Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview
Abstract
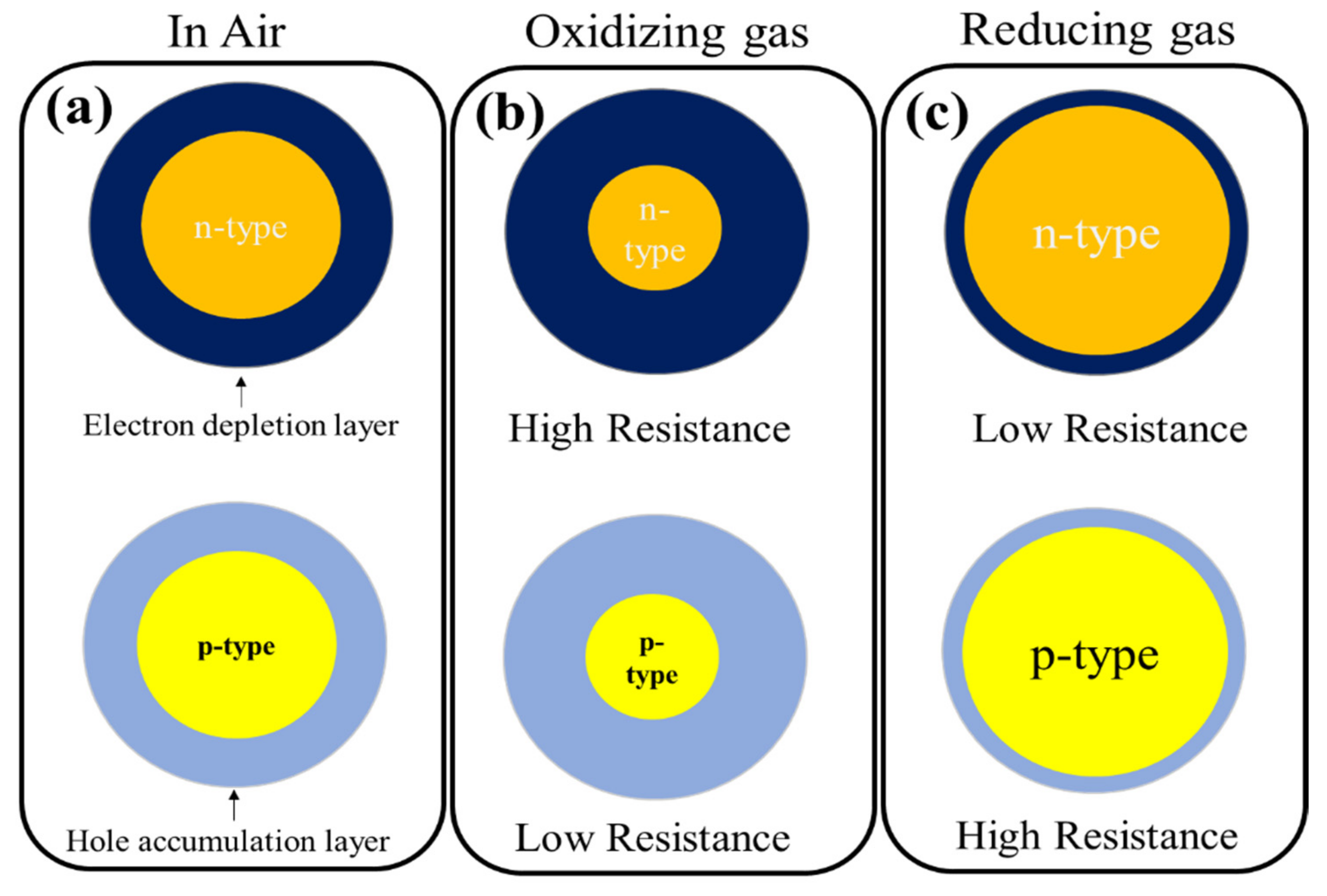
:1. Semiconducting Metal Oxide (SMO)-Based Gas Sensors
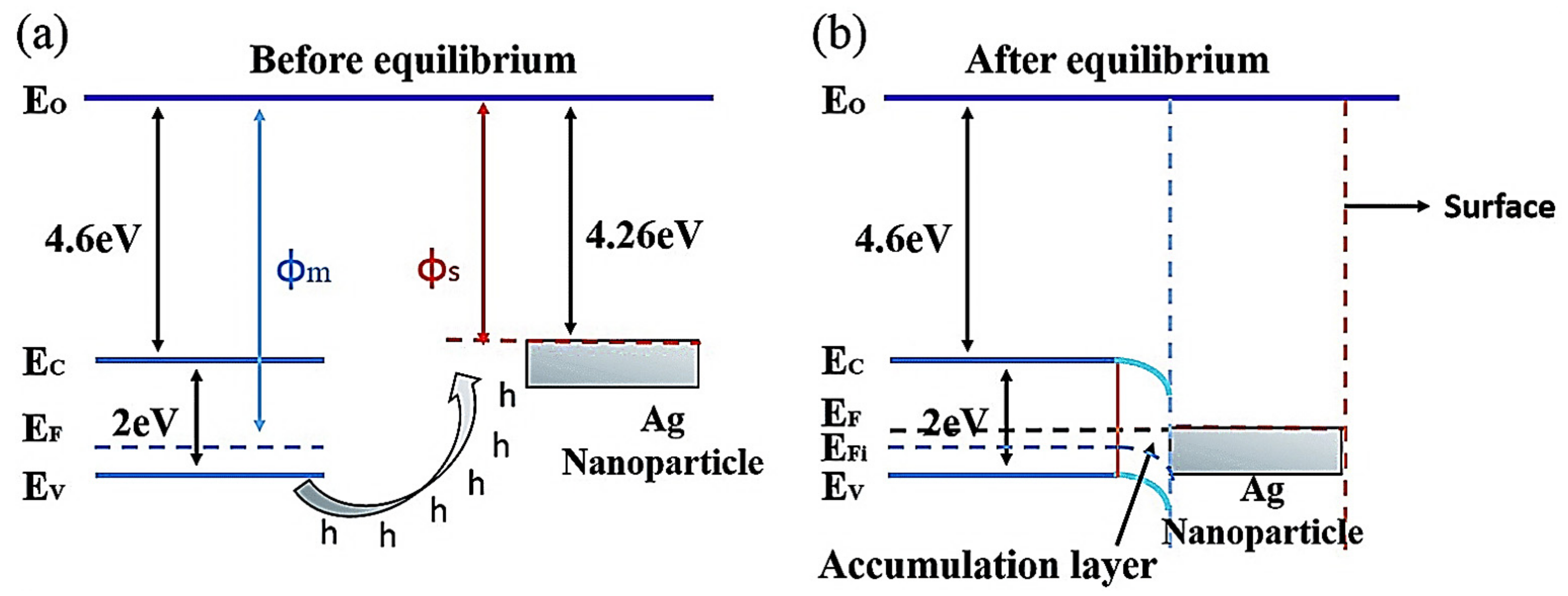
2. Noble Metal Decoration
3. Ag-Decorated/Loaded Gas Sensors
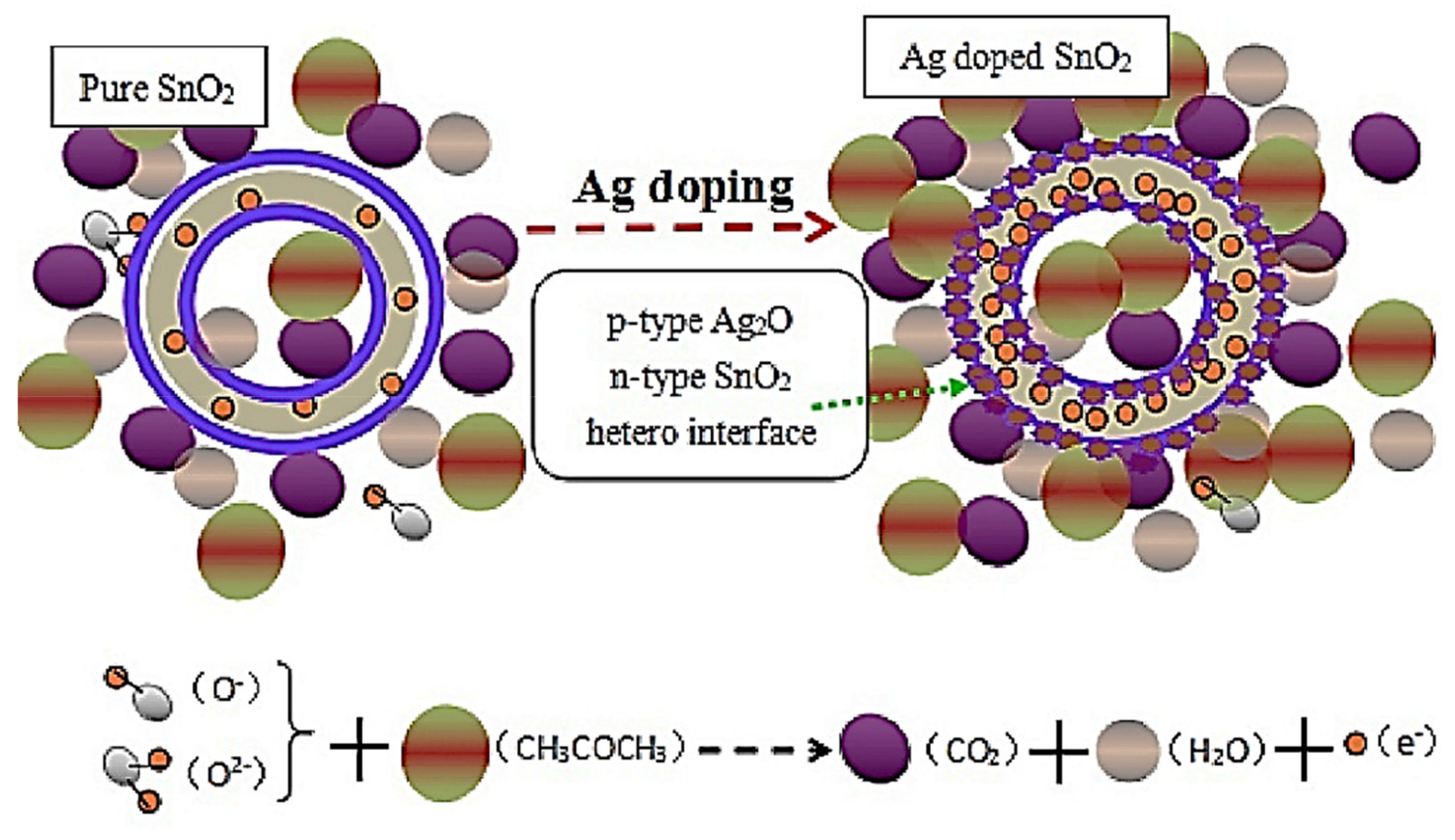
3.1. Ag-Decorated/Loaded Acetone (CH3COCH3) Gas Sensors
3.2. Ag-Decorated/Loaded Chlorine (Cl2) Gas Sensors
3.3. Ag-Decorated/Loaded Acetylene (C2H2) Gas Sensors
3.4. Ag-Decorated/Loaded Triethylamine (TEA) Gas Sensors
3.5. Ag-Decorated/Loaded Formaldehyde (HCHO) Gas Sensors
3.6. Ag-Decorated/Loaded Carbon Monoxide (CO) Gas Sensors
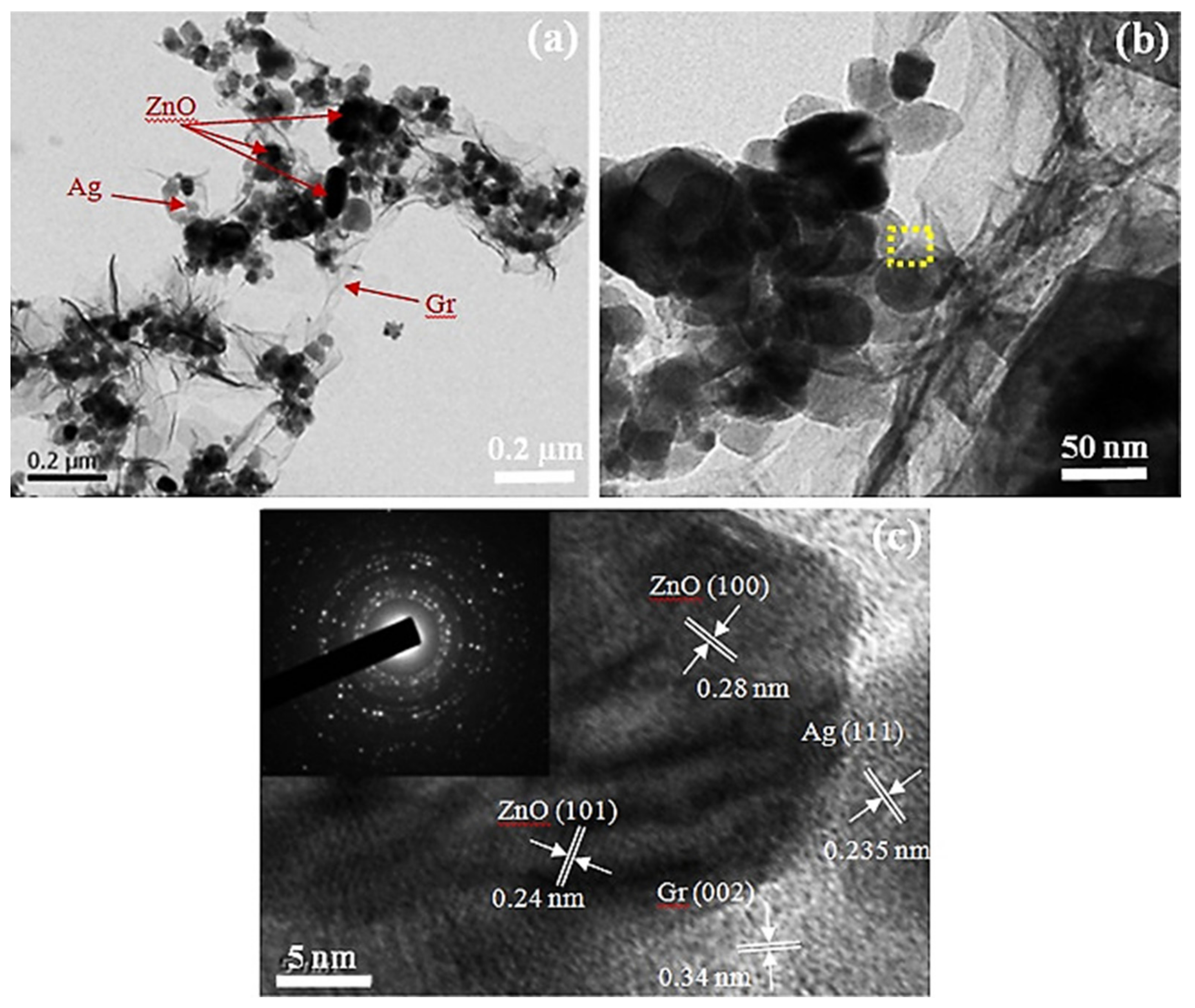
3.7. Ag-Decorated/Loaded Ethanol (C2H5OH) Gas Sensors
3.8. Ag-Decorated/Loaded Nitrogen Dioxide (NO2) Gas Sensors
3.9. Ag-Decorated/Loaded Methyl Mercaptan (CH3SH) Gas Sensors
3.10. Ag-Decorated/Loaded Xylene (C8H10) Gas Sensors
3.11. Ag-Decorated/Loaded Ammonia (NH3) Gas Sensors
3.12. Summary of Ag-Decorated Gas Sensors
4. Ag-Doped Gas Sensors
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, R.; Wang, Z.; Zhou, X.; Huang, L.; Chi, L. Gas-sensing performance and operation mechanism of organic π-conjugated materials. ChemPlusChem 2019, 84, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, W.; Song, J.; Jang, H.J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-effect transistors. Chem. Rev. 2018, 119, 3–35. [Google Scholar] [CrossRef]
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S.J. Recent advances in perylene diimide-based active materials in electrical mode gas sensing. Chemosensors 2021, 9, 30. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Amini, A. Metal oxide-based gas sensors for the detection of exhaled breath markers. Med. Devices Sens. 2021, 4, e10161. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Deng, Z.; Liao, J. Electrically transduced gas sensors based on semiconducting metal oxide nanowires. Sensors 2020, 20, 6781. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Bhowmik, B. Micro/nanostructured gas sensors: The physics behind the nanostructure growth, sensing and selectivity mechanisms. Nanoscale Adv. 2021, 3, 73–93. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Navale, S.; Kim, H.W.; Kim, S.S. Boosting the sensing properties of resistive-based gas sensors by irradiation techniques: A review. Nanoscale 2021, 13, 4728–4757. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godse, P.R.; Mane, A.T.; Navale, Y.H.; Navale, S.T.; Mulik, R.N.; Patil, V.B. Hydrothermally grown 1D ZnO nanostructures for rapid detection of NO2 gas. SN Appl. Sci. 2021, 3, 360. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Navale, S.T.; Yang, Z.B.; Liu, C.; Cao, P.J.; Patil, V.B.; Ramgir, N.S.; Mane, R.S.; Stadler, F.J. Enhanced acetone sensing properties of titanium dioxide nanoparticles with a sub-ppm detection limit. Sens. Actuators B Chem. 2018, 255, 1701–1710. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor metal oxides as chemoresistive sensors for detecting volatile organic compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Gui, X.; Navale, S.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.X.; Liu, W.; Zhu, D.; et al. Design of flower-like V2O5 hierarchical nanostructures by hydrothermal strategy for the selective and sensitive detection of xylene. J. Alloy. Compd. 2020, 815, 152378. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Meng, F.; Hou, N.; Jin, Z.; Sun, B.; Guo, Z.; Kong, L.; Xiao, X.; Wu, H.; Li, M.; Liu, J. Ag-decorated ultra-thin porous single-crystalline ZnO nanosheets prepared by sunlight induced solvent reduction and their highly sensitive detection of ethanol. Sens. Actuators B Chem. 2015, 209, 975–982. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Nanocomposite based flexible ultrasensitive resistive gas sensor for chemical reactions studies. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef]
