Mechanosensory Hairs and Hair-like Structures in the Animal Kingdom: Specializations and Shared Functions Serve to Inspire Technology Applications
Abstract
1. Introduction
2. Mechanosensory Feedback for Coordinated Locomotion
3. Navigation and Exploration
4. Prey Capture and Feeding
5. Engineering Applications of Biologically Inspired Hair Sensors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfeifer, R.; Lungarella, M.; Iida, F. Self-Organization, Embodiment, and Biologically Inspired Robotics. Science 2007, 318, 1088–1093. [Google Scholar] [CrossRef]
- Solomon, J.H.; Hartmann, M.J. Robotic whiskers used to sense features. Nature 2006, 443, 525. [Google Scholar] [CrossRef]
- Solomon, J.; Hartmann, M. Artificial Whiskers Suitable for Array Implementation: Accounting for Lateral Slip and Surface Friction. IEEE Trans. Robot. 2008, 24, 1157–1167. [Google Scholar] [CrossRef]
- Han, Z.; Liu, L.; Wang, K.; Song, H.; Chen, D.; Wang, Z.; Niu, S.; Zhang, J.; Ren, L. Artificial Hair-Like Sensors Inspired from Nature: A Review. J. Bionic Eng. 2018, 15, 409–434. [Google Scholar] [CrossRef]
- Asterindi Blandin, A.A.; Bernardeschi, I.; Beccai, L. Biomechanics in Soft Mechanical Sensing: From Natural Case Studies to the Artificial World. Biomimetics 2018, 3, 32. [Google Scholar] [CrossRef]
- Yu, S.W.Y.; Graff, M.M.; Hartmann, M.J.Z. Mechanical responses of rat vibrissae to airflow. J. Exp. Biol. 2016, 219, 937–948. [Google Scholar] [CrossRef]
- Crish, C.M.; Crish, S.D.; Comer, C. Tactile Sensing in the Naked Mole Rat. In Scholarpedia of Touch; Prescott, T., Ahissar, E., Izhikevich, E., Eds.; Atlantis Press: Paris, France, 2016; pp. 95–101. ISBN 978-94-6239-132-1. [Google Scholar]
- Weis-Fogh, T. An Aerodynamic Sense Organ Stimulating and Regulating Flight in Locusts. Nature 1949, 164, 873–874. [Google Scholar] [CrossRef]
- Sterbing-D’Angelo, S.J.; Chadha, M.; Marshall, K.L.; Moss, C.F. Functional role of airflow-sensing hairs on the bat wing. J. Neurophysiol. 2017, 117, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, J.C.; Bauer, G.B.; Mann, D.A.; Boerner, K.; Denum, L.; Frances, C.; Reep, R.L. Detection of hydrodynamic stimuli by the postcranial body of Florida manatees (Trichechus manatus latirostris). J. Comp. Physiol. A 2017, 203, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mcgovern, K.A.; Marshall, C.D.; Davis, R.W. Are Vibrissae Viable Sensory Structures for Prey Capture in Northern Elephant Seals, Mirounga angustirostris? Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2015, 298, 750–760. [Google Scholar] [CrossRef]
- Gorb, S.N. The design of the fly adhesive pad: Distal tenent setae are adapted to the delivery of an adhesive secretion. Proc. R. Soc. B Boil. Sci. 1998, 265, 747–752. [Google Scholar] [CrossRef]
- Wong, R.K.; Pearson, K.G. Properties of the trochanteral hair plate and its function in the control of walking in the cockroach. J. Exp. Biol. 1976, 64, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Barth, F.G. The slightest whiff of air: Airflow sensing in arthropods. In Flow Sensing in Air and Water; Springer: Berlin/Heidelberg, Germany, 2014; pp. 169–196. [Google Scholar]
- Barth, F.G. Spider senses–technical perfection and biology. Zoology 2002, 105, 271–285. [Google Scholar] [CrossRef]
- Barth, F.G. Spider mechanoreceptors. Curr. Opin. Neurobiol. 2004, 14, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Gronenberg, W.; Tautz, J. The sensory basis for the trap-jaw mechanism in the ant Odontomachus bauri. J. Comp. Physiol. Ser. A 1994, 174, 49–60. [Google Scholar] [CrossRef]
- Gottschaldt, K.-M.; Iggo, A.; Young, D.W. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J. Physiol. 1973, 235, 287–315. [Google Scholar] [CrossRef]
