Application of Chemical Sensors and Olfactometry Method in Ecological Audits of Degraded Areas
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Similä, J.; Jokinen, M. Governing conflicts between mining and tourism in the Arctic. Arct. Rev. Law Politics 2018, 9, 148–173. [Google Scholar] [CrossRef]
- United Nations. Transforming or World. The 2030 Agenda for Sustainable Development; A/RES/70/1; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerdà, A.; Montanarella, L.; Quinton, J.N.; Pachepsky, Y.; van der Putten, W.H.; et al. The significance of soils and soil science towards realization of the United Nations Sustainable Development Goals. Soil 2016, 2, 111–128. [Google Scholar] [CrossRef]
- Zhenqi, H.; Peijun, W.; Jing, L. Ecological restoration of abandoned mine land in China. J. Resour. Ecol. 2012, 3, 289–296. [Google Scholar] [CrossRef]
- Biały, W.; Mroczkowska, P. Influence of coal waste heaps on water environment in Upper Silesian borderland areas—Case study. In Proceedings of the 15th SGEM GeoConference on Science and Technologies, Albena, Bulgaria, 18–24 June 2015; pp. 675–682, ISBN 978-619-7105-33-9, ISSN 1314-2704. [Google Scholar]
- Siuta, J.; Dylewski, J.; Żukowski, B. Reclamation and development of post-mining lands in Poland. J. Min. Geoengin. 2012, 36, 5–7. [Google Scholar]
- Mazur-Belzyt, K. Re-thinking Post-Mining Areas Reclamation in 21st Century. IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 102055. [Google Scholar] [CrossRef]
- Pactwa, K.; Woźniak, J.; Dudek, M. Coal mining waste in Poland in reference to circular economy principles. Fuel 2020, 270, 117493. [Google Scholar] [CrossRef]
- Chuman, T. Restoration practices used on post mining sites and industrial deposits in the Czech Republic with an example of natural restoration of granodiorite quarries and spoil heaps. J. Landsc. Ecol. 2015, 8, 29–46. [Google Scholar] [CrossRef]
- Kabrna, M. Studies of land restoration on spoil heaps from brown coal mining in the Czech Republic—A literature review. J. Lands. Stud. 2011, 4, 59–69. [Google Scholar]
- Prach, K.; Šebelíkov´a, L.; Řehounkov´a, K.; del Moral, R. Possibilities and limitations of passive restoration of heavily disturbed sites. Landsc. Res. 2019, 45, 247–253. [Google Scholar] [CrossRef]
- Sekba, D.U.G.; Acar, C. Determining usages in post-mining sites according to landscape design approaches. Land Degrad Dev. 2021, 32, 2661–2676. [Google Scholar] [CrossRef]
- Konovalov, V.E.; Semyachkov, A.I.; Pochechun, V.A. Concept of mining landscape rehabilitation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 451, 012208. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Lelicińska-Serafin, K. The Role and Effectiveness of the MBT Installation in Poland Based on Selected Examples. Civ. Environ. Eng. Rep. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Kwiatkowska-Malina, J.; Wyszomierska, M. Redevelopment of natural aggregates post-mining areas: The SITNO deposit—Case study. Infrastruct. Ecol. Rural Areas 2014, 2, 705–717. [Google Scholar]
- Biały, W.; Moni, V. Rehabilitation of Post-Mining Areas in the Bohumin City Area (Czech Republic). Case Study. New Trends Prod. Eng. 2020, 3, 367–378. [Google Scholar] [CrossRef]
- Hendrychov’a, M.; Svobodova, K.; Kabrna, M. Mine reclamation planning and management: Integrating natural habitats into post-mining land use. Resour. Policy 2020, 69, 101882. [Google Scholar] [CrossRef]
- Supreme Audit Office. Land Reclamation after Exploitation of the Minerals Covered by Ownership of Land; Information on the Results of the Audit; Supreme Audit Office: Warsaw, Poland, 2018.