- Chen, L.; Tsang, S.C. Ag doped WO3-based powder sensor for the detection of NO gas in air. Sens. Actuators B Chem. 2003, 89, 68–75. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kang, S.Y.; Mirzaei, A.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of gas sensing properties by the functionalization of ZnO-branched SnO2 nanowires with Cr2O3 nanoparticles. Sens. Actuators B Chem. 2017, 249, 656–666. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yoon, J.-W.; Choi, K.-I.; Jang, H.W.; Umar, A.; Lee, J.-H. Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 2013, 5, 7066–7073. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; Maggio, F.D.; Vallejos, S.; Vilic, T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-assisted CVD-grown PdO nanoparticle decorated tungsten oxide nanoneedles extremely sensitive and selective to hydrogen. ACS Appl. Mater. Inter. 2016, 8, 10413–10421. [Google Scholar] [CrossRef]
- Mirzaei, A.; Bang, J.H.; Kim, S.S.; Kim, H.W. Effect of noble metals on hydrogen sensing properties of metal oxide-based gas sensors. J. Sens. Sci. Tech. 2020, 29, 365–368. [Google Scholar]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches for the improvement of conductometric gas sensor parameters: Part 1. Improvement of sensor sensitivity and selectivity (short survey). Sens. Actuators B Chem. 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Rzaij, J.M.; Abass, A.M. Review on: TiO2 thin film as a metal oxide gas sensor. J. Chem. Rev. 2020, 2, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, R.; Yao, L.; Ran, Y.; Zhang, X.; Wang, Y. A review on WO3 based gas sensors: Morphology control and enhanced sensing properties. J. Alloy. Compd. 2020, 820, 153194. [Google Scholar] [CrossRef]
- Hariharan, V.; Gnanavel, B.; Sathiyapriya, R.; Aroulmoji, V. A review on tungsten oxide (WO3) and their derivatives for sensor applications. Int. J. Adv. Sci. Eng. 2019, 5, 1163–1168. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2020, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.U.; Kim, J.H.; Lee, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun metal oxide composite nanofibers gas sensors: A review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Sheikhi, M.H. A review of nanostructured resistive-based vanadium oxide gas sensors. Chemosensors 2020, 8, 105. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of oxygen vacancies in nanostructured metal-oxide gas sensors: A review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.H.; Kim, J.H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrog. Energy 2019, 44, 20552–20571. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrog. Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Cattabiani, N.; Baratto, C.; Zappa, D.; Comini, E.; Donarelli, M.; Ferroni, M.; Ponzoni, A.; Faglia, G. Tin oxide nanowires decorated with Ag nanoparticles for visible light-enhanced hydrogen sensing at room temperature: Bridging conductometric gas sensing and plasmon-driven catalysis. J. Phys. Chem. C 2018, 122, 5026–5031. [Google Scholar] [CrossRef]
- Conner, W.C., Jr.; Falconer, J.L. Spillover in heterogeneous catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Inter. J. Hydrog. Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Orlandi, O.L. Investigation of electronic and chemical sensitization effects promoted by Pt and Pd nanoparticles on single crystalline SnO nanobelt-based gas sensors. Sens. Actuators B Chem. 2019, 301, 127055. [Google Scholar] [CrossRef]
- Molavi, R.; Sheikhi, M.H. Low temperature carbon monoxide gas sensor based on Ag-Co3O4 thick film nanocomposite. Mater. Lett. 2018, 233, 74–77. [Google Scholar] [CrossRef]