- Brecht, M.; Preilowski, B.; Merzenich, M.M. Functional architecture of the mystacial vibrissae. Behav. Brain Res. 1997, 84, 81–97. [Google Scholar] [CrossRef]
- Taylor, G.K.; Krapp, H.G. Sensory Systems and Flight Stability: What do Insects Measure and Why? In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 34, pp. 231–316. ISBN 978-0-12-373714-4. [Google Scholar]
- Chiba, A.; Kämper, G.; Murphey, R.K. Response Properties of Interneurons of the Cricket Cercal Sensory System are Conserved in Spite of Changes in Peripheral Receptors during Maturation. J. Exp. Biol. 1992, 164, 205–226. [Google Scholar] [CrossRef]
- Jacobs, G.A.; Miller, J.P.; Aldworth, Z. Computational mechanisms of mechanosensory processing in the cricket. J. Exp. Biol. 2008, 211, 1819–1828. [Google Scholar] [CrossRef][Green Version]
- Sterbing-D’Angelo, S.J.; Liu, H.; Yu, M.; Moss, C.F. Morphology and deflection properties of bat wing sensory hairs: Scanning electron microscopy, laser scanning vibrometry and mechanics model. Bioinspir. Biomim. 2016, 11, 56008. [Google Scholar] [CrossRef]
- Tautz, J.; Masters, W.M.; Aicher, B.; Märkl, H. A new type of water vibration receptor on the crayfish antenna. J. Comp. Physiol. A 1981, 144, 533–541. [Google Scholar] [CrossRef]
- Tautz, J.; Sandeman, D.C. The Detection of Waterborne Vibration by Sensory Hairs on the Chelae of the Crayfish. J. Exp. Biol. 1980, 88, 351–356. [Google Scholar] [CrossRef]
- Drake, S.E.; Crish, S.D.; George, J.C.; Stimmelmayr, R.; Thewissen, J. Sensory Hairs in the Bowhead Whale, Balaena mysticetus (Cetacea, Mammalia): Sensory Hairs in the Bowhead Whale. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2015, 298, 1327–1335. [Google Scholar] [CrossRef]
- Dougill, G.; Starostin, E.L.; Milne, A.O.; Van Der Heijden, G.H.M.; Goss, V.G.A.; Grant, R.A. Ecomorphology reveals Euler spiral of mammalian whiskers. J. Morphol. 2020, 281, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Reep, R.L.; Marshall, C.D.; Stoll, M.L.; Whitaker, D.M. Distribution and Innervation of Facial Bristles and Hairs in the Florida Manatee (Trichechus Manatus Latirostris). Mar. Mammal. Sci. 1998, 14, 257–273. [Google Scholar] [CrossRef]
- Reep, R.L.; Marshall, C.D.; Stoll, M.L. Tactile Hairs on the Postcranial Body in Florida Manatees: A Mammalian Lateral Line? Brain Behav. Evol. 2002, 59, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Krueger, S.; Heys, J.J.; Gedeon, T. Quantitative Characterization of the Filiform Mechanosensory Hair Array on the Cricket Cercus. PLoS ONE 2011, 6, e27873. [Google Scholar] [CrossRef] [PubMed]
- Pringle, J.W.S. Proprioception in Insects I: A New Type of Mechanical Receptor from the Palps of the Cockroach. J. Exp. Biol. 1937, 13, 101–103. [Google Scholar]
- Tuthill, J.C.; Wilson, R.I. Mechanosensation and Adaptive Motor Control in Insects. Curr. Biol. 2016, 26, R1022–R1038. [Google Scholar] [CrossRef]
- Dean, J.; Wendler, G. Stick Insect Locomotion on a Walking Wheel: Interleg Coordination of Leg Position. J. Exp. Biol. 1983, 103, 75–94. [Google Scholar] [CrossRef]
- Kuenzi, F.; Burrows, M. Central connections of sensory neurones from a hair plate proprioceptor in the thoraco-coxal joint of the locust. J. Exp. Biol. 1995, 198, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Büschges, A. Sensory Control and Organization of Neural Networks Mediating Coordination of Multisegmental Organs for Locomotion. J. Neurophysiol. 2005, 93, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, A.; Hooper, S.L.; Büschges, A. Sensory Feedback Induced by Front-Leg Stepping Entrains the Activity of Central Pattern Generators in Caudal Segments of the Stick Insect Walking System. J. Neurosci. 2009, 29, 2972–2983. [Google Scholar] [CrossRef]
