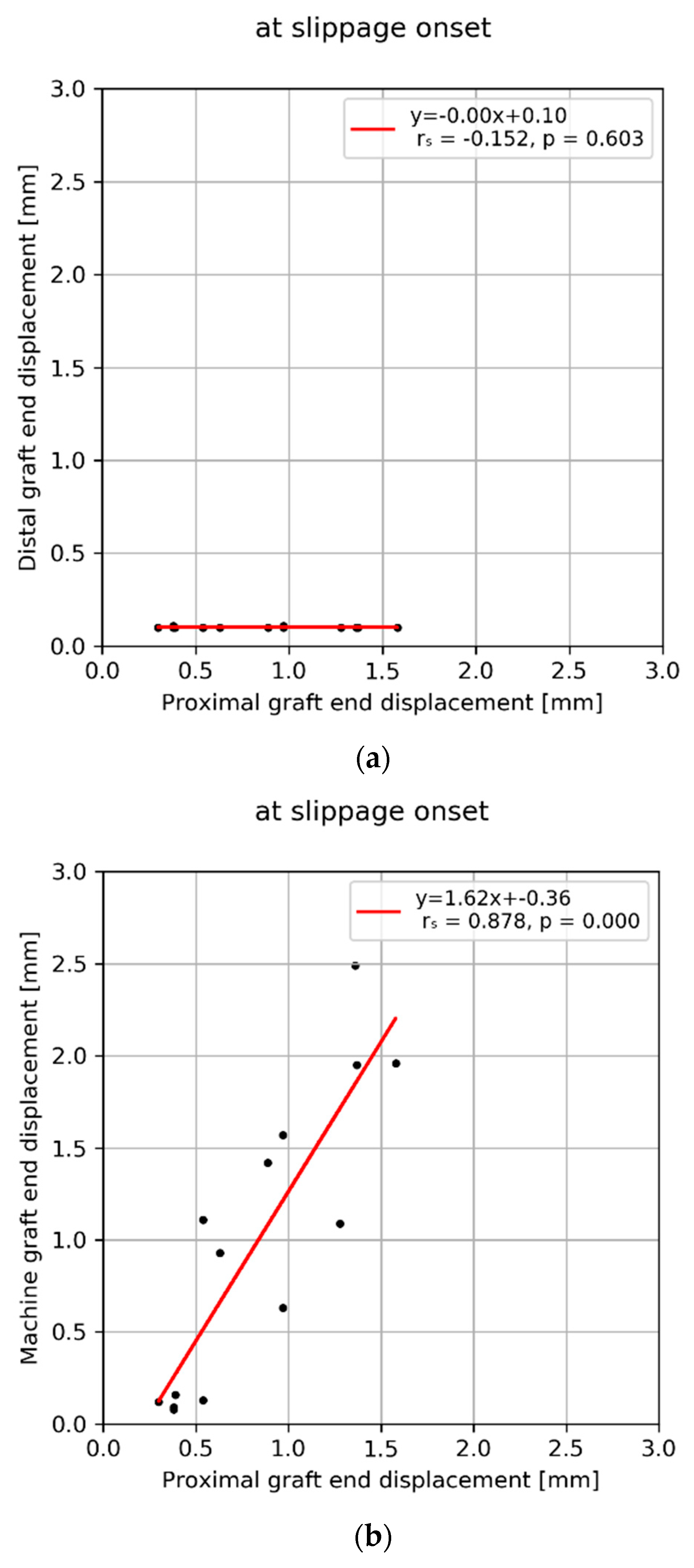
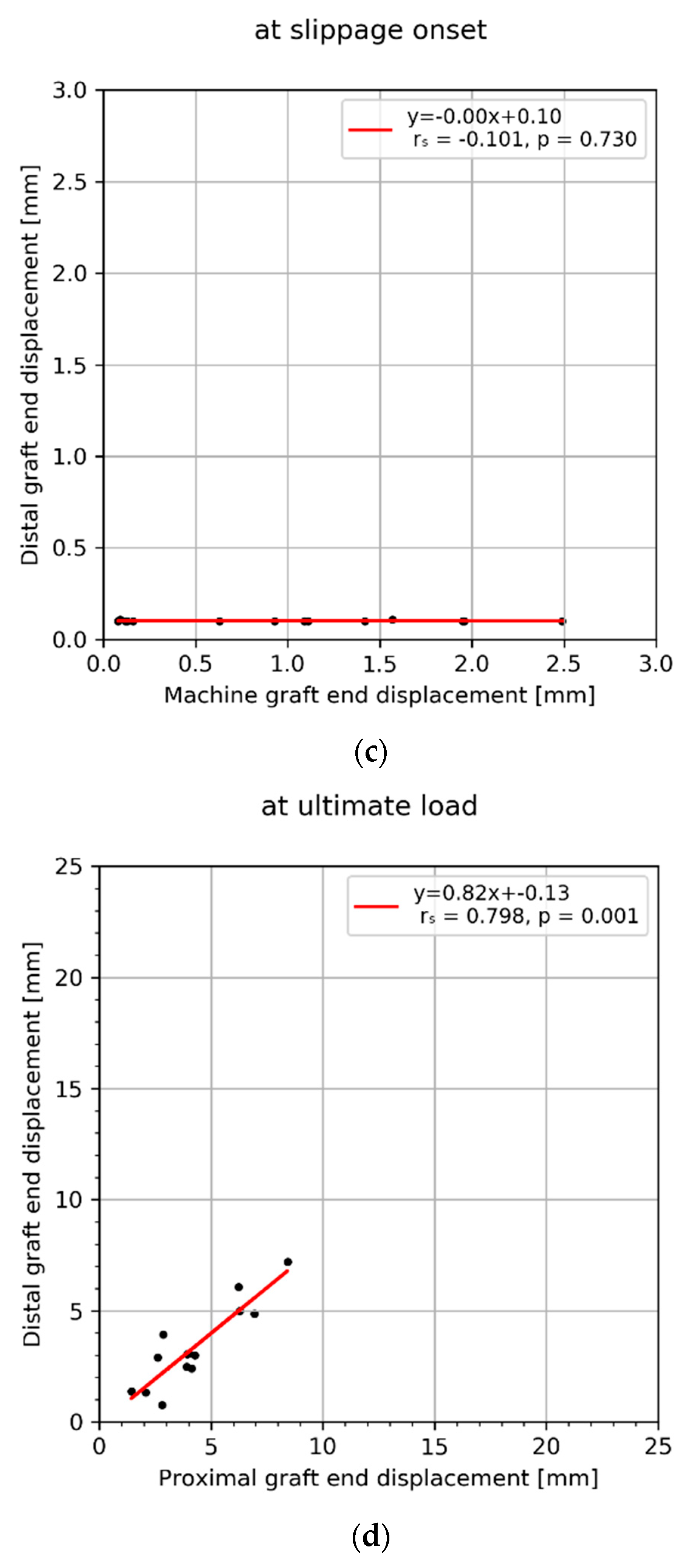
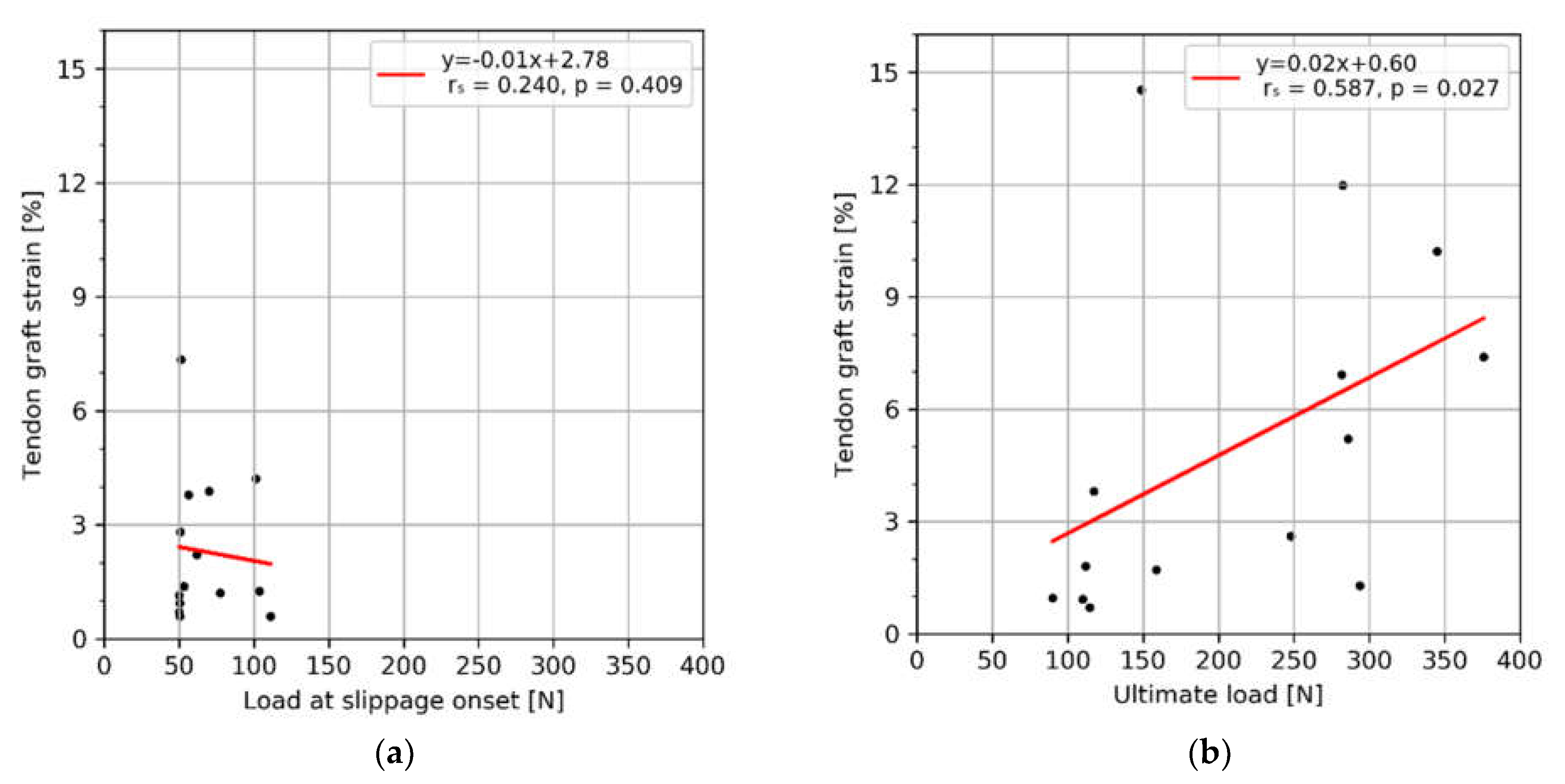
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, T.O.; Postle, K.; Penny, F.; McNamara, I.; Mann, C.J.V. Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta-analysis comparing anterior cruciate ligament reconstruction versus non-operative treatment. Knee 2014, 21, 462–470. [Google Scholar]
- Delay, B.S.; Smolinski, R.J.; Wind, W.M.; Bowman, D.S. Current practices and opinions in ACL reconstruction and rehabilitation: Results of a survey of the American Orthopaedic Society for Sports Medicine. Am. J. Knee Surg. 2001, 14, 85–91. [Google Scholar] [PubMed]
- Halonen, K.; Mononen, M.; Töyräs, J.; Kröger, H.; Joukainen, A.; Korhonen, R. Optimal graft stiffness and pre-strain restore normal joint motion and cartilage responses in ACL reconstructed knee. J. Biomech. 2016, 49, 2566–2576. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, S.; Kvist, J. Dynamic and static tibial translation in patients with anterior cruciate ligament deficiency initially treated with a structured rehabilitation protocol. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2337–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barenius, B.; Ponzer, S.; Shalabi, A.; Bujak, R.; Norlén, L.; Eriksson, K. Increased Risk of Osteoarthritis After Anterior Cruciate Ligament Reconstruction:A 14-Year Follow-up Study of a Randomized Controlled Trial. Am. J. Sports Med. 2014, 42, 1049–1057. [Google Scholar] [CrossRef]
- Fu, F.H.; Bennett, C.H.; Lattermann, C.; Ma, C.B. Current Trends in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 1999, 27, 821–830. [Google Scholar] [CrossRef]
- Pasquali, M.; Plante, M.J.; Monchik, K.O.; Spenciner, D.B. A comparison of three adjustable cortical button ACL fixation devices. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1613–1616. [Google Scholar] [CrossRef]