- Li, M.; Zhu, H.; Wang, B.; Cheng, J.; Yan, W.; Xia, S.; Tang, Z. Ultrasensitive and highly selective detection of methoxy propanol based on Ag-decorated SnO2 hollow nanospheres. Sens. Actuators B Chem. 2016, 232, 545–556. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhang, G.; Ma, S.; Lu, Y.; Bian, H.; Chen, Q. Highly sensitive VOCs-acetone sensor based on Ag-decorated SnO2 hollow nanofibers. J. Alloy. Compd. 2017, 703, 572–579. [Google Scholar] [CrossRef]
- Kılıç, A.; Alev, O.; Özdemir, O.; Arslan, L.Ç.; Büyükköse, S.; Öztürk, Z.Z. The effect of Ag loading on gas sensor properties of TiO2 nanorods. Thin Solid Film. 2021, 726, 138662. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Wang, C.; Ma, J.; Ning, L.; Fan, H. Ag modified bismuth ferrite nanospheres as a chlorine gas sensor. RSC Adv. 2018, 8, 33156–33163. [Google Scholar] [CrossRef] [Green Version]
- Iftekhar Uddin, A.S.M.; Phan, D.-T.; Chung, G.-S. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 207, 362–369. [Google Scholar] [CrossRef]
- Lee, K.-W.; Uddin, A.S.M.I.; Phan, D.-T.; Chung, G.-S. Fabrication of low-temperature acetylene gas sensor based on Ag nanoparticles-loaded hierarchical ZnO nanostructures. Electron. Lett. 2014, 51, 572–574. [Google Scholar] [CrossRef]
- Gupta Chatterjee, S.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Yaqoob, U.; Phan, D.-T.; Chung, G.-S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2015, 222, 536–543. [Google Scholar] [CrossRef]
- Espinosa, E.H.; Ionescu, R.; Bittencourt, C.; Felten, A.; Erni, R.; Van Tendeloo, G.; Pireaux, J.J.; Llobet, E. Metal-decorated multi-wall carbon nanotubes for low temperature gas sensing. Thin Solid Film. 2007, 515, 8322–8327. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Zhang, Z.C.; Xu, Q.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Near room temperature, fast-response, and highly sensitive triethylamine sensor assembled with Au-loaded ZnO/SnO2 core-shell nanorods on flat alumina substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Kubotera, Y.; Yano, K.; Hashimoto, Y.; Kon, T.; Nakakura, S.; Nishi, Y.; Endo, H. Trimethylamine biosensor with flavin-containing monooxygenase type 3 (FMO3) for fish-freshness analysis. Sens. Actuators B Chem. 2004, 103, 463–467. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Yu, H.; Zhai, T.; Xu, Q.; Yang, X.; Wang, J.; Cao, B. Different morphologies of ZnO and their triethylamine sensing properties. J. Alloy. Compd. 2017, 706, 461–469. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Mi, R.; Liu, M.; Chen, Y.; Chen, C.; Ruan, S. On the high response towards TEA of gas sensors based on Ag-loaded 3D porous ZnO microspheres. Sens. Actuators B Chem. 2018, 270, 492–499. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Mandayo, G.G.; Castaño, E. Conductometric formaldehyde gas sensors. A review: From conventional films to nanostructured materials. Thin Solid Film. 2013, 548, 665–676. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, P.; Huang, S.; Zhang, Y. Comprehensive influence of environmental factors on the emission rate of formaldehyde and VOCs in building materials: Correlation development and exposure assessment. Environ. Res. 2016, 151, 734–741. [Google Scholar] [CrossRef]
- Wang, J.; Yunus, R.; Li, J.; Li, P.; Zhang, P.; Kim, J. In situ synthesis of manganese oxides on polyester fiber for formaldehyde decomposition at room temperature. Appl. Surf. Sci. 2015, 357, 787–794. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Han, B.; Deng, S.; Xiao, X.; Wang, Y. Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens. Actuators B Chem. 2016, 224, 193–200. [Google Scholar] [CrossRef]