- Knebel, D.; Wörner, J.; Rillich, J.; Nadler, L.; Ayali, A.; Couzin-Fuchs, E. The subesophageal ganglion modulates locust inter-leg sensory-motor interactions via contralateral pathways. J. Insect Physiol. 2018, 107, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Knebel, D.; Rillich, J.; Nadler, L.; Pflüger, H.-J.; Ayali, A. The functional connectivity between the locust leg pattern generators and the subesophageal ganglion higher motor center. Neurosci. Lett. 2019, 692, 77–82. [Google Scholar] [CrossRef]
- Knebel, D.; Ayali, A.; Pflüger, H.-J.; Rillich, J. Rigidity and Flexibility: The Central Basis of Inter-Leg Coordination in the Locust. Front. Neural Circuits 2017, 10, 112. [Google Scholar] [CrossRef]
- Gorb, S.N.; Beutel, R.G.; Gorb, E.V.; Jiao, Y.; Kastner, V.; Niederegger, S.; Popov, V.L.; Scherge, M.; Schwarz, U.; Vötsch, W. Structural Design and Biomechanics of Friction-Based Releasable Attachment Devices in Insects. Integr. Comp. Biol. 2002, 42, 1127–1139. [Google Scholar] [CrossRef]
- Bullock, J.M.R.; Drechsler, P.; Federle, W. Comparison of smooth and hairy attachment pads in insects: Friction, adhesion and mechanisms for direction-dependence. J. Exp. Biol. 2008, 211, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuijsen, L.I.; van Trimmer, B.A. Locomotion in caterpillars. Biol. Rev. 2014, 89, 656–670. [Google Scholar] [CrossRef]
- Weeks, J.C.; Jacobs, G.A. A reflex behavior mediated by monosynaptic connections between hair afferents and motoneurons in the larval tobacco hornworm, Manduca sexta. J. Comp. Physiol. A 1987, 160, 315–329. [Google Scholar] [CrossRef]
- Newland, P. Physiological properties of afferents from tactile hairs on the hindlegs of the locust. J. Exp. Biol. 1991, 155, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, T.; Murakami, J.; Kumagai, T. Cricket Wind Receptors: Thermal Noise for the Highest Sensitivity Known. In Sensors and Sensing in Biology and Engineering; Barth, F.G., Humphrey, J.A.C., Secomb, T.W., Eds.; Springer: Vienna, Austria, 2003; pp. 145–157. [Google Scholar] [CrossRef]
- Boyd, K.; Ewer, D.W. Flight Responses in Grasshoppers. S. Afr. Sci. 1949, 2, 168–169. [Google Scholar]
- Weis-Fogh, T.; Pringle, J.W.S. Biology and physics of locust flight IV. Notes on sensory mechanisms in locust flight. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1956, 239, 553–584. [Google Scholar] [CrossRef]
- Camhi, J.M. Yaw-Correcting Postural Changes in Locusts. J. Exp. Biol. 1970, 52, 519–531. [Google Scholar] [CrossRef]
- Camhi, J.M. Sensory Control of Abdomen Posture in Flying Locusts. J. Exp. Biol. 1970, 52, 533–537. [Google Scholar] [CrossRef]
- Neese, V. Zur Funktion der Augenborsten bei der Honigbiene. J. Comp. Physiol. A 1965, 49, 543–585. [Google Scholar] [CrossRef]
- Boyan, G.S.; Ashman, S.; Ball, E.E. Initiation and modulation of flight by a single giant interneuron in the cercal system of the locust. Naturwissenschaften 1986, 73, 272–274. [Google Scholar] [CrossRef]
- Landolfa, M.; Jacobs, G. Direction sensitivity of the filiform hair population of the cricket cereal system. J. Comp. Physiol. A 1995, 177, 759–766. [Google Scholar] [CrossRef]
- Gnatzy, W.; Tautz, J. Ultrastructure and mechanical properties of an insect mechanoreceptor: Stimulus-transmitting structures and sensory apparatus of the cereal filiform hairs of Gryllus. Cell Tissue Res. 1980, 213, 441–463. [Google Scholar] [CrossRef]
- Fraser, P.J. Cercal ablation modifies tethered flight behaviour of cockroach. Nature 1977, 268, 523–524. [Google Scholar] [CrossRef]
- Leem, J.W.; Willis, W.D.; Chung, J.M. Cutaneous sensory receptors in the rat foot. J. Neurophysiol. 1993, 69, 1684–1699. [Google Scholar] [CrossRef]