- Colón, J.; Alvarez, C.; Vinot, M.; Lafuente, F.J.; Ponsá, S.; Sánchez, A.; Gabriel, D. Characterization of odorous compounds and odor load in indoor air of modern complex MBT facilities. Characterization of odorous compounds and odor load in indoor air of modern complex MBT facilities. Chem. Eng. J. 2017, 313, 1311–1319. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. The Use of Chemical Sensors to Monitor Odour Emissions at Municipal Waste Biogas Plants. Appl. Sci. 2021, 11, 3916. [Google Scholar] [CrossRef]
- Duan, Z.; Kjeldsen, P.; Scheutz, C. Trace gas composition in landfill gas at Danish landfills receiving low-organic waste. Waste Manag. 2021, 122, 113–123. [Google Scholar] [CrossRef]
- Smet, E.; Van Langenhove, H.; De Bo, I. The emission of volatile compounds during the aerobic and the combined anaerobic aerobic composting of biowaste. Atmos. Environ. 1999, 33, 1295–1303. [Google Scholar] [CrossRef]
- Staley, B.F.; Xu, F.; Cowie, S.J.; Barlaz, M.A.; Hater, G.R. Release of trace organic compounds during the decomposition of municipal solid waste components. Environ. Sci. Technol. 2006, 40, 5984–5991. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Domestic waste composting facilities: A review of human health risks. Environ. Int. 2009, 35, 382–389. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. The Impact of Technological Processes on Odorant Emissions at Municipal Waste Biogas Plants. Sustainability 2020, 12, 5457. [Google Scholar] [CrossRef]
- Fischedick, M.; Roy, J.; Abdel-Aziz, A.; Acquaye, A.; Allwood, J.; Ceron, J.-P.; Geng, Y.; Kheshgi, H.; Lanza, A.; Perczyk, D.; et al. Industry. In Climate Change 2014: Mitigation of Climate Change; Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 2014; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyb, K., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- European Environment Agency. Municipal Waste Management across European Countries. 2016. Available online: https://www.eea.europa.eu/themes/waste/municipal-waste/municipal-waste-management-acrosseuropean-countries (accessed on 18 June 2020).
- Pawlowska, M.; Czerwinski, J.; Stepniewski, W. Variability of the nonmethane volatile organic compounds (NMVOC) composition in biogas from sorted and unsorted landfill material. Arch. Environ. Prot. 2008, 34, 287–298. [Google Scholar]
- Duan, Z.; Scheutz, C.; Kjeldsen, P. Trace gas emissions from municipal solid waste landfills: A review. Waste Manag. 2021, 119, 39–62. [Google Scholar] [CrossRef]
- Young, P.J.; Parker, A. The identification and possible environmental impact of trace gases and vapours in landfill gas. Waste Manag. Res. 1983, 1, 213–226. [Google Scholar] [CrossRef]
- Dincer, F.; Odabasi, M.; Muezzinoglu, A. Chemical characterization of odorous gases at a landfill site by gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1122, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.P. Trace Constituents in Landfill Gas. Task Report on Inventory and Assessment of Cleaning Technologies; Final Report, May 1984–February 1987; Energy Research Corporation: Danbury, CT, USA, 1988. [Google Scholar]
- Komilis, D.P.; Ham, R.K.; Park, J.K. Emission of volatile organic compounds during composting of municipal solid wastes. Water Res. 2004, 38, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.; Dottridge, J.; Kelly, S. Investigation of the Composition and Emissions of Trace Components in Landfill Gas; R&D Technical Report P1–438/TR; Environment Agency: Bristol, UK, 2002.
- Wiśniewska, M.; Szyłak-Szydłowski, M. The Air and Sewage Pollutants from Biological Waste Treatment. Processes 2021, 9, 250. [Google Scholar] [CrossRef]
- Kulig, A.; Szyłak-Szydłowski, M. Assessment of the Effects of Wastewater Treatment Plant Modernization by Means of the Field Olfactometry Method. Water 2019, 11, 2367. [Google Scholar] [CrossRef]
- Wiśniewska, M. Methods of Assessing Odour Emissions from Biogas Plants Processing Municipal Waste. J. Ecol. Eng. 2020, 21, 140–147. [Google Scholar] [CrossRef]
- Szyłak-Szydłowski, M. Odour Samples Degradation during Detention in Tedlar® Bags. Water Air Soil Pollut. 2015, 226, 227. [Google Scholar] [CrossRef][Green Version]
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Wilson, D. Chemical Sensors for Farm-to-Table Monitoring of Fruit Quality. Sensors 2021, 21, 1634. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.B.; Schmitt, A.; Berger, F.; Mavon, C. Silicon-micromachined gas chromatographic columns for the development of portable detection device. J. Sens. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Malcolm, A.; Wright, S.; Syms, R.R.A.; Dash, N.; Schwab, M.A.; Finlay, A. Miniature mass spectrometer system based on a microengineered quadrupole filter. Anal. Chem. 2010, 82, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Ho, C.K.; Itamura, M.T.; Kelley, M.; Hughes, R.C. Review of Chemical Sensors for In-Situ Monitoring of Volatile Contaminants; Sandia National Laboratories: Albuquerque, NM, USA, 2001.