- Xing, X.; Xiao, X.; Wang, L.; Wang, Y. Highly sensitive formaldehyde gas sensor based on hierarchically porous Ag-loaded ZnO heterojunction nanocomposites. Sens. Actuators B Chem. 2017, 247, 797–806. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, B.; Yang, T.; Wang, P.; Xiao, C.; Li, Z.; Zhao, R.; Zhang, M. Enhanced HCHO gas sensing properties by Ag-loaded sunflower-like In2O3 hierarchical nanostructures. J. Mater. Chem. A 2014, 2, 6598–6604. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Na, S.-I.; Yu, Y.T.; Suh, E.-k.; Hahn, Y.-B. Fabrication and enhanced carbon monoxide gas sensing performance of p-CuO/n-TiO2 heterojunction device. Colloids Surf. A Physicochem. Eng. 2020, 612, 125962. [Google Scholar] [CrossRef]
- Niakan, H.; Zhang, C.; Hu, Y.; Szpunar, J.A.; Yang, Q. Thermal stability of diamond-like carbon–MoS2 thin films in different environments. Thin Solid Film 2014, 562, 244–249. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.E.; Jiang, C.; Yao, Y.; Wang, D.; Zhang, Y. Room-temperature highly sensitive CO gas sensor based on Ag-loaded zinc oxide/molybdenum disulfide ternary nanocomposite and its sensing properties. Sens. Actuators B Chem. 2017, 253, 1120–1128. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Meng, X.; Zhang, Z.; Cao, J. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH4. Surf. Interfaces 2021, 24, 101110. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol–gel method. Ceram. Inter. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Kailasam, K. Near-room-temperature ethanol detection using Ag-loaded mesoporous carbon nitrides. ACS Omega 2017, 2, 3658–3668. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, P.; Hu, W.; Li, J.; Liu, Y.; Liu, Y.; Yu, F.; Zhang, C.; Xu, M. Performance degradation mechanism of the light-activated room temperature NO2 gas sensor based on Ag-ZnO nanoparticles. Appl. Surf. Sci. 2021, 541, 148418. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Yang, Q.; Liu, J.; Gao, Y.; Sun, P.; Lu, G. Preparation of Ag-loaded mesoporous WO3 and its enhanced NO2 sensing performance. Sens. Actuators B Chem. 2016, 225, 544–552. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Facile synthesis and UV-activated gas sensing performance of Ag: ZnO nano-ellipsoids. ECS J. Solid State Sci. Technol. 2018, 7, 3089. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xie, G.; Xu, M.; Yu, S.; Tai, H.; Du, H.; Jiang, Y. Visible light-assisted room temperature gas sensing with ZnO-Ag heterostructure nanoparticles. Sens. Actuators B Chem. 2018, 259, 269–281. [Google Scholar] [CrossRef]
- Garcia, D.; Picasso, G.; Hidalgo, P.; Peres, H.E.M.; Sun Kou, R.; Gonçalves, J.M. Sensors based on Ag-loaded hematite (α-Fe2O3) nanoparticles for methyl mercaptan detection at room temperature. Anal. Chem. Res. 2016, 12, 74–81. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Tian, H.; Qiao, L.; Zeng, Y.; Liu, C. Bimetal carbonaceous templates for multi-shelled NiCo2O4 hollow sphere with enhanced xylene detection. Sens. Actuators B Chem. 2021, 339, 129862. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Zhou, L.; Du, L.; Liu, F.; Xiang, L.; Gao, Y.; Liu, F.; Yan, X.; Lu, G. Preparation of silver-loaded titanium dioxide hedgehog-like architecture composed of hundreds of nanorods and its fast response to xylene. J. Colloid Interface Sci. 2019, 536, 215–223. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Aan, V.; Berg, D. Ammonia sensors and their applications-a review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E.; Çelikkan, H.; Erk, N.; Acar, S. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloy. Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Qin, Y.; Zang, J.; Wen, Z. Synergistic functionalization of aligned silicon nanowires by Ag nanoparticles & PPy wrapping for improving gas-sensing response at high humidity level. Phys. E Low-Dimens. Syst. Nanostructures 2020, 118, 113957. [Google Scholar]
- Yoon, J.-W.; Hong, Y.J.; Chan Kang, Y.; Lee, J.-H. High performance chemiresistive H2S sensors using Ag-loaded SnO2 yolk-shell nanostructures. RSC Adv. 2014, 4, 16067–16074. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Lee, K.-W.; Chung, G.-S. Acetylene gas sensing properties of an Ag-loaded hierarchical ZnO nanostructure-decorated reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 216, 33–40. [Google Scholar] [CrossRef]