- Manfredi, L.R.; Baker, A.T.; Elias, D.O.; Dammann, J.F.; Zielinski, M.; Polashock, V.S.; Bensmaia, S.J. The Effect of Surface Wave Propagation on Neural Responses to Vibration in Primate Glabrous Skin. PLoS ONE 2012, 7, e31203. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Vallbo, B. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983, 6, 27–32. [Google Scholar] [CrossRef]
- Sterbing-D’Angelo, S.; Chadha, M.; Chiu, C.; Falk, B.; Xian, W.; Barcelo, J.; Zook, J.M.; Moss, C.F. Bat wing sensors support flight control. Proc. Natl. Acad. Sci. USA 2011, 108, 11291–11296. [Google Scholar] [CrossRef]
- Kang, C.; Reep, R. Post-Cranial Hairs in Four Families of Bats. Acta Chiropterologica 2013, 15, 153–161. [Google Scholar] [CrossRef]
- Maxim, H. Preventing Collisions at Sea: A Mechanical Application of the Bat’s Sixth Sense. Sci. Am. 1912, 27, 80–81. [Google Scholar] [CrossRef]
- Zook, J.; Fowler, B. A Specialized Mechanosensory Array of the Bat Wing. Myotis 1986, 23, 31–36. [Google Scholar]
- Marshall, K.L.; Chadha, M.; Desouza, L.A.; Sterbing-D’Angelo, S.J.; Moss, C.F.; Lumpkin, E.A. Somatosensory Substrates of Flight Control in Bats. Cell Rep. 2015, 11, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.; Liu, C.; Krijnen, G. Biomimetic Flow Sensors. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 264–276. ISBN 978-90-481-9751-4. [Google Scholar]
- Sandeman, D.C. Crayfish antennae as tactile organs: Their mobility and the responses of their proprioceptors to displacement. J. Comp. Physiol. A 1985, 157, 363–373. [Google Scholar] [CrossRef]
- McMahon, A.; Patullo, B.W.; Macmillan, D.L. Exploration in a T-Maze by the CrayfishCherax destructorSuggests Bilateral Comparison of Antennal Tactile Information. Biol. Bull. 2005, 208, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, K.; Ohnishi, M. Responses from Tactile Receptors in the Antenna of the Spiny Lobster Panulirus japonicus. Comp. Biochem. Physiol. Part A Physiol. 1974, 47, 1323–1327. [Google Scholar] [CrossRef]
- Vincent, S.B. The Function of Vibrissae in the Behavior of the White Rat; University of Chicago: Chicago, IL, USA, 1912; Volume 1. [Google Scholar]
- Diamond, M.E.; von Heimendahl, M.; Knutsen, P.M.; Kleinfeld, D.; Ahissar, E. Where and what in the whisker sensorimotor system. Nat. Rev. Neurosci. 2008, 9, 601–612. [Google Scholar] [CrossRef]
- Yu, Y.S.W.; Graff, M.M.; Bresee, C.S.; Man, Y.B.; Hartmann, M.J.Z. Whiskers aid anemotaxis in rats. Sci. Adv. 2016, 2, e1600716. [Google Scholar] [CrossRef]
- Hartmann, M.J. Active Sensing Capabilities of the Rat Whisker System. Auton. Robot. 2001, 11, 249–254. [Google Scholar] [CrossRef]
- Welker, W. Analysis of Sniffing of the Albino Rat 1. Behaviour 1964, 22, 223–244. [Google Scholar] [CrossRef]
- Carvell, G.; Simons, D. Biometric analyses of vibrissal tactile discrimination in the rat. J. Neurosci. 1990, 10, 2638–2648. [Google Scholar] [CrossRef]
- Berg, R.W.; Kleinfeld, D. Rhythmic Whisking by Rat: Retraction as Well as Protraction of the Vibrissae Is Under Active Muscular Control. J. Neurophysiol. 2003, 89, 104–117. [Google Scholar] [CrossRef]
- Hartmann, M.J.; Johnson, N.J.; Towal, R.B.; Assad, C. Mechanical Characteristics of Rat Vibrissae: Resonant Frequencies and Damping in Isolated Whiskers and in the Awake Behaving Animal. J. Neurosci. 2003, 23, 6510–6519. [Google Scholar] [CrossRef]
- Quist, B.W.; Faruqi, R.A.; Hartmann, M.J. Variation in Young’s modulus along the length of a rat vibrissa. J. Biomech. 2011, 44, 2775–2781. [Google Scholar] [CrossRef]