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef]
- CEN. Air Quality—Determination of Odour Concentration by Dynamic Olfactometry; Standard EN 13725: 2003; CEN: Brussels, Belgium, 2003. [Google Scholar]
- Maurer, D.L.; Bragdon, A.M.; Short, B.C.; Ahn, H.; Koziel, J.A. Improving environmental odor measurements: Comparison of lab-based standard method and portable odor measurement technology. Arch. Environ. Prot. 2018, 44, 100–107. [Google Scholar] [CrossRef]
- Szyłak-Szydłowski, M. Comparison of two types of field olfactometers for assessing odours in laboratory and field tests. Chem. Eng. Trans. 2014, 40, 67–72. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. Olfactometric testing as a method for assessing odour nuisance of biogas plants processing municipal waste. Arch. Environ. Prot. 2020, 46, 60–68. [Google Scholar] [CrossRef]
- Jia, C.; Cao, K.; Valaulikar, R.; Fu, X.; Sorin, A.B. Variability of Total Volatile Organic Compounds (TVOC) in the indoor air of retail stores. Int. J. Environ. Res. Public Health 2019, 16, 4622. [Google Scholar] [CrossRef]
- Sangseok, L.; Dong, K.L. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and chromium from aqueous solutions using red mud-an aluminum industry waste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef]
- Montanaro, L.; Bianchini, N.; Rincon, J.M.; Romero, M. Sintering behaviour of pressed red mud wastes from zinc hydrometallurgy. Ceram. Int. 2001, 27, 29–37. [Google Scholar] [CrossRef]
- Rashchi, F.; Dashti, A.; Arabpour-Yazdi, M.; Abdizadeh, A. Anglesite flotation: A study for lead recovery from zinc leach residue. Miner. Eng. 2005, 18, 205–212. [Google Scholar] [CrossRef]
- Moors, E.H.M.; Dijkema, G.P.J. Embedded industrial production systems: Lessons from waste management in zinc production. Technol. Forecast. Soc. Chang. 2006, 73, 250–265. [Google Scholar] [CrossRef]
- Kaur, H.; Siddique, R.; Rajor, A. Removal of alkalinity and metal toxicity from incinerated biomedical waste ash by using Bacillus halodurans. Bioremediation J. 2021, 1–24. [Google Scholar] [CrossRef]
- Pierucci, P.; Porazzi, E.; Martinez, M.P.; Adani, F.; Carati, C.; Rubino, F.M.; Colombi, A.; Calcaterra, E.; Benfenati, E. Volatile organic compounds produced during the aerobic biological processing of municipal solid waste in a pilot plant. Chemosphere 2005, 59, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.I.; Arnáiz, N.; Font, R.; Carratalá, A. Chemical characterization of emissions from a municipal solid waste treatment plant. Waste Manag. 2014, 34, 2393–2399. [Google Scholar] [CrossRef]
- Pagans, E.; Barrena, R.; Font, X.; Sánchez, A. Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere 2006, 62, 1534–1542. [Google Scholar] [CrossRef]
- Higgins, M.J.; Chen, Y.-C.; Yarosz, D.P.; Murthy, S.N.; Maas, N.A.; Glindemann, D.; Novak, J.T. Cycling of volatile organic sulfur compounds in anaerobically digested biosolids and its implications for odors. Water Environ. Res. 2006, 78, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.; Caselli, M.; De Gennaro, G.; Solito, M.; Tutino, M. Monitoring of odor compounds produced by solid waste treatment plants with diffusive samplers. Waste Manag. 2007, 27, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Gutierrez, M.C.; Siles, J.A.; Martin, M.A. Full-scale composting of sewage sludge and market waste: Stability monitoring and odor dispersion modelling. Environ. Res. 2018, 167, 739–750. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. The Importance of the Microclimatic Conditions Inside and Outside of Plant Buildings in Odorants Emission at Municipal Waste Biogas Installations. Energies 2020, 13, 6463. [Google Scholar] [CrossRef]
- Australian Government. Mine Rehabilitation. Leading Practice Sustainable Development Program for Mining Industry; Australian Government: Canberra, Australia, 2016.