- Kousa, P.; Järvinen, T.L.N.; Vihavainen, M.; Kannus, P.; Järvinen, M. The Fixation Strength of Six Hamstring Tendon Graft Fixation Devices in Anterior Cruciate Ligament Reconstruction: Part I: Femoral Site. Am. J. Sports Med. 2003, 31, 174–181. [Google Scholar] [CrossRef]
- Kousa, P.; Järvinen, T.L.N.; Vihavainen, M.; Kannus, P.; Järvinen, M. The Fixation Strength of Six Hamstring Tendon Graft Fixation Devices in Anterior Cruciate Ligament Reconstruction: Part II: Tibial Site. Am. J. Sports Med. 2003, 31, 182–188. [Google Scholar] [CrossRef]
- Milano, G.; Mulas, P.D.; Ziranu, F.; Deriu, L.; Fabbriciani, C. Comparison of femoral fixation methods for anterior cruciate ligament reconstruction with patellar tendon graft: A mechanical analysis in porcine knees. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 733–738. [Google Scholar] [CrossRef]
- Milano, G.; Mulas, P.D.; Ziranu, F.; Piras, S.; Manunta, A.; Fabbriciani, C. Comparison Between Different Femoral Fixation Devices for ACL Reconstruction With Doubled Hamstring Tendon Graft: A Biomechanical Analysis. Arthroscopy 2006, 22, 660–668. [Google Scholar] [CrossRef]
- Scannell, B.P.; Loeffler, B.J.; Hoenig, M.; Peindl, R.D.; D’Alessandro, D.F.; Connor, P.M.; Fleischli, J.E. Biomechanical comparison of hamstring tendon fixation devices for anterior cruciate ligament reconstruction: Part 1. Five femoral devices. Am. J. Orthop. 2015, 44, 32–36. [Google Scholar] [PubMed]
- Scannell, B.P.; Loeffler, B.J.; Hoenig, M.; Peindl, R.D.; D’Alessandro, D.F.; Connor, P.M.; Fleischli, J.E. Biomechanical comparison of hamstring tendon fixation devices for anterior cruciate ligament reconstruction: Part 2: Four tibial devices. Am. J. Orthop. 2015, 44, 82–85. [Google Scholar] [PubMed]