- Zhou, L.; Bai, J.; Liu, Y.; Liu, F.; Wang, H.; Zhang, Y.; Lu, G. Highly sensitive C2H2 gas sensor based on Ag modified ZnO nanorods. Ceram. Inter. 2020, 46, 15764–15771. [Google Scholar] [CrossRef]
- Yousefi, H.R.; Hashemi, B.; Mirzaei, A.; Roshan, H.; Sheikhi, M.H. Effect of Ag on the ZnO nanoparticles properties as an ethanol vapor sensor. Mater. Sci. Semicond. Proc. 2020, 117, 105172. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Bateer, D.R.; Tamboli, M.S.; Mulla, I.S.; Suryavanshi, S.S. A greener approach towards the development of graphene-Ag loaded ZnO nanocomposites for acetone sensing applications. RSC Adv. 2019, 9, 33602–33606. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Q.; Zheng, X.; Wang, C.; Ma, J.; Yan, B.; Du, Z.; Li, M.; Wang, W.; Fan, H. 3D porous flower-like ZnO microstructures loaded by large-size Ag and their ultrahigh sensitivity to ethanol. J. Alloy. Compd. 2020, 829, 154453. [Google Scholar] [CrossRef]
- Xue, Y.-Y.; Wang, J.-L.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C.; Zhai, Q.-G. Mesoporous Ag/In2O3 composite derived from indium organic framework as high performance formaldehyde sensor. J. Solid State Chem. 2017, 251, 170–175. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, D.; Yin, N.; Yao, Y.; Shaymurat, T.; Zhou, X. Acetylene gas-sensing properties of layer-by-layer self-assembled Ag-decorated tin dioxide/graphene nanocomposite film. Nanomaterials 2017, 7, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhang, D.; Hu, J.; Wang, H.; Zhang, Y.; Li, K.; Rong, Q.; Zhou, S.; Zhang, J.; Zhu, Z.; et al. Excellent toluene gas sensing properties of molecular imprinted Ag-LaFeO3 nanostructures synthesized by microwave-assisted process. Mater. Res. Bull. 2019, 111, 320–328. [Google Scholar] [CrossRef]
- Liu, M.; Song, P.; Liang, D.; Yang, Z.; Wang, Q. Highly sensitive and selective triethylamine gas sensor based on Ag nanoparticles-decorated MoO3 nanobelts. Mater. Res. Express 2019, 6, 125910. [Google Scholar] [CrossRef]
- Liu, H.; Shen, W.; Chen, X. A room temperature operated ammonia gas sensor based on Ag-decorated TiO2 quantum dot clusters. RSC Adv. 2019, 9, 24519–24526. [Google Scholar] [CrossRef] [Green Version]
- Kamble, C.; Panse, M.; Nimbalkar, A. Ag decorated WO3 sensor for the detection of sub-ppm level NO2 concentration in air. Mater. Sci. Semicond. Proc. 2019, 103, 104613. [Google Scholar] [CrossRef]
- Ghanbari, R.; Safaiee, R.; Sheikhi, M.H.; Golshan, M.M.; Horastani, Z.K. Graphene decorated with silver nanoparticles as a low-temperature methane gas sensor. ACS Appl. Mater. Interfaces 2019, 11, 21795–21806. [Google Scholar] [CrossRef]
- Liu, D.; Pan, J.; Tang, J.; Liu, W.; Bai, S.; Luo, R. Ag decorated SnO2 nanoparticles to enhance formaldehyde sensing properties. J. Phys. Chem. Solids 2019, 124, 36–43. [Google Scholar] [CrossRef]
- Lin, Z.D.; Young, S.J.; Hsiao, C.H.; Chang, S.J. Adsorption sensitivity of Ag-decorated carbon nanotubes toward gas-phase compounds. Sens. Actuators B Chem. 2013, 188, 1230–1234. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, G.; Gao, W.; Zhao, R.; Wang, Y. Ag decorated ZnO nanocrystallines synthesized by a low-temperature solvothermal method and their application for high response H2 gas sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 18959–18969. [Google Scholar]
- Shim, Y.S.; Zhang, L.; Kim, Y.H.; Choi, Y.R.; Nahm, S.H.; Kang, C.Y.; Lee, W.; Jang, H.W. Highly sensitive and selective H2 and NO2 gas sensors based on surface-decorated WO3 nanoigloos. Sens. Actuators B Chem. 2014, 198, 294–301. [Google Scholar] [CrossRef]
- Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B Chem. 2018, 255, 672–683. [Google Scholar]