- DeChant, H.-E.; Rammerstorfer, F.; Barth, F. Arthropod touch reception: Stimulus transformation and finite element model of spider tactile hairs. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2001, 187, 313–322. [Google Scholar] [CrossRef]
- Yu, Y.S.W.; Bush, N.E.; Hartmann, M.J.Z. Whisker Vibrations and the Activity of Trigeminal Primary Afferents in Response to Airflow. J. Neurosci. 2019, 39, 5881–5896. [Google Scholar] [CrossRef]
- Wineski, L.E. Movements of the cranial vibrissae in the Golden hamster (Mesocricetus auratus). J. Zool. 1983, 200, 261–280. [Google Scholar] [CrossRef]
- Crish, S.D.; Rice, F.L.; Park, T.J.; Comer, C.M. Somatosensory organization and behavior in naked mole-rats I: Vibrissa-like body hairs comprise a sensory array that mediates orientation to tactile stimuli. Brain Behav. Evol. 2003, 62, 141–151. [Google Scholar] [CrossRef]
- Mitchinson, B.; Grant, R.A.; Arkley, K.; Rankov, V.; Perkon, I.; Prescott, T.J. Active Vibrissal Sensing in Rodents and Mar-supials. Philos. Trans. R Soc. B Biol. Sci. 2011, 366, 3037–3048. [Google Scholar] [CrossRef]
- Schmidberger, G. About the Importance of Whiskers in Cats. Z. Vgl. Physiol. 1932, 17, 387–407. [Google Scholar]
- Nilsson, B.Y. Effects of Sympathetic Stimulation on Mechanoreceptors of Cat Vibrissae. Acta Physiol. Scand. 1972, 85, 390–397. [Google Scholar] [CrossRef]
- Schultz, W.; Galbraith, G.C.; Gottschaldt, K.-M.; Creutzfeldt, O.D. A comparison of primary afferent and cortical neurone activity coding sinus hair movements in the cat. Exp. Brain Res. 1976, 24, 365–381. [Google Scholar] [CrossRef]
- Williams, C.M.; Kramer, E.M. The Advantages of a Tapered Whisker. PLoS ONE 2010, 5, e8806. [Google Scholar] [CrossRef]
- Nilsson, B.Y.; Skoglund, C.R. The Tactile Hairs on the Cat’s Foreleg. Acta Physiol. Scand. 1965, 65, 364–369. [Google Scholar] [CrossRef]
- Nilsson, B.Y. Structure and Function of the Tactile Hair Receptors on the Cat’s Foreleg. Acta Physiol. Scand. 1969, 77, 396–416. [Google Scholar] [CrossRef]
- Fitzgerald, O. Discharges from the sensory organs of the cat’s vibrissae and the modification in their activity by ions. J. Physiol. 1940, 98, 163–178. [Google Scholar] [CrossRef]
- Dehnhardt, G.; Mauck, B.; Bleckmann, H. Seal whiskers detect water movements. Nature 1998, 394, 235–236. [Google Scholar] [CrossRef]
- Dehnhardt, G.; Mauck, B.; Hanke, W.; Bleckmann, H. Hydrodynamic Trail-Following in Harbor Seals (Phoca vitulina). Science 2001, 293, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Mass, A.M.; Ketten, D.R.; Odell, D.K.; Supin, A.Y. Ganglion Cell Distribution and Retinal Resolution in the Florida Manatee, Trichechus Manatus Latirostris. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 177–186. [Google Scholar] [CrossRef]
- Bauer, G.B.; Colbert-Luke, D.; Gaspard, J.; Littlefield, B.; Fellner, W. Underwater Visual Acuity of Florida Manatees (Trich-echus manatus latirostris). Int. J. Comp. Psychol. 2003, 16, 130–142. [Google Scholar]
- Reep, R.L.; Stoll, M.L.; Marshall, C.D.; Homer, B.L.; Samuelson, D.A. Microanatomy of facial vibrissae in the Florida manatee: The basis for specialized sensory function and oripulation. Brain Behav. Evol. 2001, 58, 1–14. [Google Scholar] [CrossRef]
- Gaspard, J.C.; Bauer, G.B.; Reep, R.L.; Dziuk, K.; Read, L.; Mann, D.A. Detection of hydrodynamic stimuli by the Florida manatee (Trichechus manatus latirostris). J. Comp. Physiol. A 2013, 199, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.D.; Huth, G.D.; Edmonds, V.M.; Halin, D.L.; Reep, R.L. Prehensile Use of Perioral Bristles During Feeding and Associated Behaviors of the Florida Manatee (Trichechus manatus latirostris). Mar. Mammal. Sci. 1998, 14, 274–289. [Google Scholar] [CrossRef]