- Karkosch, R.F.; Ettinger, M.; Bachmaier, S.; Wijdicks, C.A.; Smith, T. Adjustable-length loop cortical button versus interference screw fixation in quadriceps tendon anterior cruciate ligament reconstruction—A biomechanical in vitro study. Clin. Biomech. 2018, 60, 60–65. [Google Scholar] [CrossRef]
- Smith, P.A.; Piepenbrink, M.; Smith, S.K.; Bachmaier, S.; Bedi, A.; Wijdicks, C.A. Adjustable- Versus Fixed-Loop Devices for Femoral Fixation in ACL Reconstruction: An In Vitro Full-Construct Biomechanical Study of Surgical Technique–Based Tibial Fixation and Graft Preparation. Orthop. J. Sports Med. 2018, 6, 2325967118768743. [Google Scholar] [CrossRef] [Green Version]
- Nye, D.D.; Mitchell, W.R.; Liu, W.; Ostrander, R.V. Biomechanical Comparison of Fixed-Loop and Adjustable-Loop Cortical Suspensory Devices for Metaphyseal Femoral-Sided Soft Tissue Graft Fixation in Anatomic Anterior Cruciate Ligament Reconstruction Using a Porcine Model. Arthroscopy 2017, 33, 1225–1232.e1221. [Google Scholar] [CrossRef] [PubMed]
- Kamelger, F.S.; Onder, U.; Schmoelz, W.; Tecklenburg, K.; Arora, R.; Fink, C. Suspensory Fixation of Grafts in Anterior Cruciate Ligament Reconstruction: A Biomechanical Comparison of 3 Implants. Arthroscopy 2009, 25, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Mayr, R.; Heinrichs, C.H.; Eichinger, M.; Coppola, C.; Schmoelz, W.; Attal, R. Biomechanical Comparison of 2 Anterior Cruciate Ligament Graft Preparation Techniques for Tibial Fixation:Adjustable-Length Loop Cortical Button or Interference Screw. Am. J. Sports Med. 2015, 43, 1380–1385. [Google Scholar] [CrossRef]
- Petre, B.M.; Smith, S.D.; Jansson, K.S.; de Meijer, P.-P.; Hackett, T.R.; LaPrade, R.F.; Wijdicks, C.A. Femoral Cortical Suspension Devices for Soft Tissue Anterior Cruciate Ligament Reconstruction:A Comparative Biomechanical Study. Am. J. Sports Med. 2013, 41, 416–422. [Google Scholar] [CrossRef]