- Bokowa, A.; Diaz, C.; Koziel, J.A.; McGinley, M.; Barclay, J.; Schauberger, G.; Guillot, J.M.; Sneath, R.; Capelli, L.; Zorich, V.; et al. Summary and Overview of the Odour Regulations Worldwide. Atmosphere 2021, 12, 206. [Google Scholar] [CrossRef]
- ÖAW. Umweltwissenschaftliche Grundlagen und Zielsetzungen im Rahmen des Nationalen Umweltplans für die Bereiche Klima, Luft, Geruch und Lärm; Schriftenreihe der Sektion I. Band, 17; Kommissionfür Reinhaltung der Luft der Österreichische Akademie der Wissenschaften, Ed.; Bundesministeriums für Umwelt, Jugend und Familie: Vienna, Austria, 1994; Volume 17. [Google Scholar]
- Amoore, J.E.; Hautala, E. Odour as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 1983, 3, 272–290. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Research Council, Division of Medical Sciences. Hydrogen Sulfide; Health Effects Research Laboratory: Research Triangle Park, NC, USA, 1978.
- American Society of Civil Engineers. Environmental Engineering Division Sulfide in Wastewater Collection and Treatment Systems; 536; American Society of Civil Engineers: New York, NY, USA, 1989; ISBN 978-0-87262-681-2. [Google Scholar]
- Helmers, S.; Top, F.H.; Knapp, L.W. Ammonia injuries in agriculture. J. Iowa Med. Soc. 1971, 61, 271–280. [Google Scholar]
- John, F.; Finklea, M.D. NIOSH recommended standards for occupational exposure to ammonia and benzene. Int. J. Occup. Health Saf. 1974, 43, 32–33. [Google Scholar]