- Sarko, D.K.; Reep, R.L.; Mazurkiewicz, J.E.; Rice, F.L. Adaptations in the structure and innervation of follicle-sinus complexes to an aquatic environment as seen in the Florida manatee (Trichechus manatus latirostris). J. Comp. Neurol. 2007, 504, 217–237. [Google Scholar] [CrossRef]
- Barth, F.G. A Spider’s World: Senses and Behavior; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-540-42046-0. [Google Scholar]
- Barth, F.G. Geethabali Spider vibration receptors: Threshold curves of individual slits in the metatarsal lyriform organ. J. Comp. Physiol. A 1982, 148, 175–185. [Google Scholar] [CrossRef]
- French, A.S.; Torkkeli, P.H.; Ernst-August, S. From stress and strain to spikes: Mechanotransduction in spider slit sensilla. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2002, 188, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Friedel, T.; Barth, F.G. Wind-sensitive interneurones in the spider CNS (Cupiennius salei): Directional information processing of sensory inputs from trichobothria on the walking legs. J. Comp. Physiol. A 1997, 180, 223–233. [Google Scholar] [CrossRef]
- Barth, F.G.; Humphrey, J.A.; Wastl, U.; Halbritter, J.; Brittinger, W. Dynamics of Arthropod Filiform Hairs. III. Flow Patterns Related to Air Movement Detection in a Spider (Cupiennius salei Keys.). Philos. Trans. R Soc. B Biol. Sci. 1995, 347, 397–412. [Google Scholar]
- Barth, F.G.; Höller, A.; Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.-T.; Augustine, G.J.; Greengard, P. Dynamics of arthropod filiform hairs. V. The response of spider trichobothria to natural stimuli. Philos. Trans. R. Soc. B: Biol. Sci. 1999, 354, 183–192. [Google Scholar] [CrossRef]
- Albert, J.; Friedrich, O.; DeChant, H.-E.; Barth, F. Arthropod touch reception: Spider hair sensilla as rapid touch detectors. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2001, 187, 303–312. [Google Scholar] [CrossRef]
- Gronenberg, W.; Tautz, J.; Hölldobler, B. Fast Trap Jaws and Giant Neurons in the Ant Odontomachus. Science 1993, 262, 561–563. [Google Scholar] [CrossRef]
- Just, S.; Gronenberg, W. The control of mandible movements in the ant Odontomachus. J. Insect Physiol. 1999, 45, 231–240. [Google Scholar] [CrossRef]
- Aonuma, H.; Osuka, K.; Ohkawara, K. Mechanisms of Ultra-High Speed Movement in the Trap Jaw Ant. In Proceedings of the 2017 56th Annual Conference of the Society of Instrument and Control Engineers of Japan (SICE), Kanazawa, Japan, 19–22 September 2017; pp. 15–18. [Google Scholar]
- Larabee, F.J.; Gronenberg, W.; Suarez, A.V. Performance, morphology and control of power-amplified mandibles in the trap-jaw ant Myrmoteras (Hymenoptera: Formicidae). J. Exp. Biol. 2017, 220, 3062–3071. [Google Scholar] [CrossRef]
- Patek, S.N.; Baio, J.E.; Fisher, B.L.; Suarez, A.V. Multifunctionality and mechanical origins: Ballistic jaw propulsion in trap-jaw ants. Proc. Natl. Acad. Sci. USA 2006, 103, 12787–12792. [Google Scholar] [CrossRef]
- Jaffé, K.; Marcuse, M. Nestmate recognition and territorial behaviour in the antOdontomachus bauri emery (Formicidae: Ponerinae). Insectes Sociaux 1983, 30, 466–481. [Google Scholar] [CrossRef]
- Murphy, C.T.; Eberhardt, W.C.; Calhoun, B.H.; Mann, K.A.; Mann, D.A. Effect of Angle on Flow-Induced Vibrations of Pinniped Vibrissae. PLoS ONE 2013, 8, e69872. [Google Scholar] [CrossRef] [PubMed]
- Larabee, F.J.; Suarez, A.V. Mandible-Powered Escape Jumps in Trap-Jaw Ants Increase Survival Rates during Predator-Prey Encounters. PLoS ONE 2015, 10, e0124871. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Spagna, J.C. Jump Performance in Trap-Jaw Ants: Beyond Trigger Hairs. Bull. NJ Acad. Sci. 2015, 60, 1–4. [Google Scholar]