- Yin, Y.; Li, F.; Zhang, N.; Ruan, S.; Zhang, H.; Chen, Y. Improved gas sensing properties of silver-functionalized ZnSnO3 hollow nanocubes. Inorg. Chem. Front. 2018, 5, 2123–2131. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Ahn, J.; Choi, S.J.; Lee, K.H. Ag-functionalized SnO2 nanowires-based sensor for NO2 detection at low operating temperature. J. Microelectron. Packag. Soc. 2020, 27, 11–17. [Google Scholar]
- Park, S.; An, S.; Ko, H.; Jin, C.; Lee, C. Enhancement of ethanol sensing of TeO2 nanorods by Ag functionalization. Curr. Appl. Phys. 2013, 13, 576–580. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, M.; Lu, Q.; Zhang, Y.; Zhang, J.; Li, B.; Wei, H.; Hu, J.; Wang, H.; Liu, Q. Ag nanoparticles sensitized In2O3 nanograin for the ultrasensitive HCHO detection at room temperature. Nanoscale Res. Lett. 2019, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. A high-response formaldehyde sensor based on fibrous Ag-ZnO/In2O3 with multi-level heterojunctions. J. Hazard. Mater. 2021, 413, 125352. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Li, Z.; Tian, Y.; Yang, Z. Enhanced formaldehyde sensing performance based on Ag@WO3 2D nanocomposite. Powder Tech. 2019, 343, 1–10. [Google Scholar] [CrossRef]
- Feng, D.L.; Zhu, Z.Y.; Du, L.L.; Xing, X.; Wang, C.; Chen, J.; Tian, Y.T.; Yang, D.C. Improved sensing performance of WO3 nanoparticles decorated with Ag and Pt nanoparticles. Rare Met. 2021, 40, 1642–1650. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal-oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Ristein, J. Surface transfer doping of semiconductors. Sci. N. Y. Wash. 2006, 313, 1057. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Haz. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.-S.; Tsymbalenko, O.; Lan, P.-T.; Moon, M.-W.; Lee, K.-B. Highly sensitive chemiresistive H2S gas sensor based on graphene decorated with Ag nanoparticles and charged impurities. Sens. Actuators B Chem. 2018, 257, 278. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Koinkar, P.M.; Maiti, N.; Sonawane, K.M. Synthesis of Ag doped SnO2 thin films for the evaluation of H2S gas sensing properties. Phys. B Condens. Matter 2017, 524, 90–96. [Google Scholar] [CrossRef]
- Anand, K.; Kaur, J.; Singh, R.C.; Thangaraj, R. Preparation and characterization of Ag-doped In2O3 nanoparticles gas sensor. Chem. Phys. Lett. 2017, 682, 140–146. [Google Scholar] [CrossRef]
- Ding, M.; Xie, N.; Wang, C.; Kou, X.; Zhang, H.; Guo, L.; Sun, Y.; Chuai, X.; Gao, Y.; Liu, F.; et al. Enhanced NO2 gas sensing properties by Ag-doped hollow urchin-like In2O3 hierarchical nanostructures. Sens. Actuators B Chem. 2017, 252, 418–427. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Ibrahim, A.A.; Al-Heniti, S.H.; Kumar, R.; Baskoutas, S.; Raffah, B.M. Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles. Ceram. Inter. 2017, 43, 6765–6770. [Google Scholar] [CrossRef]
- Hong, C.; Zhou, Q.; Lu, Z.; Umar, A.; Kumar, R.; Wei, Z.; Wu, X.; Xu, L.; Kim, S.H. Ag-doped ZnO nanoellipsoids based highly sensitive gas sensor. Mater. Express 2017, 7, 380–388. [Google Scholar] [CrossRef]
- Wei, W.; Guo, S.; Chen, C.; Sun, L.; Chen, Y.; Guo, W.; Ruan, S. High sensitive and fast formaldehyde gas sensor based on Ag-doped LaFeO3 nanofibers. J. Alloy. Compd. 2017, 695, 1122–1127. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Cao, J.; Kong, F.; Xia, H.; Zhang, J.; Zhu, B.; Wang, S.; Wu, S. Low-temperature H2S sensors based on Ag-doped α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2008, 131, 183–189. [Google Scholar] [CrossRef]