- Padappayil, R.P.; Borger, J. Ammonia toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Leonardos, G.; Kendall, D.; Barnard, N. Odor threshold determinations of 53 odorant chemicals. J. Air Pollut. Control Assoc. 1969, 19, 91–95. [Google Scholar] [CrossRef]
- DHHS. Criteria for a Recommended Standard: Occupational Exposure to Phenol; DHHS (NIOSH) Publication: Spokane, WA, USA, 1976; pp. 76–196.
- Gebicki, J.; Dymerski, T.; Namieśnik, J. Investigation of air quality beside a municipal landfill: The fate of malodour compounds as a model VOC. Environments 2017, 4, 7. [Google Scholar] [CrossRef]
- Kulig, A.; Ossowska-Cypryk, K.; Skorupski, W.; Wieczorek, B. Simple methods for ambient air testing. Atmos. Prot. Probl. 1984. (In Polish) [Google Scholar]
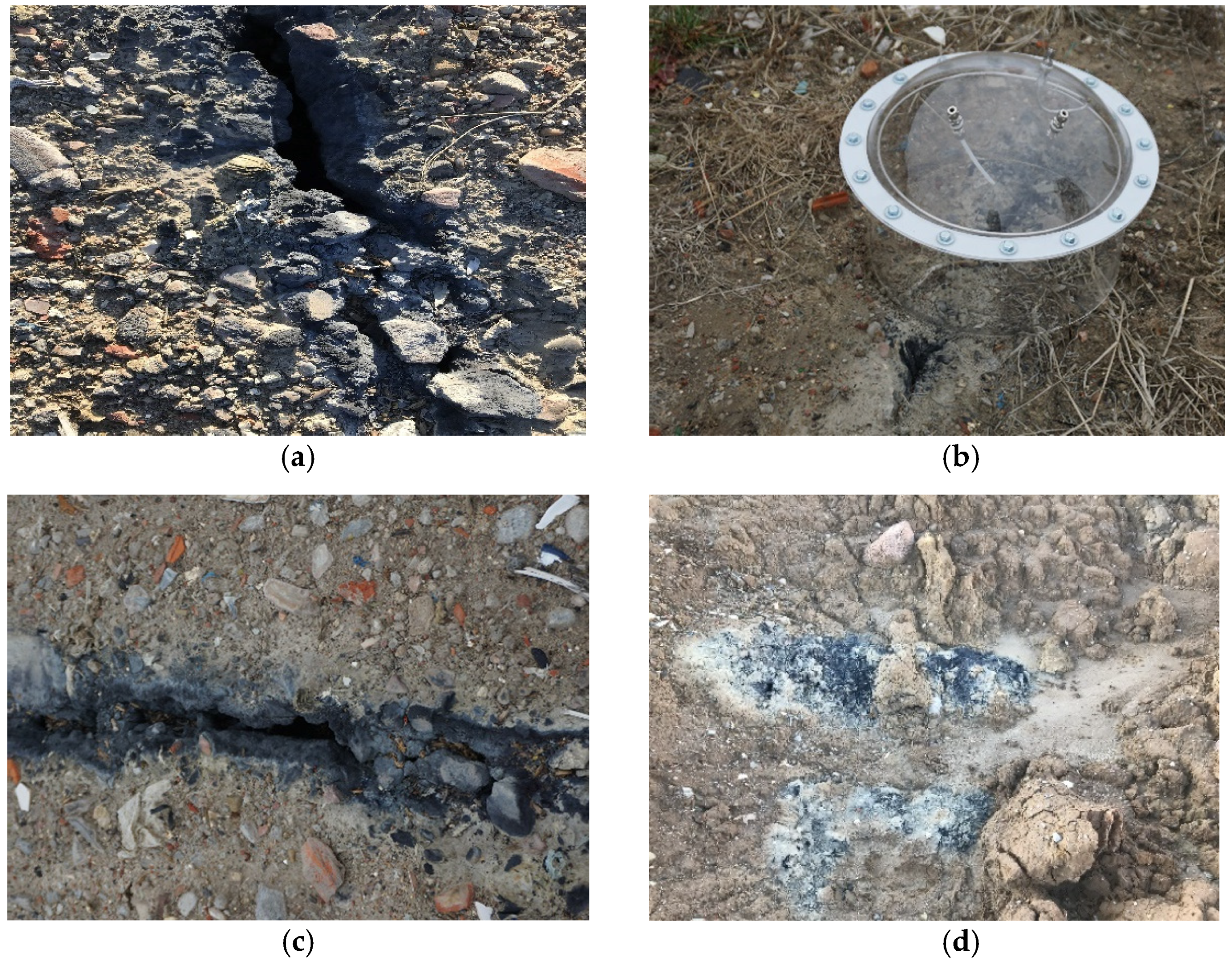
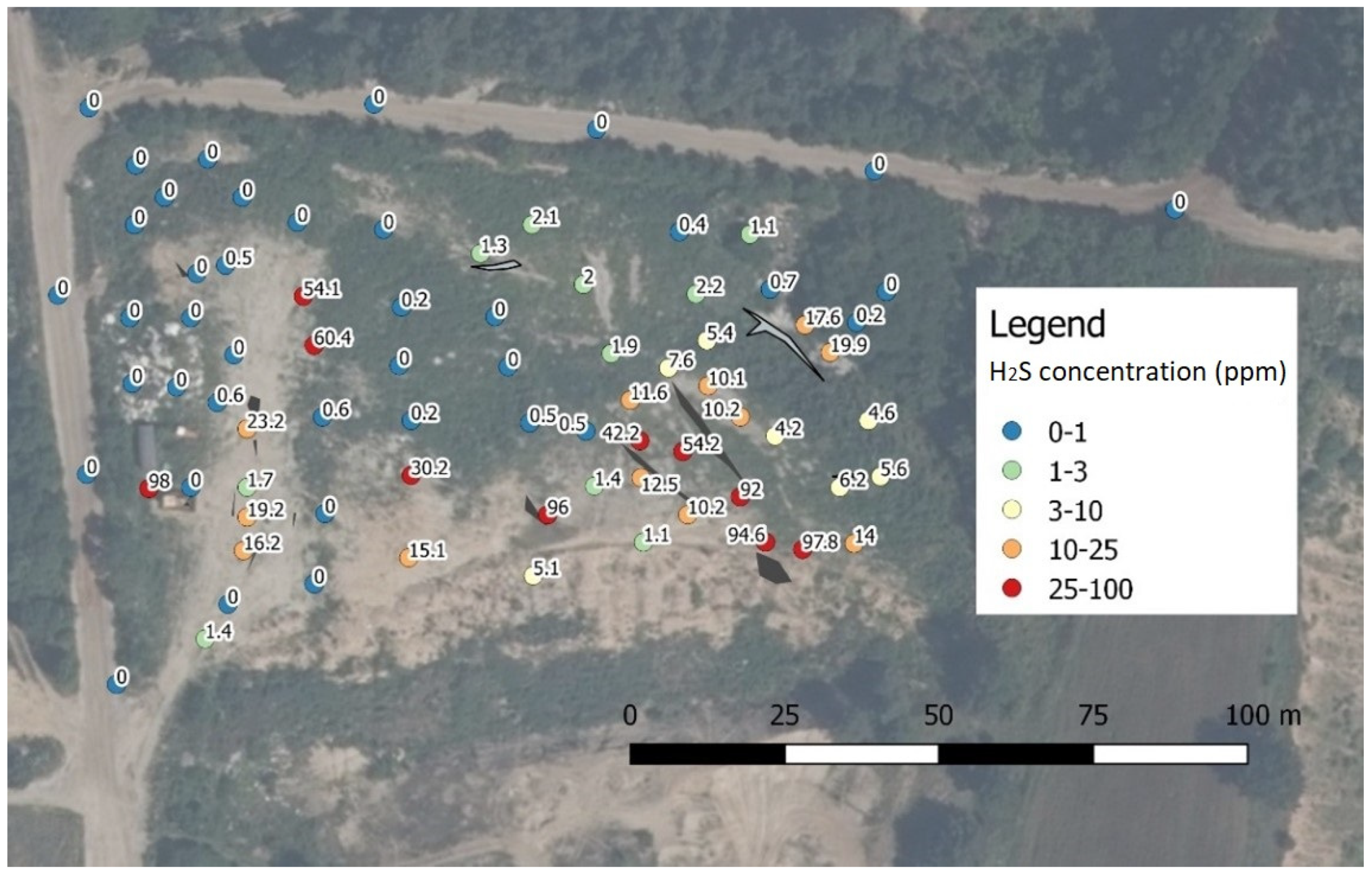

| Compound | Sensor Type | Range | Resolution | Ionization Energy (IE) | Response Time (RT) | Calibration Gas |
|---|---|---|---|---|---|---|
| VOCs | photoionization detector (PID) | 0–1000 ppm | 0.01 ppm | 10.6 eV | 15 s | C4H8 (10.02 ppm; 100 ppm) |
| NH3 | electrochemical (EC) | 0–100 ppm | 1 ppm | Not applicable | 60 s | NH3 (46.44 ppm) |
| H2S | 0–100 ppm | 0.1 ppm | Not applicable | 35 s | H2S (25.8 ppm) | |
| CH3SH | 0–10 ppm | 0.1 ppm | Not applicable | <35 s | CH3SH (5 ppm) | |
| CH4 | protected catalytic bead | 0–100% | 0.1% | Not applicable | 30 s | 50% CH4 |
| No | Date | Temperature (°C) | Humidity (%) | Wind Speed (m/s) |
|---|---|---|---|---|
| Mean | ||||
| 1 | 20 November 2019 | 2.1 | 78.4 | 2.9 |
| 2 | 15 January 2020 | 0.2 | 82.3 | 4.9 |