- Trump, M.; Palathinkal, D.M.; Beaupre, L.; Otto, D.; Leung, P.; Amirfazli, A. In vitro biomechanical testing of anterior cruciate ligament reconstruction: Traditional versus physiologically relevant load analysis. Knee 2011, 18, 193–201. [Google Scholar] [CrossRef]
- Mickelson, D.T.; Lefebvre, T.; Gall, K.; Riboh, J.C. Adjustable-Loop Femoral Cortical Suspensory Fixation for Patellar Tendon Anterior Cruciate Ligament Reconstruction: A Time Zero Biomechanical Comparison With Interference Screw Fixation. Am. J. Sports Med. 2018, 46, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.; Labianca, L.; Speranza, A.; Agrò, A.M.; Camillieri, G.; D’Arrigo, C.; Ferretti, A. Biomechanical evaluation of different anterior cruciate ligament fixation techniques for hamstring graft. J. Orthop. Sci. 2010, 15, 125–131. [Google Scholar] [CrossRef]
- Efe, T.; Bauer, J.; Herdrich, S.; Gotzen, L.; El-Zayat, B.F.; Schmitt, J.; Schofer, M.D. Comparison between bovine bone and titanium interference screws for implant fixation in ACL reconstruction: A biomechanical study. Arch. Orthop. Trauma Surg. 2010, 130, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, P.; Bahr, R.; Landreau, P.; Miladi, R.; Witvrouw, E. Likelihood of ACL graft rupture: Not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br. J. Sports Med. 2016, 50, 946–951. [Google Scholar] [CrossRef]
- Bourke, H.E.; Gordon, D.J.; Salmon, L.; Waller, A.; Linklater, J.; Pinczewski, L. The outcome at 15 years of endoscopic anterior cruciate ligament reconstruction using hamstring tendon autograft for ‘isolated’ anterior cruciate ligament rupture. J. Bone Jt. Surg. Br. Vol. 2012, 94-B, 630–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.D.; Gerdeman, A.C.; Bennett, C.G.; Hart, J.M.; Miller, M.D. A Biomechanical Comparison of the EndoButton CL Using Transtibial Drilling and EndoButton Direct Using Anteromedial Arthroscopic Drilling. Arthroscopy 2010, 26, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.A.; Kwak, J.H.; Yang, S.H.; Lee, B.K. Comparative biomechanical study of the Ligament Plate® and other fixation devices in ACL reconstruction. Int. Orthop. 