- Fay, F.H. Ecology and Biology of the Pacific Walrus, Odobenus rosmarus divergensIlliger. N. Am. Fauna 1982, 74, 1–279. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Stevens, S.; Mosterd, P. The Tactile Sensitivity of the Mystacial Vibrissae of a Pacific Walrus (Odobenus rosmarus divergens). Part 2: Masking. Aquat. Mamm. 1990, 16, 78–87. [Google Scholar]
- Bauer, G.B.; Reep, R.L.; Marshall, C.D. The Tactile Senses of Marine Mammals. Int. J. Comp. Psychol. 2018, 31. [Google Scholar] [CrossRef]
- Chapman, H.C. Observations on the Structure of the Manatee. Proc. Acad. Nat. Sci. Phila. 1875, 27, 452–462. [Google Scholar]
- Pearson, M.J.; Pipe, A.G.; Melhuish, C.; Mitchinson, B.; Prescott, T. Whiskerbot: A Robotic Active Touch System Modeled on the Rat Whisker Sensory System. Adapt. Behav. 2007, 15, 223–240. [Google Scholar] [CrossRef]
- Takei, K.; Yu, Z.; Zheng, M.; Ota, H.; Takahashi, T.; Javey, A. Highly sensitive electronic whiskers based on patterned carbon nanotube and silver nanoparticle composite films. Proc. Natl. Acad. Sci. USA 2014, 111, 1703–1707. [Google Scholar] [CrossRef]
- Amoli, V.; Kim, S.Y.; Kim, J.S.; Choi, H.; Koo, J.; Kim, D.H. Biomimetics for high-performance flexible tactile sensors and advanced artificial sensory systems. J. Mater. Chem. C 2019, 7, 14816–14844. [Google Scholar] [CrossRef]
- Ko, H.; Song, H.; Im, S.; Kim, H.; Jang, B.; ShimHyungbo, H.; Cho, D.-I.; Hyoungho, K.; Haryong, S.; Seunghyun, I.; et al. Bioinspired Piezoresistive Acceleration Sensor Using Artificial Filiform Sensillum Structure. Sens. Mater. 2015, 27, 437–445. [Google Scholar] [CrossRef]
- Maschmann, M.R.; Ehlert, G.J.; Dickinson, B.T.; Phillips, D.M.; Ray, C.W.; Reich, G.W.; Baur, J.W. Bioinspired Carbon Nanotube Fuzzy Fiber Hair Sensor for Air-Flow Detection. Adv. Mater. 2014, 26, 3230–3234. [Google Scholar] [CrossRef]
- Brown, E.; Rodenberg, N.; Amend, J.; Mozeika, A.; Steltz, E.; Zakin, M.R.; Lipson, H.; Jaeger, H.M. Universal robotic gripper based on the jamming of granular material. Proc. Natl. Acad. Sci. USA 2010, 107, 18809–18814. [Google Scholar] [CrossRef]
- Syed, T.N.; Jizhan, L.; Xin, Z.; Shengyi, Z.; Yan, Y.; Mohamed, S.H.A.; Lakhiar, I.A. Seedling-lump integrated non-destructive monitoring for automatic transplanting with Intel RealSense depth camera. Artif. Intell. Agric. 2019, 3, 18–32. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Y.; Zhou, J.; Wang, K.; Zhang, Z. State-of-the-art robotic grippers, grasping and control strategies, as well as their applications in agricultural robots: A review. Comput. Electron. Agric. 2020, 177, 105694. [Google Scholar] [CrossRef]
- Colorado, J.; Barrientos, A.; Rossi, C.; Breuer, K. Biomechanics of smart wings in a bat robot: Morphing wings using SMA actuators. Bioinspir. Biomim. 2012, 7, 036006. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Mintchev, S.; Su, Y.; Shaw, E.; Breuer, K. A bioinspired Separated Flow wing provides turbulence resilience and aerodynamic efficiency for miniature drones. Sci. Robot. 2020, 5, eaay8533. [Google Scholar] [CrossRef]
- Quist, B.W.; Hartmann, M.J.Z. Mechanical signals at the base of a rat vibrissa: The effect of intrinsic vibrissa curvature and implications for tactile exploration. J. Neurophysiol. 2012, 107, 2298–2312. [Google Scholar] [CrossRef]
- Quist, B.W.; Seghete, V.; Huet, L.A.; Murphey, T.D.; Hartmann, M.J.Z. Modeling Forces and Moments at the Base of a Rat Vibrissa during Noncontact Whisking and Whisking against an Object. J. Neurosci. 2014, 34, 9828–9844. [Google Scholar] [CrossRef]
- Huet, L.A.; Rudnicki, J.W.; Hartmann, M.J. Tactile Sensing with Whiskers of Various Shapes: Determining the Three-Dimensional Location of Object Contact Based on Mechanical Signals at the Whisker Base. Soft Robot. 2017, 4, 88–102. [Google Scholar] [CrossRef]