| 3 | 11 March 2020 | 6.2 | 77.5 | 3.6 |
| 4 | 3 June 2020 | 14.7 | 62.8 | 2.7 |
| 5 | 17 June 2020 | 20.1 | 75.2 | 2.1 |
| 6 | 24 June 2020 | 17.4 | 90.1 | 5.6 |
| Concentration | |||||
|---|---|---|---|---|---|
| cod [ou/m3] | NH3 [ppm] | VOC [ppm] | H2S [ppm] | CH3SH [ppm] | |
| Sample size | 380 | 380 | 380 | 380 | 380 |
| Maximum | 7500 | 98 | 171 | 99.6 | 10.0 |
| Mean | 1237 | 23.8 | 11.5 | 13.7 | 3.89 |
| Median | 111 | 5.00 | 1.60 | 2.45 | 2.85 |
| Minimum | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Standard deviation | 2025 | 32.9 | 26.2 | 27.2 | 3.77 |
| Concentration | F | p |
|---|---|---|
| cod [ou/m3] | 0.3167 | 0.867 |
| NH3 [ppm] | 7.8263 | <0.001 |
| H2S [ppm] | 0.0578 | 0.994 |
| CH3SH [ppm] | 1.0628 | 0.376 |
| VOC [ppm] | 3.9615 | 0.004 |
| Coefficients | Series | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Mean difference | — | −15.9 ** | −16.895 ** | −16.9342 ** | −15.816 ** |
| p-value | — | 0.006 | 0.003 | 0.003 | 0.006 | |
| 2 | Mean difference | — | −0.974 | −1.0132 | 0.105 | |
| p-value | — | 1 | 1 | 1 | ||
| 3 | Mean difference | — | −0.0395 | 1.079 | ||
| p-value | — | 1 | 1 | |||
| 4 | Mean difference | — | 1.118 | |||
| p-value | — | 1 | ||||
| 5 | Mean difference | — | ||||
| p-value | — | |||||
| Concentration | ||||||
|---|---|---|---|---|---|---|
| Concentration | Coefficients | cod [ou/m3] | NH3 [ppm] | VOC [ppm] | H2S [ppm] | CH3SH [ppm] |
| cod [ou/m3] | Pearson’s r | — | ||||
| 95% CI Upper | — | |||||
| 95% CI Lower | — | |||||