2009, 33, 1269–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, J.T.; Howell, L.C.S.M.; Hull, M.L. Contributions of Femoral Fixation Methods to the Stiffness of Anterior Cruciate Ligament Replacements at Implantation. Arthrosc. J. Arthrosc. Relat. Surg. 1999, 15, 379–387. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Cheng, C.-K. Stiffness and shape of the ACL graft affects tunnel enlargement and graft wear. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2184–2193. [Google Scholar] [CrossRef]
- Magen, H.E.; Howell, S.M.; Hull, M.L. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am. J. Sports Med. 1999, 27, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.J.; Bae, T.S.; Moon, Y.-W.; Ahn, J.H.; Wang, J.H. A Comparative Biomechanical Study of Femoral Cortical Suspension Devices for Soft-Tissue Anterior Cruciate Ligament Reconstruction: Adjustable-Length Loop Versus Fixed-Length Loop. Arthroscopy 2018, 34, 566–572. [Google Scholar] [CrossRef] [PubMed]



| Machine Displacement [mm] | Proximal Graft End Displacement [mm] | Distal Graft End Displacement [mm] | Abs. Vertical Bone Displacement [mm] | Graft Strain [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specimen ID | At Slippage Onset | At Ultimate Load | At Slippage Onset | At Ultimate Load | At Slippage Onset | At Ultimate Load | At Slippage Onset | At Ultimate Load | At Slippage Onset | At Ultimate Load |
| 17709 | 2.5 | 6.7 | 1.4 | 2.8 | 0.1 | 0.7 | 0.0 | 0.0 | 1.2 | 1.7 |
| 17724 | 0.1 | 5.6 | 0.4 | 2.1 | 0.1 | 1.3 | 0.0 | 0.0 | 0.7 | 1.0 |
| 17725 | 0.1 | 13.4 | 0.4 | 6.2 | 0.1 | 6.1 | 0.1 | 1.0 | 0.6 | 2.6 |
| 17726 | 0.1 | 16.8 | 0.3 | 2.6 | 0.1 | 2.9 | 0.0 | 0.4 | 1.1 | 0.7 |
| 17733 | 2.0 | 9.5 | 1.4 | 4.1 | 0.1 | 2.4 | 0.0 | 0.2 | 0.6 | 1.3 |
| 17767 | 0.6 | 7.9 | 1.0 | 4.0 | 0.1 | 3.1 | 0.3 | 0.8 | 1.4 | 1.8 |