- Natkaeo, A.; Phokharatkul, D.; Hodak, J.H.; Wisitsoraat, A.; Hodak, S.K. Highly selective sub-10 ppm H2S gas sensors based on Ag-doped CaCu3Ti4O12 films. Sens. Actuators B Chem. 2018, 260, 571–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Z.; Yang, F. Highly sensitive and selective alcohol sensors based on Ag-dped In2O3 Coating. Indus. Eng. Chem. Res. 2010, 49, 3539–3543. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.Y.; Zhang, Q.X.; Zhu, K.M.; Tie, Y.; Pei, S.T.; Wang, B.J.; Zhang, J.L. Highly sensitive formaldehyde gas sensors based on Ag doped Zn2SnO4/SnO2 hollow nanospheres. Mater. Lett. 2019, 254, 178–181. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Q.; Xu, L.; Gui, Y.; Zhao, Z.; Tang, C.; Chen, W. Synthesis and characterization of highly sensitive hydrogen (H2) sensing device based on Ag doped SnO2 nanospheres. Materials 2018, 11, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, D.; Kim, K.; Park, S.-i.; Kim, Y.-h.; Kim, S.; Kim, S.-I. Characteristics of Ga and Ag-doped ZnO-based nanowires for an ethanol gas sensor prepared by hot-walled pulsed laser deposition. Res. Chem. Intermed. 2014, 40, 97–103. [Google Scholar] [CrossRef]
- Wang, J.; Zou, B.; Ruan, S.; Zhao, J.; Chen, Q.; Wu, F. HCHO sensing properties of Ag-doped In2O3 nanofibers synthesized by electrospinning. Mater. Lett. 2009, 63, 1750–1753. [Google Scholar] [CrossRef]
- Adilakshmi, G.; Reddy, R.S.; Reddy, A.S.; Reddy, P.S.; Reddy, C.S. Ag-doped WO3 nanostructure films for organic volatile gas sensor application. J. Mater. Sci. Mater. Electron. 2020, 31, 12158–12168. [Google Scholar] [CrossRef]
- Postica, V.; Vahl, A.; Santos-Carballal, D.; Dankwort, T.; Kienle, L.; Hoppe, M.; Cadi-Essadek, A.; De Leeuw, N.H.; Terasa, M.I.; Adelung, R.; et al. Tuning ZnO sensors reactivity toward volatile organic compounds via Ag doping and nanoparticle functionalization. Acs Appl. Mater. Interfaces 2019, 11, 31452–31466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abideen, Z.U.; Kim, J.-H.; Kim, S.S. Optimization of metal nanoparticle amount on SnO2 nanowires to achieve superior gas sensing properties. Sens. Actuators B Chem. 2016, 238, 374–380. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, H.; Sun, Y.; Li, M.; Liu, J. UV-activated room temperature single-sheet ZnO gas sensor. Micro Nano Lett. 2017, 12, 813–817. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, J.; Li, J.; Zhang, N.; Hong, R.; Deng, X.; Tang, P.; Li, D. Hydrogen sensing properties of Pt-Au bimetallic nanoparticles loaded on ZnO nanorods. Sens. Actuators B Chem. 2017, 241, 895–903. [Google Scholar] [CrossRef]
- Mitri, F.; Iacovo, A.; Luca, M.; Pecora, A.; Colace, L. Lead sulphide colloidal quantum dots for room temperature NO2 gas sensors. Sci. Reports 2020, 10, 12556. [Google Scholar]
- Wu, T.; Wang, Z.; Tian, M.; Miao, J.; Zhang, H.; Sun, J. UV excitation NO2 gas sensor sensitized by ZnO quantum dots at room temperature. Sens. Actuators B Chem. 2018, 259, 526–531. [Google Scholar] [CrossRef]
- Ayhan, B.; Kwan, C.; Zhou, J.; Kish, L.B.; Benkstein, K.D.; Rogers, P.H.; Semancik, S. Fluctuation enhanced sensing (FES) with a nanostructured, semiconducting metal oxide film for gas detection and classification. Sens. Actuators B Chem. 2013, 188, 651–660. [Google Scholar] [CrossRef]
- Kish, L.B.; Smulko, J.; Heszler, P.; Granqvist, C.G. On the sensitivity, selectivity, sensory information and optimal size of resistive chemical sensors. Nanotechnol. Percept. 2007, 3, 43–52. [Google Scholar] [CrossRef]
- Ederth, J.; Smulko, J.M.; Kish, L.B.; Heszler, P.; Granqvist, C.G. Comparison of classical and fluctuation-enhanced gas sensing with PdxWO3 nanoparticle films. Sens. Actuators B Chem. 2006, 113, 310–315. [Google Scholar] [CrossRef]
- Kish, L.B.; Vajtai, R.; Granqvist, C.G. Extracting information from the noise spectra of chemical sensors: Electronic nose and tongue by one sensor. Sens. Actuators B Chem. 2000, 71, 55–59. [Google Scholar] [CrossRef]

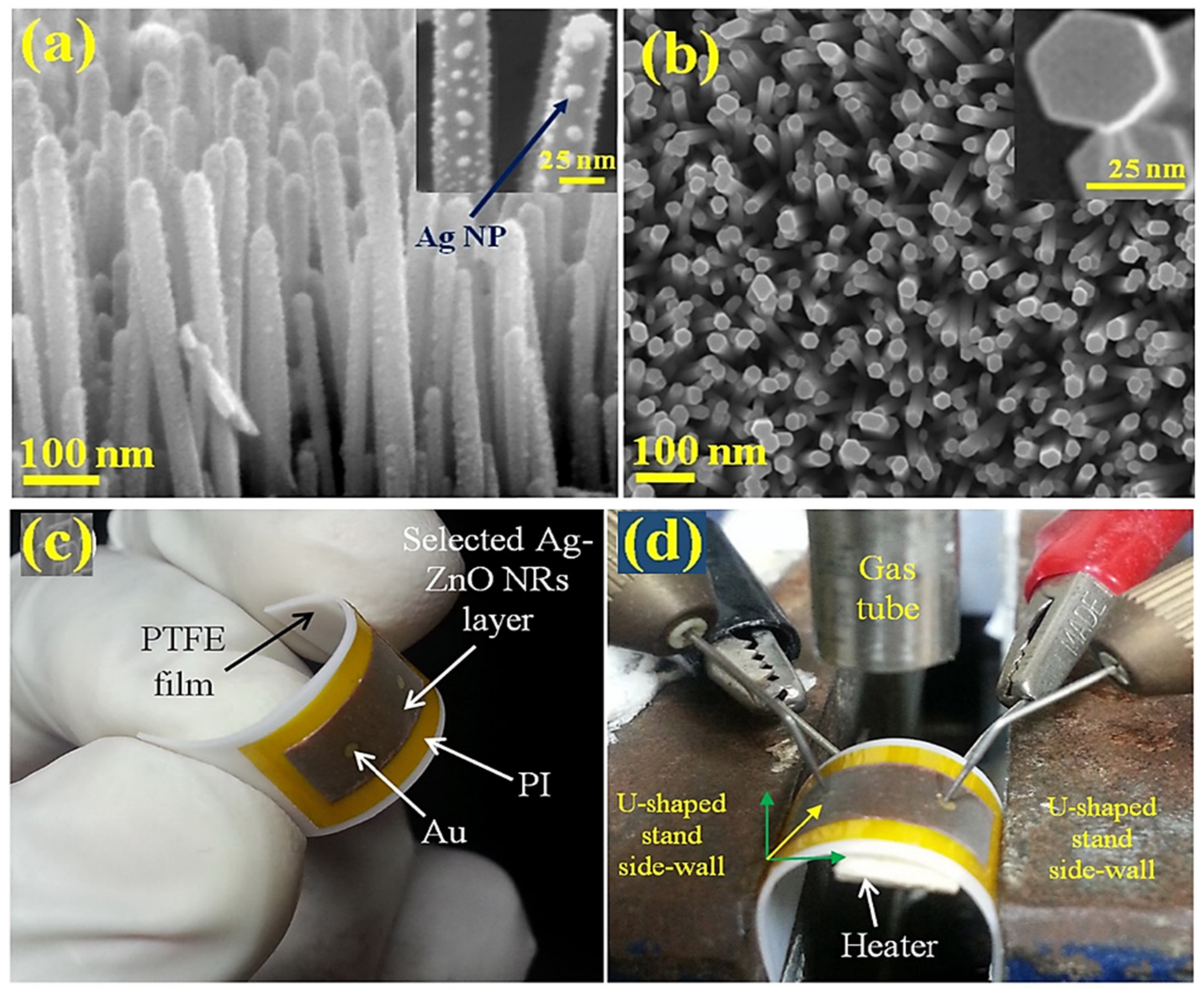
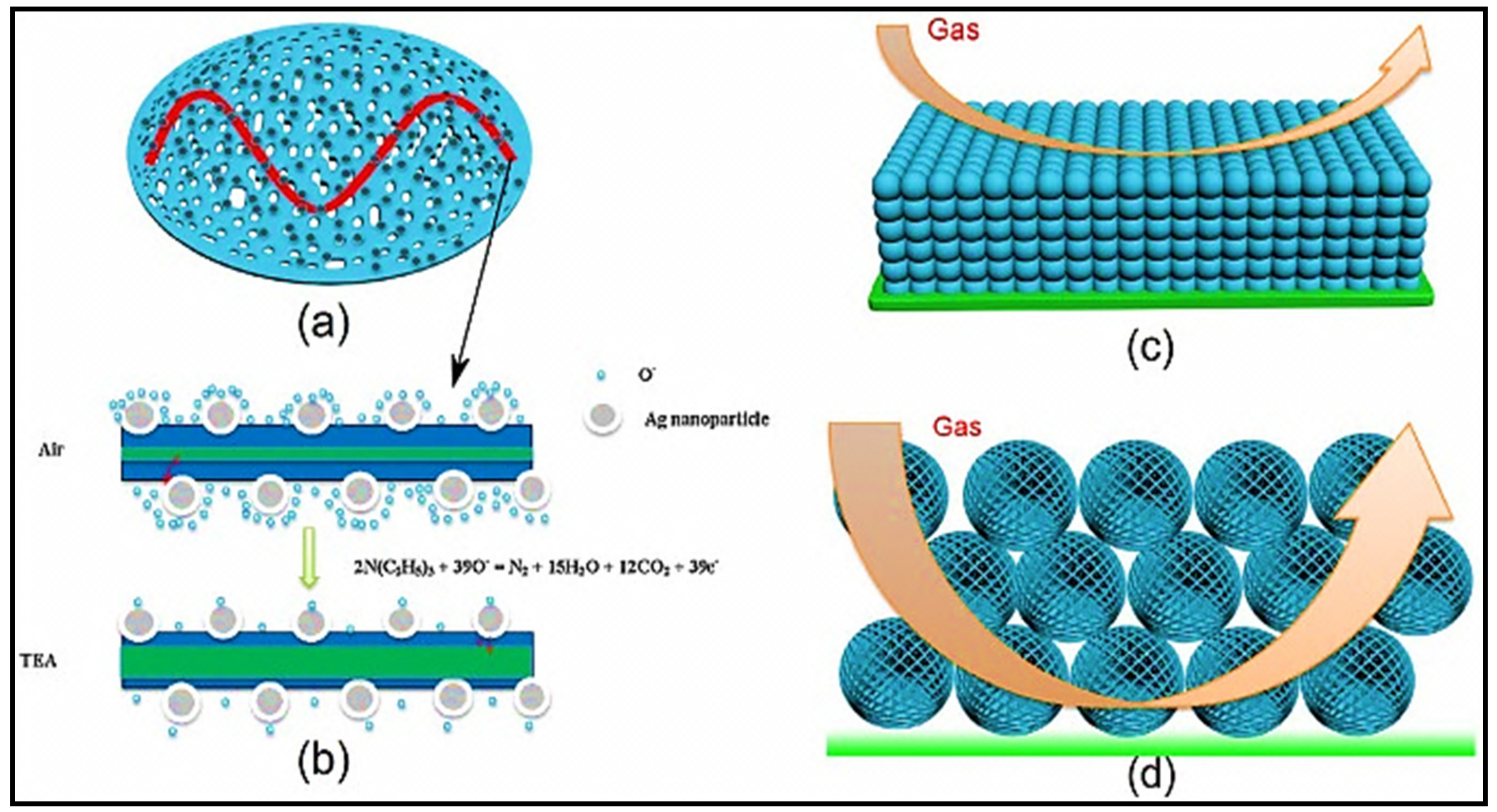
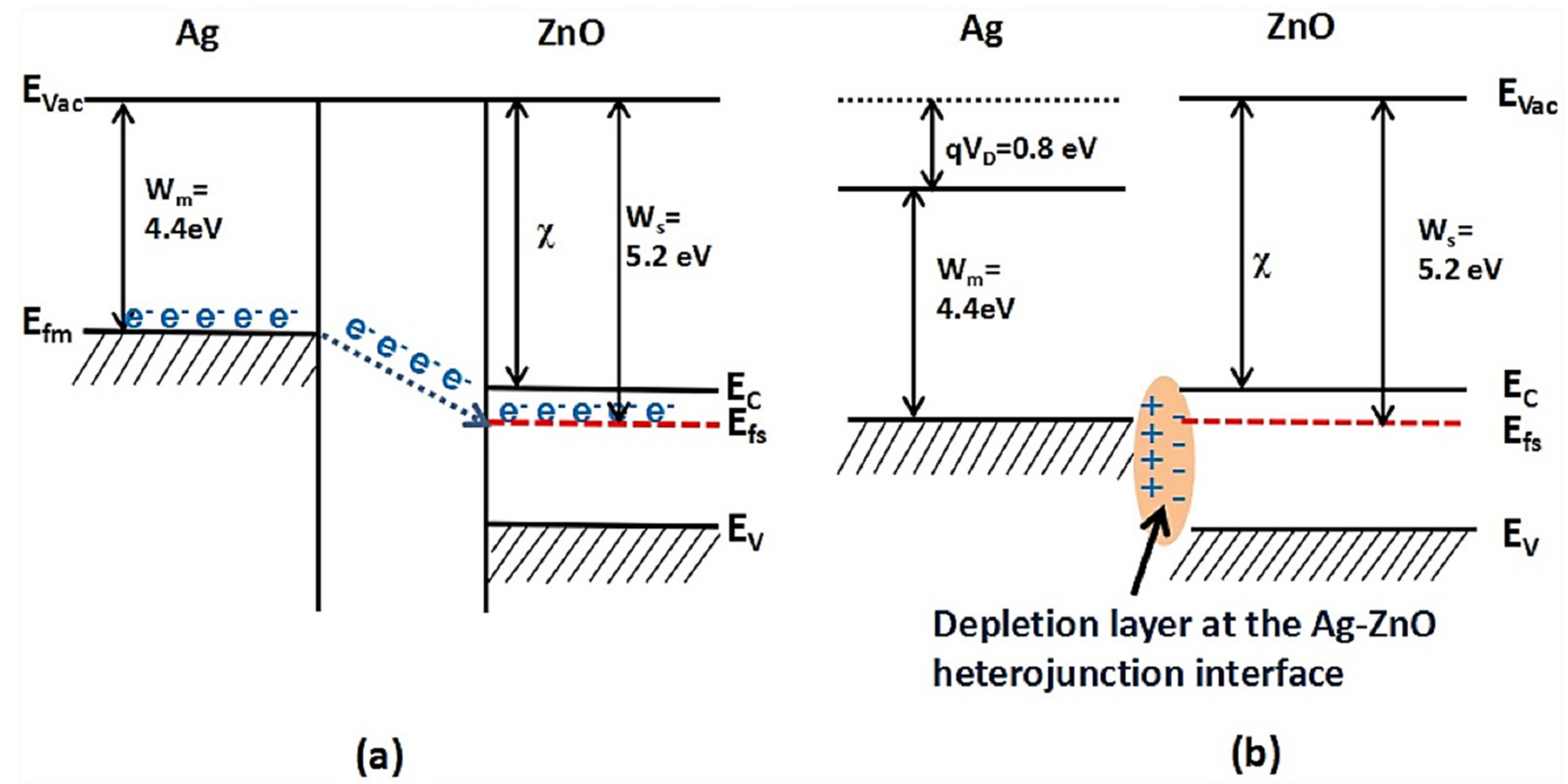