| NH3 [ppm] | Pearson’s r | 0.552 *** | — | |||
| 95% CI Upper | 0.619 | — | ||||
| 95% CI Lower | 0.478 | — | ||||
| VOC [ppm] | Pearson’s r | 0.656 *** | 0.582 *** | — | ||
| 95% CI Upper | 0.71 | 0.645 | — | |||
| 95% CI Lower | 0.594 | 0.512 | — | |||
| H2S [ppm] | Pearson’s r | 0.864 *** | 0.567 *** | 0.786 *** | — | |
| 95% CI Upper | 0.888 | 0.632 | 0.822 | — | ||
| 95% CI Lower | 0.836 | 0.495 | 0.744 | — | ||
| CH3SH [ppm] | Pearson’s r | 0.658 *** | 0.632 *** | 0.421 *** | 0.472 *** | — |
| 95% CI Upper | 0.712 | 0.689 | 0.5 | 0.546 | — | |
| 95% CI Lower | 0.597 | 0.568 | 0.334 | 0.39 | — | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulig, A.; Szyłak-Szydłowski, M.; Wiśniewska, M. Application of Chemical Sensors and Olfactometry Method in Ecological Audits of Degraded Areas. Sensors 2021, 21, 6190. https://doi.org/10.3390/s21186190
Kulig A, Szyłak-Szydłowski M, Wiśniewska M. Application of Chemical Sensors and Olfactometry Method in Ecological Audits of Degraded Areas. Sensors. 2021; 21(18):6190. https://doi.org/10.3390/s21186190
Chicago/Turabian StyleKulig, Andrzej, Mirosław Szyłak-Szydłowski, and Marta Wiśniewska. 2021. "Application of Chemical Sensors and Olfactometry Method in Ecological Audits of Degraded Areas" Sensors 21, no. 18: 6190. https://doi.org/10.3390/s21186190
APA StyleKulig, A., Szyłak-Szydłowski, M., & Wiśniewska, M. (2021). Application of Chemical Sensors and Olfactometry Method in Ecological Audits of Degraded Areas. Sensors, 21(18), 6190. https://doi.org/10.3390/s21186190