| 17768 | 2.0 | 23.4 | 1.6 | 8.4 | 0.1 | 7.2 | 0.2 | 1.2 | 4.2 | 10.2 |
| 17777 | 0.1 | 9.8 | 0.5 | 4.3 | 0.1 | 3.0 | 0.2 | 1.6 | 2.8 | 6.9 |
| 17791 | 0.2 | 21.6 | 0.4 | 6.3 | 0.1 | 5.0 | 0.0 | 0.1 | 0.9 | 0.9 |
| 17796 | 1.1 | 7.6 | 0.5 | 1.4 | 0.1 | 1.4 | 0.1 | 0.2 | 7.3 | 14.5 |
| 17798 | 1.6 | 16.0 | 1.0 | 6.9 | 0.1 | 4.9 | 0.2 | 0.9 | 1.3 | 5.2 |
| 17874 | 1.1 | 11.9 | 1.3 | 3.9 | 0.1 | 2.5 | 0.0 | 0.0 | 3.8 | 3.8 |
| 17875 | 0.9 | 11.4 | 0.6 | 2.9 | 0.1 | 3.9 | 0.1 | 0.2 | 2.2 | 12.0 |
| 17876 | 1.4 | 9.4 | 0.9 | 3.9 | 0.1 | 3.0 | 0.3 | 2.1 | 3.9 | 7.4 |
| Mean ± SD (Min–MAX) | 1.0 ± 0.8 (0.1–2.5) | 12.2 ± 5.3 (5.6–23.4) | 0.8 ± 0.4 (0.3–1.6) | 4.3 ± 1.9 (1.4–8.4) | 0.1 ± 0.0 (0.1–0.1) | 3.4 ± 1.8 (0.7–7.2) | 0.1 ± 0.1 (0.0–0.3) | 0.6 ± 0.6 (0.0–2.1) | 2.3 ± 1.9 (0.6–7.3) | 5.0 ± 4.4 (0.7–14.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benca, E.; Zderic, I.; Caspar, J.; Knegsel, K.v.; Hirtler, L.; Gueorguiev, B.; Widhalm, H.; Windhager, R.; Varga, P. On Measuring Implant Fixation Stability in ACL Reconstruction. Sensors 2021, 21, 6632. https://doi.org/10.3390/s21196632
Benca E, Zderic I, Caspar J, Knegsel Kv, Hirtler L, Gueorguiev B, Widhalm H, Windhager R, Varga P. On Measuring Implant Fixation Stability in ACL Reconstruction. Sensors. 2021; 21(19):6632. https://doi.org/10.3390/s21196632
Chicago/Turabian StyleBenca, Emir, Ivan Zderic, Jan Caspar, Kenneth van Knegsel, Lena Hirtler, Boyko Gueorguiev, Harald Widhalm, Reinhard Windhager, and Peter Varga. 2021. "On Measuring Implant Fixation Stability in ACL Reconstruction" Sensors 21, no. 19: 6632. https://doi.org/10.3390/s21196632
APA StyleBenca, E., Zderic, I., Caspar, J., Knegsel, K. v., Hirtler, L., Gueorguiev, B., Widhalm, H., Windhager, R., & Varga, P. (2021). On Measuring Implant Fixation Stability in ACL Reconstruction. Sensors, 21(19), 6632. https://doi.org/10.3390/s21196632