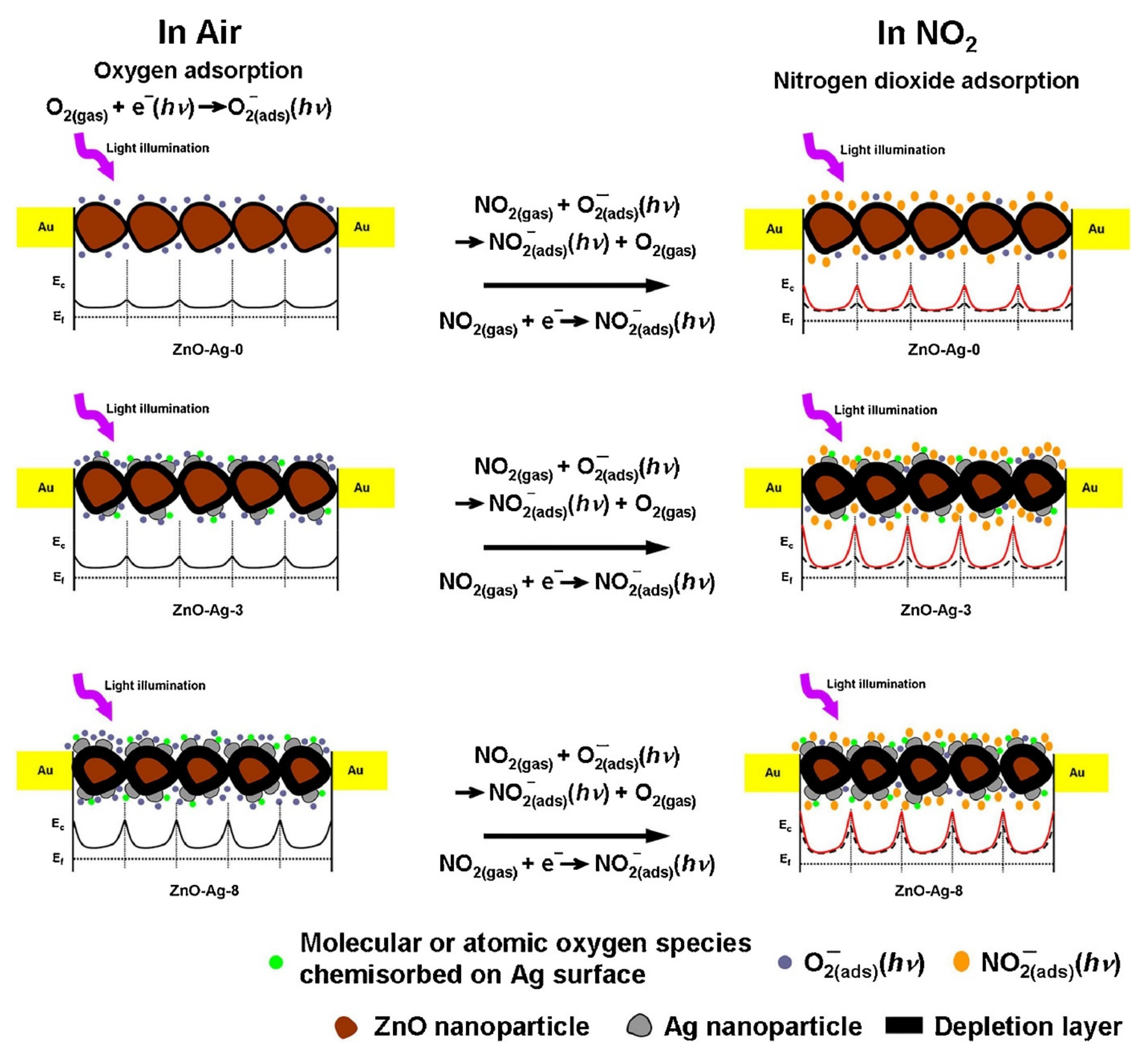

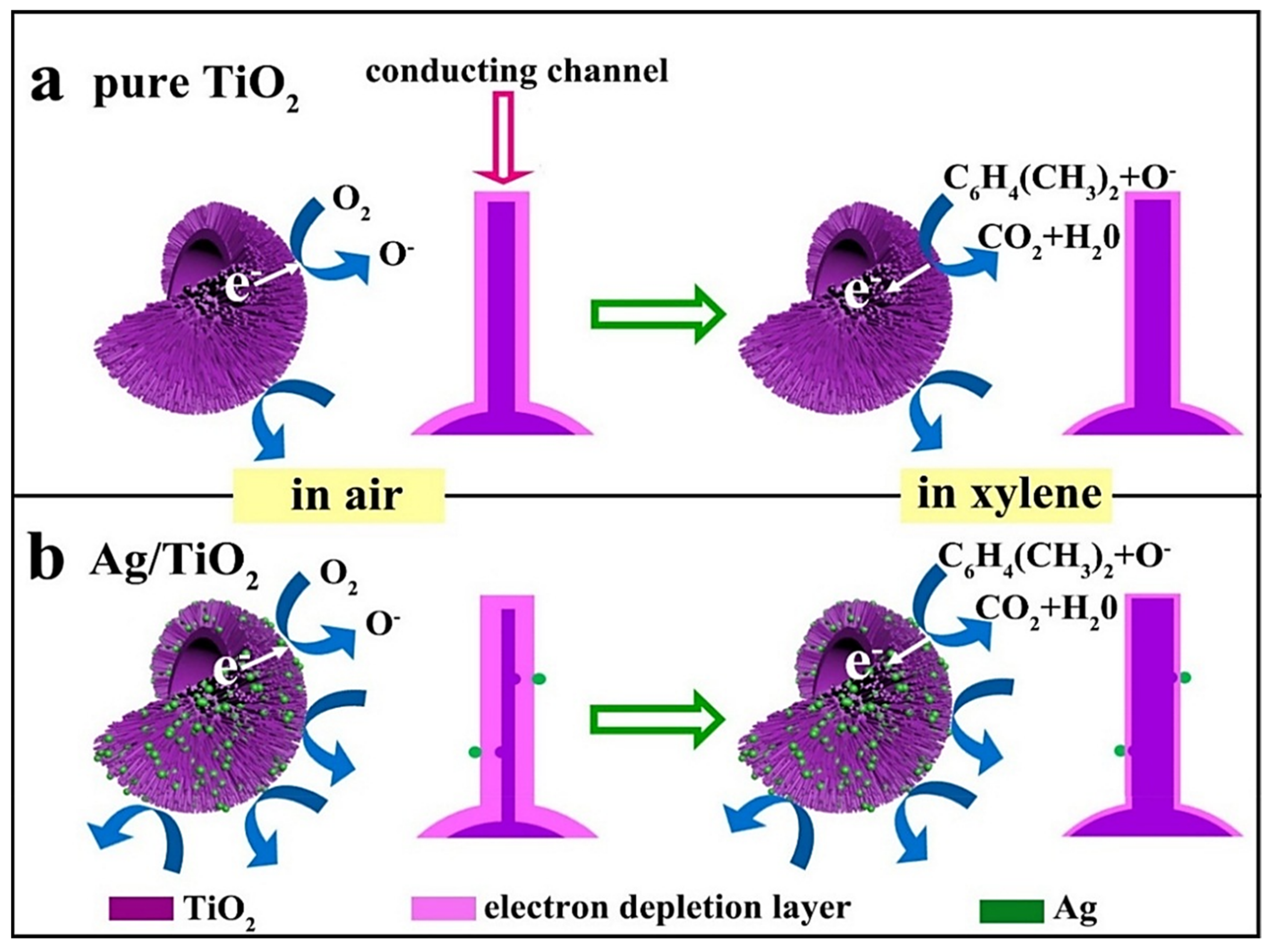
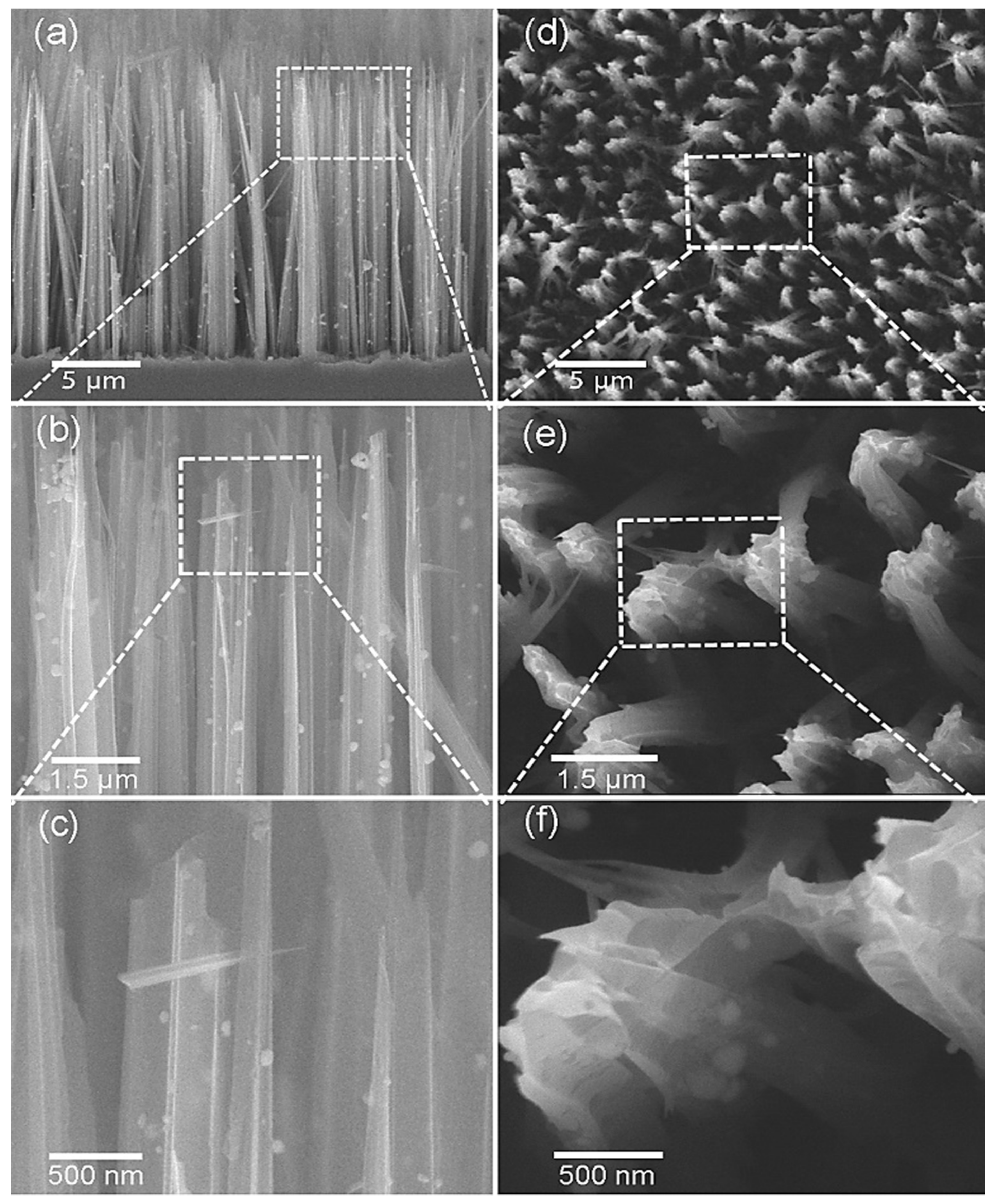

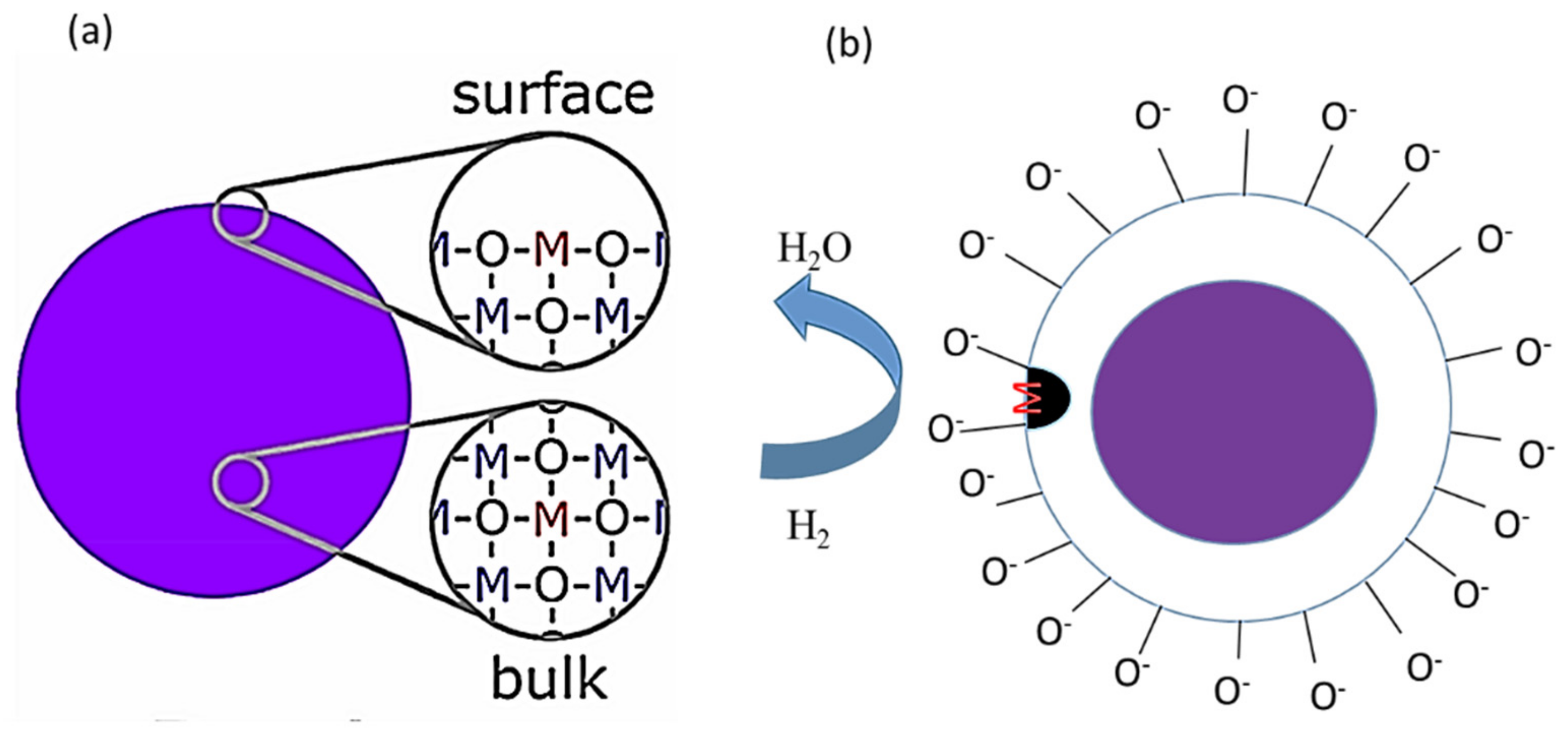

| Sensing Materials | Gas Type | GC (ppm) | T (°C) | Response | Ref. |
|---|---|---|---|---|---|
| Ag-loaded SnO2 hollow NFs | CH3COCH3 | 50 | 160 | 42 | [48] |
| Ag-decorated TiO2 NRs | CH3COCH3 | 3.8 | 200 | 7.31 (ΔI)/I0 | [49] |
| Ag-loaded BiFe2O4 NPs | Cl2 | 10 | 240 | 72.62 | [50] |
| Ag-decorated ZnO/G nanocomposite | C2H2 | 100 | 150 | 21.2 | [51] |
| 5 wt.% Ag-loaded ZnO NPs | C2H2 | 100 | 200 | ≈80% | [52] |
| Ag-loaded ZnO NRs | C2H2 | 1000 | 200 | 27.2 | [54] |
| Ag-loaded ZnO NPs | TEA | 100 | 183.5 | 6043 | [60] |
| Ag-loaded Porous ZnO NPs | HCHO | 100 | 240 | 180.4 | [65] |
| Ag-loaded In2O3 sunflower structure | HCHO | 20 | 240 | 11.3 | [66] |
| Ag-loaded ZnO/MoS2 | CO | 100 | 25 | 6 | [69] |
| Ag-loaded ZnO NPs | CO | 100 | 130 | 24.17 | [70] |
| Ag-loaded ZnO NPs | CH4 | 5000 | 200 | 20.15 | [70] |
| Ag/g-CN | C2H5OH | 50 | 40 | 1.3 | [72] |
| Ag-loaded mesoporous WO3 | NO2 | 1 | 75 | 40 | [74] |
| Ag-loaded ZnO NPs | NO2 | 5 | 25 (UV light) | 1.545 | [76] |
| Ag-loaded Fe2O3 | CH3SH | 80 | 25 | 72% | [77] |
| Ag-loaded TiO2 hedgehog-like architecture | C8H10 | 100 | 375 | 6.9 | [80] |
| Ag-loaded SnO2 yolk-shell nanostructures | H2S | 5 | 350 | 614.9 | [85] |
| Ag-loaded ZnO/rGO | C2H2 | 1000 | 200 | 33 | [86] |
| Ag-loaded ZnO NRs | C2H2 | 100 | 250 | 255 | [87] |
| 3.5 wt.% Ag-decorated ZnO | C2H5OH | 50 | 325 | 32.5 | [88] |
| Ag-decorated ZnO/Graphene nanocomposite | CH3COCH3 | 1000 | 175 | 71 | [89] |
| Ag-loaded 3D porous flower-like ZnO NPs | C2H5OH | 200 | 300 | 268 | [90] |
| Ag-loaded In2O3 NPs | HCHO | 50 | 210 | 156.9 | [91] |
| Ag-loaded SnO2-rGO nanocomposite | C2H2 | 500 | 90 | 26 (%) | [92] |
| Ag-loaded LaFeO3 NPs | C7H8 | 5 | 215 | 24 | [93] |
| Ag-loaded MoO3 nanobelts | TEA | 100 | 240 | 26.58 | [94] |
| Ag-decorated TiO2 QDs | NH3 | 20 | 25 | 25.1 | [95] |
| Sensing Materials | Gas Type | GC (ppm) | T (°C) | Response | Ref. |
|---|---|---|---|---|---|
| Ag-doped graphene | H2S | 50 | 25 | 140% | [113] |
| Ag-doped SnO2 | H2S | 450 | 200 | 1.32 | [114] |
| 3% Ag-doped In2O3 | C2H5OH | 1000 | 300 | 175 | [115] |
| Ag-doped In2O3 NPs | NO2 | 1 | 62 | 190.1 | [116] |
| Ag-doped ZnO nanoneedles | CH3COCH3 | 100 | 370 | 19 | [117] |
| Ag-doped ZnO nanoellipsoids | CH3OH | 200 | 370 | 15.8 | [118] |
| Ag-doped LaFeO3 NPs | HCHO | 100 | 230 | 20 | [119] |
| Ag-doped Fe2O3 NPs | H2S | 100 | 400 | 220 | [120] |
| Ag-doped CaCu3Ti4O12 NPs | H2S | 10 | 250 | 110 | [121] |
| Ag-doped In2O3 NPs | C2H5OH | 150 | 100 | 100 | [122] |
| Ag-doped Zn2SnO4/SnO2 hollow NPs | HCHO | 140 | 50 | 62.2 | [123] |
| Ag-doped SnO2 NPs | H2 | 50 | 300 | 25 | [124] |
| Ag-doped ZnO NWs | C2H5OH | 1 | 300 | 203% | [125] |
| Ag-doped In2O3 NFs | HCHO | 600 | 120 | 130 | [126] |
| Ag-doped WO3 | C2H5OH | 100 | 300 | 65 | [127] |
| Ag-decorated/Ag-doped ZnO columnar films | C2H5OH | 100 | 250 | 145 | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navale, S.; Shahbaz, M.; Mirzaei, A.; Kim, S.S.; Kim, H.W. Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview. Sensors 2021, 21, 6454. https://doi.org/10.3390/s21196454
Navale S, Shahbaz M, Mirzaei A, Kim SS, Kim HW. Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview. Sensors. 2021; 21(19):6454. https://doi.org/10.3390/s21196454
Chicago/Turabian StyleNavale, Sachin, Mehrdad Shahbaz, Ali Mirzaei, Sang Sub Kim, and Hyoun Woo Kim. 2021. "Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview" Sensors 21, no. 19: 6454. https://doi.org/10.3390/s21196454
APA StyleNavale, S., Shahbaz, M., Mirzaei, A., Kim, S. S., & Kim, H. W. (2021). Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview. Sensors, 21(19), 6454. https://doi.org/10.3390/s21196454









