Random Forest for Automatic Feature Importance Estimation and Selection for Explainable Postural Stability of a Multi-Factor Clinical Test
Abstract
1. Introduction
2. Materials and Methods
2.1. General Approach
2.2. Subjects
2.3. Clinical Tests
- The Timed Up and Go test (TUG) [40] is a common clinical test of gait and mobility. Different geriatric institutions recommend its implementation for fall-risk screening [41]. Previous studies have determined the effectiveness of using an inertial sensor during a TUG test to measure mobility [42], as well as to detect frailty [43] which could potentially result in a fall. It has also been proven to be an accurate measurement tool for predicting falls among community-dwelling elder adults [44]. Physicians commonly employ this clinical test in community settings due to its ease of implementation. Before starting the TUG test, subjects sit on a chair in a comfortable position, facing an object on a floor, which is located 3 m in front of them. When the test starts, subjects are asked to stand up, walk naturally towards the object, then return to the chair at their natural pace and sit down. The total time the subjects require to perform this test is recorded and used to label the subjects that performed the test in over 12.47 s as having mobility problems [44]. A summary of the label distribution for each clinical test can be observed in Table 2.
- The Short-Form Berg Balance Scale (SFBBS) [45] is the simplified version of the Berg Balance Scale (BBS) [46] which is used to assess balance. It is easier to perform as it has half the number of activities, greatly reducing the time required to assess a subject. These activities include (i) bending your back forward with outstretched arms, (ii) standing with both feet while keeping eyes closed, (iii) standing with one foot in front of the other, (iv) turning the back and neck to look backwards without moving the feet or knees, (v) bending down to pick up an object from the floor, (vi) standing on one foot while having the other foot in the air, and (vii) standing up from a chair and sitting down again. While subjects perform a SFBBS test, a medical expert evaluates their performance by assigning scores to each activity. The performance criteria states that a score between zero points (subject was unable to perform the activity) and four points (subject completed the activity without problems) is assigned based on the expert’s observations. Therefore, in this study, subjects who scored 28 points were considered to have correct balance since they were able to perform all seven tasks without problems. Meanwhile, subjects with a score below 23 were labeled as having balance problems [47,48].
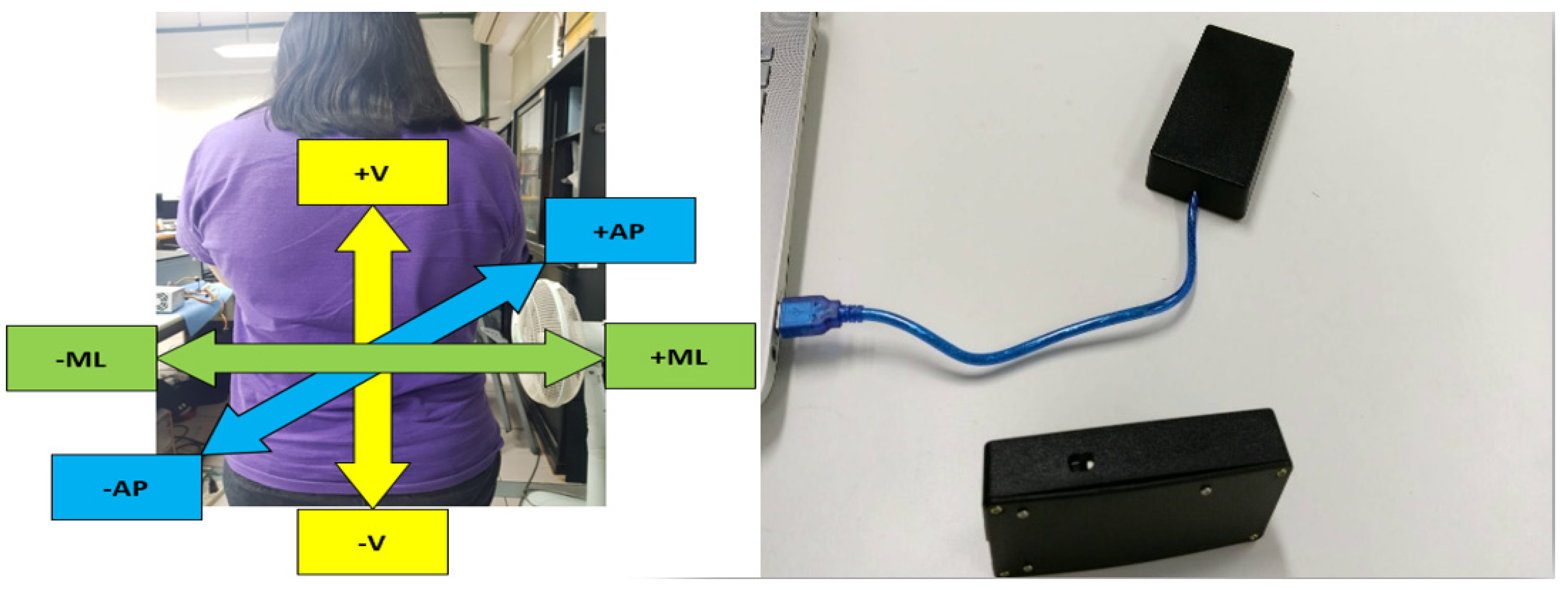
2.4. Wearable Accelerometer
2.5. Data Analysis
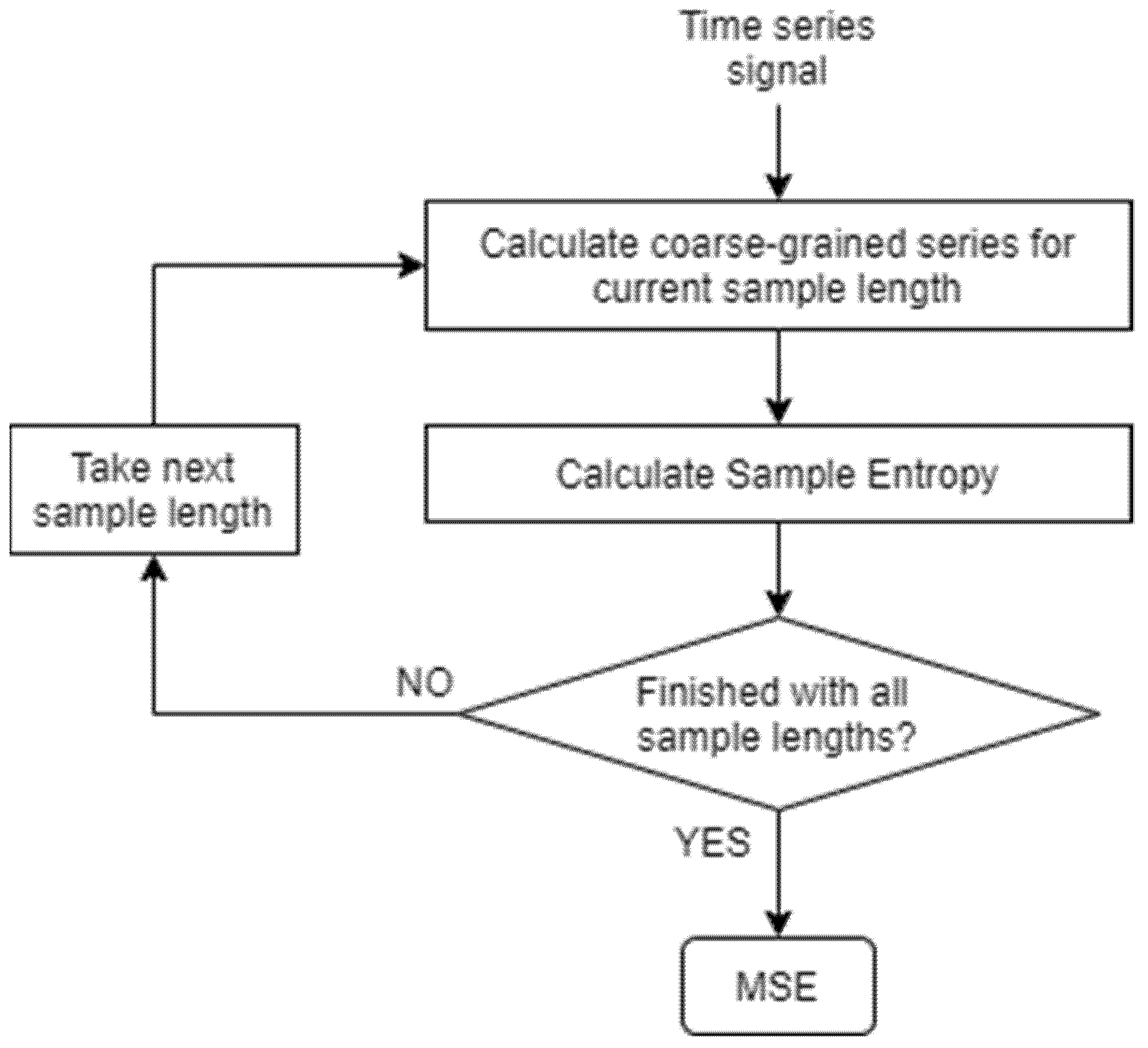