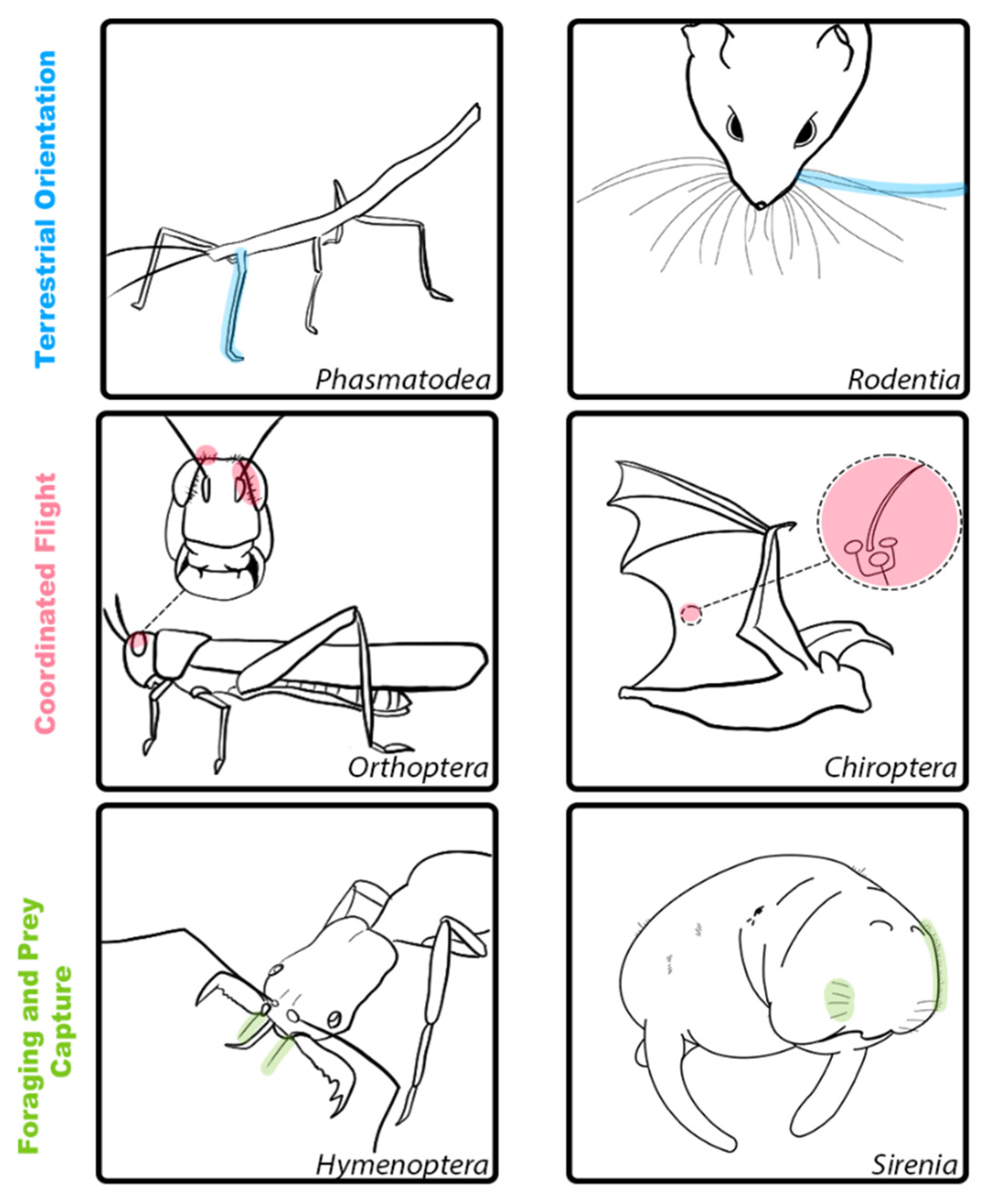
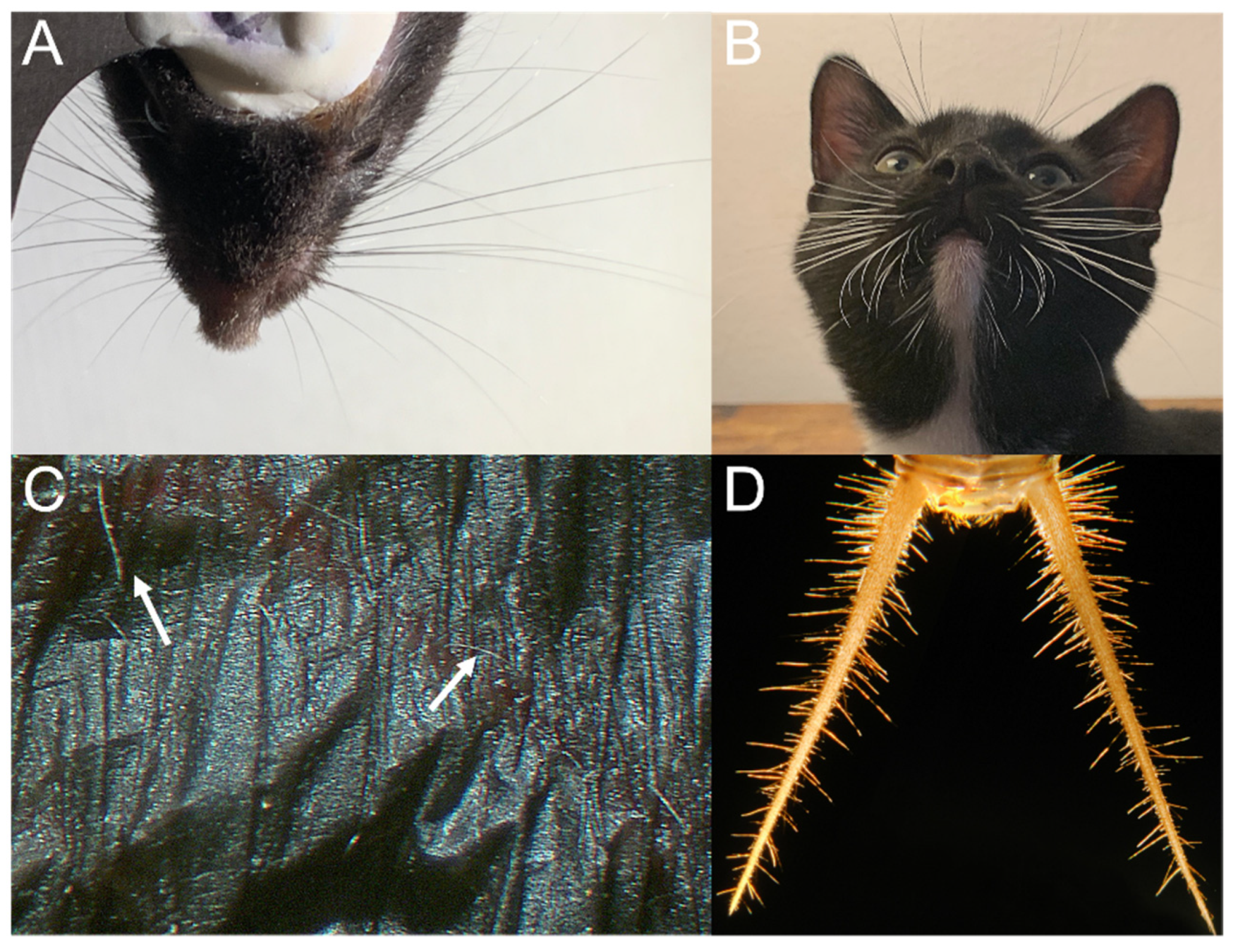

| Category | Order | Species | Hair Structure | Hair Length (mm) | Location | Function |
|---|---|---|---|---|---|---|
| Terrestrial Invertebrates | Diptera | Brachycera (fly) | Setae | 0.0339 ± 0.00471 [12] | Basal part of pulvillus | Attachment to surfaces |
| Blattodea | Periplaneta americana (Cockroach) | Sensilla of hair plates (short/long) | Short: 0.005–0.03 Long: 0.03–0.07 [13] | Leg joints | Limit detectors for coordinated movements | |
| Aranneae | Cupiennius salei (Wandering spider) | Trichobothria (filiform hairs) | 0.1–1.4 [14,15,16] | Body/legs | Prey detection | |
| Hymenoptera | Odontomachus bauri (trap jaw ant) | Hair-like sensilla/bristles | 0.6–1.2 [17] | Mandibles | Prey detection | |
| Terrestrial Vertebrates | Carnivora | Felis catus (Domestic cat) | Capral vibrissae | 10–20 [18] | Forelimbs | Coordinated movement |
| Carnivora | Felis catus (Domestic cat) | Mystacial vibrissae | 40–70 [18] | Face | Coordinated movement | |
| Rodentia | Mus musculus (Mouse) | Mystacial vibrissae | 30 [19] | Face | Coordinated movement | |
| Rodentia | Rattus norvegicus domestica (rat) | Mystacial vibrissae | 10–60 [6] | Face | Coordinated movement | |
| Flying Invertebrates | Orthoptera | Locusts | Trichoid sensilla | 0.03–0.35 [20] | Head capsule | Airflow sensing |
| Orthoptera | Crickets | Cerci | 0.03–1.5 [21,22] | Body | Airflow sensing | |
| Flying Vertebrates | Chiroptera | Eptesicus fuscus (Big brown bat) | Sensory hairs | 0.08–1 [23] | Wing/tail membrane | Airflow sensing |
| Aquatic Invertebrates | Decapoda | Astacus leptodactylus (Crayfish) | Conical/ Feathered hairs | Conical: 0.4–0.8 Feathered: 0.9–1.2 [24] | Flagellum of antennae | Fluid sensing |
| Decapoda | Cherax destructor (Crayfish) | Sensory hairs | 0.02 [25] | Chelae | Fluid sensing | |
| Aquatic Vertebrates | Cetacea | Balaena mysticetus (Bowhead whale) | Sensory hairs | 3–46 [26] | Lips, caudal blowhole | Fluid sensing |
| Carnivora | Mirounga angustirostris (Northern elephant seal) | Facial vibrissae | 7.54–138.14 [11] | Face | Foraging | |
| Carnivora | Phoca vitulina (Harbor seal) | Facial vibrissae | 22.9 [27] | Face | Fluid sensing, foraging | |
| Sirenia | Trichechus manatus latirostris (Florida manatee) | Facial vibrissae/bristles | 1–10 [28] | Face | Foraging, detection, discrimination, | |
| Sirenia | Trichechus manatus latirostris (Florida manatee) | Postcranial vibrissae | 2–9 [29] | Body | Detection and localization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boublil, B.L.; Diebold, C.A.; Moss, C.F. Mechanosensory Hairs and Hair-like Structures in the Animal Kingdom: Specializations and Shared Functions Serve to Inspire Technology Applications. Sensors 2021, 21, 6375. https://doi.org/10.3390/s21196375
Boublil BL, Diebold CA, Moss CF. Mechanosensory Hairs and Hair-like Structures in the Animal Kingdom: Specializations and Shared Functions Serve to Inspire Technology Applications. Sensors. 2021; 21(19):6375. https://doi.org/10.3390/s21196375
Chicago/Turabian StyleBoublil, Brittney L., Clarice Anna Diebold, and Cynthia F. Moss. 2021. "Mechanosensory Hairs and Hair-like Structures in the Animal Kingdom: Specializations and Shared Functions Serve to Inspire Technology Applications" Sensors 21, no. 19: 6375. https://doi.org/10.3390/s21196375
APA StyleBoublil, B. L., Diebold, C. A., & Moss, C. F. (2021). Mechanosensory Hairs and Hair-like Structures in the Animal Kingdom: Specializations and Shared Functions Serve to Inspire Technology Applications. Sensors, 21(19), 6375. https://doi.org/10.3390/s21196375