2.5.1. MSE Calculation
2.5.2. Permutation Entropy Calculation
2.6. Random Forest for Feature Importance and Classification
3. Results and Discussion
3.1. Feature Selection for Each Clinical Test
3.2. Classification Performance for Each Clinical Test under Multiple Criteria for Feature Selection
3.3. Classification Performance with and without MSE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamińska, M.S.; Brodowski, J.; Karakiewicz, B. Fall Risk Factors in Community-Dwelling Elderly Depending on Their Physical Function, Cognitive Status and Symptoms of Depression. Int. J. Environ. Res. Public Health 2015, 12, 3406–3416. [Google Scholar] [CrossRef]
- Bergland, A. Fall risk factors in community-dwelling elderly people. Nor. Epidemiol. 2012, 22, 151–164. [Google Scholar] [CrossRef][Green Version]
- Chu, L.W.; Chi, I.; Chiu, A.Y.Y. Incidence and predictors of falls in the Chinese elderly. Ann. Acad. Med. 2005, 34, 60–72. [Google Scholar]
- Stevens, J.; Corso, P.S.; Finkelstein, E.; Miller, T. The costs of fatal and non-fatal falls among older adults. Inj. Prev. 2006, 12, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Litwin, H.; Erlich, B.; Dunsky, A. The Complex Association between Fear of Falling and Mobility Limitation in Relation to Late-Life Falls: A SHARE-Based Analysis. J. Aging Health 2017, 30, 987–1008. [Google Scholar] [CrossRef] [PubMed]
- Letts, L.; Moreland, J.; Richardson, J.A.; Coman, L.; Edwards, M.; Ginis, K.M.; Wilkins, S.; Wishart, L. The physical environment as a fall risk factor in older adults: Systematic review and meta-analysis of cross-sectional and cohort studies. Aust. Occup. Ther. J. 2010, 57, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Chaudhury, H. Falls and the Physical Environment: A Review and a New Multifactorial Falls-Risk Conceptual Framework. Can. J. Occup. Ther. 2008, 75, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.C.; Murlidhar, A.A. Falls in the Elderly. Emerg. Med. Clin. N. Am. 1990, 8, 309–324. [Google Scholar] [CrossRef]
- Tinetti, M.; Speechley, M.; Ginter, S. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Duncan, P.W.; Studenski, S.; Chandler, J.; Prescott, B. Functional Reach: Predictive Validity in a Sample of Elderly Male Veterans. J. Gerontol. 1992, 47, M93–M98. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Shumway-Cook, A. Changes in Posture Control across the Life Span—A Systems Approach. Phys. Ther. 1990, 70, 799–807. [Google Scholar] [CrossRef]
- Maki, B.E.; Holliday, P.J.; Topper, A.K. Fear of Falling and Postural Performance in the Elderly. J. Gerontol. 1991, 46, M123–M131. [Google Scholar] [CrossRef]
- Mänty, M.; Heinonen, A.; Viljanen, A.; Pajala, S.; Koskenvuo, M.; Kaprio, J.; Rantanen, T. Self-reported preclinical mobility limitation and fall history as predictors of future falls in older women: Prospective cohort study. Osteoporos. Int. 2009, 21, 689–693. [Google Scholar] [CrossRef]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35, ii37–ii41. [Google Scholar] [CrossRef]
- Stubbs, B.; Schofield, P.; Binnekade, T.; Patchay, S.; Sepehry, A.; Eggermont, L. Pain Is Associated with Recurrent Falls in Community-Dwelling Older Adults: Evidence from a Systematic Review and Meta-Analysis. Pain Med. 2014, 15, 1115–1128. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Ciol, M.; Hoffman, J.; Dudgeon, B.J.; Yorkston, K.; Chan, L. Falls in the Medicare Population: Incidence, Associated Factors, and Impact on Health Care. Phys. Ther. 2009, 89, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Q. Design and Development of a Portable Fall Risk Assessment System. Master’s Thesis, Southern Taiwan University, Tainan, Taiwan, 2012. [Google Scholar]
- Montesinos, L.; Castaldo, R.; Pecchia, L. Wearable Inertial Sensors for Fall Risk Assessment and Prediction in Older Adults: A Systematic Review and Meta-Analysis. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Saber-Sheikh, K.; Bryant, E.C.; Glazzard, C.; Hamel, A.; Lee, R.Y. Feasibility of using inertial sensors to assess human movement. Man. Ther. 2010, 15, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Pavic, A.; Goodwin, V.A. Wearable inertial sensors to measure gait and posture characteristic differences in older adult fallers and non-fallers: A scoping review. Gait Posture 2019, 76, 110–121. [Google Scholar] [CrossRef]
- Wang, K.; Delbaere, K.; Brodie, A.M.D.; Lovell, N.; Kark, L.; Lord, S.R.; Redmond, S.J. Differences Between Gait on Stairs and Flat Surfaces in Relation to Fall Risk and Future Falls. IEEE J. Biomed. Health Inform. 2017, 21, 1479–1486. [Google Scholar] [CrossRef]
- Ponti, M.; Bet, P.; Oliveira, C.L.; Castro, P.C. Better than counting seconds: Identifying fallers among healthy elderly using fusion of accelerometer features and dual-task Timed up and go. PLoS ONE 2017, 12, e0175559. [Google Scholar] [CrossRef]
- Howcroft, J.; Lemaire, E.D.; Kofman, J.; McIlroy, W.E. Dual-Task Elderly Gait of Prospective Fallers and Non-Fallers: A Wearable-Sensor Based Analysis. Sensors 2018, 18, 1275. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Zhao, Y.; Huang, K.-H.; Wu, Y.-T.; Cabrera, J.; Sun, T.-L.; Tsui, K.-L. A Novel Approach for Fall Risk Prediction Using the Inertial Sensor Data from the Timed-Up-and-Go Test in a Community Setting. IEEE Sens. J. 2020, 20, 9339–9350. [Google Scholar] [CrossRef]
- Viton, F.; Elbattah, M.; Guerin, J.-L.; Dequen, G. Heatmaps for Visual Explainability of CNN-Based Predictions for Multivariate Time Series with Application to Healthcare. In Proceedings of the 2020 IEEE International Conference on Healthcare Informatics (ICHI), Oldenburg, Germany, 30 November–3 December 2020. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Wang, S.; Sun, Y.; Honavar, V. Explainable Multivariate Time Series Classification: A Deep Neural Network Which Learns to Attend to Important Variables as well as Time Intervals. In Proceedings of the 14th ACM International Conference on Web Search and Data Mining, Virtual, Online, 8–12 March 2021; pp. 607–615. [Google Scholar]
- Aicha, A.N.; Englebienne, G.; Van Schooten, K.S.; Pijnappels, M.; Kröse, B. Deep Learning to Predict Falls in Older Adults Based on Daily-Life Trunk Accelerometry. Sensors 2018, 18, 1654. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoon, S.; Kim, W. A Study on CNN-Based Berg Balance Scale Analysis for Elderly Persons. In Proceedings of the 2019 34th International Technical Conference on Circuits/Systems, Computers and Communications (ITC-CSCC), JeJu, Korea, 23–26 June 2019. [Google Scholar] [CrossRef]
- Tunca, C.; Salur, G.; Ersoy, C. Deep Learning for Fall Risk Assessment with Inertial Sensors: Utilizing Domain Knowledge in Spatio-Temporal Gait Parameters. IEEE J. Biomed. Health Inform. 2019, 24, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Kiprijanovska, I.; Gjoreski, H.; Gams, M. Detection of Gait Abnormalities for Fall Risk Assessment Using Wrist-Worn Inertial Sensors and Deep Learning. Sensors 2020, 20, 5373. [Google Scholar] [CrossRef] [PubMed]
- Aziz, W.; Arif, M. Multiscale Permutation Entropy of Physiological Time Series. In Proceedings of the 2005 Pakistan Section Multitopic Conference, Karachi, Pakistan, 24–25 December 2005. [Google Scholar] [CrossRef]
- Gruber, A.H.; Busa, M.A.; Iii, G.E.G.; Van Emmerik, R.E.; Masso, P.D.; Hamill, J. Time-to-contact and multiscale entropy identify differences in postural control in adolescent idiopathic scoliosis. Gait Posture 2011, 34, 13–18. [Google Scholar] [CrossRef]
- Costa, M.; Peng, C.-K.; Goldberger, A.L.; Hausdorff, J.M. Multiscale entropy analysis of human gait dynamics. Phys. A Stat. Mech. Appl. 2003, 330, 53–60. [Google Scholar] [CrossRef]
- Riva, F.; Toebes, M.; Pijnappels, M.; Stagni, R.; van Dieën, J. Estimating fall risk with inertial sensors using gait stability measures that do not require step detection. Gait Posture 2013, 38, 170–174. [Google Scholar] [CrossRef]
- Lee, C.-H.; Sun, T.-L.; Jiang, B.C.; Choi, V.H. Using Wearable Accelerometers in a Community Service Context to Categorize Falling Behavior. Entropy 2016, 18, 257. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation Entropy: A Natural Complexity Measure for Time Series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, S.-H.; Jiang, B.C.; Sun, T.-L. Estimating Postural Stability Using Improved Permutation Entropy via TUG Accelerometer Data for Community-Dwelling Elderly People. Entropy 2020, 22, 1097. [Google Scholar] [CrossRef]
- Palumbo, P.; Palmerini, L.; Bandinelli, S.; Chiari, L. Fall Risk Assessment Tools for Elderly Living in the Community: Can We Do Better? PLoS ONE 2015, 10, e0146247. [Google Scholar] [CrossRef] [PubMed]
- Cella, A.; De Luca, A.; Squeri, V.; Parodi, S.; Vallone, F.; Giorgeschi, A.; Senesi, B.; Zigoura, E.; Guerrero, K.L.Q.; Siri, G.; et al. Development and validation of a robotic multifactorial fall-risk predictive model: A one-year prospective study in community-dwelling older adults. PLoS ONE 2020, 15, e0234904. [Google Scholar] [CrossRef]
- Podsiadlo, S.; Richardson, D. The Timed Up and Go: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- McMurdo, M.E.T. Guideline for the prevention of falls in older persons: Essential reading. Age Ageing 2002, 31, 13–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salarian, A.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.R.; Doheny, E.P.; O’Halloran, A.; Kenny, R.A. Frailty status can be accurately assessed using inertial sensors and the TUG test. Age Ageing 2013, 43, 406–411. [Google Scholar] [CrossRef]
- Alexandre, T.S.; Meira, D.M.; Rico, N.C.; Mizuta, S.K. Accuracy of Timed Up and Go Test for screening risk of falls among community-dwelling elderly. Braz. J. Phys. Ther. 2012, 16, 381–388. [Google Scholar] [CrossRef]
- Chou, C.; Chien, C.; Hsueh, I.; Sheu, C.; Wang, C.; Hsieh, C. Developing a Short Form of the Berg. Phys. Ther. 2006, 86, 195–204. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The balance scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Karthikeyan, G.; Sheikh, S.G.; Chippala, P. Test-retest reliability of short form of berg balance scale in elderly people. J. Med. Med. Sci. 2012, 1, 139–144. [Google Scholar]
- Shahzad, A.; Ko, S.; Lee, S.; Lee, J.-A.; Kim, K. Quantitative Assessment of Balance Impairment for Fall-Risk Estimation Using Wearable Triaxial Accelerometer. IEEE Sens. J. 2017, 17, 6743–6751. [Google Scholar] [CrossRef]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients†. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Erkinjuntti, T.; Sulkava, R.; Wikström, J.; Autio, L. Short Portable Mental Status Questionnaire as a Screening Test for Dementia and Delirium among the Elderly. J. Am. Geriatr. Soc. 1987, 35, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Inouye, S.K.; Gill, T.M.; Doucette, J.T. Shared Risk Factors for Falls, Incontinence, and Functional Dependence. J. Am. Med. Assoc. 1995, 273, 1348. [Google Scholar] [CrossRef]
- Van Doorn, C.; Gruber-Baldini, A.L.; Zimmerman, S.; Hebel, J.R.; Port, C.L.; Baumgarten, M.; Quinn, C.C.; Taler, G.; May, C.; Magaziner, J.; et al. Dementia as a Risk Factor for Falls and Fall Injuries Among Nursing Home Residents. J. Am. Geriatr. Soc. 2003, 51, 1213–1218. [Google Scholar] [CrossRef]
- Oliver, D.; Connelly, J.B.; Victor, C.; Shaw, F.E.; Whitehead, A.; Genc, Y.; Vanoli, A.; Martin, F.C.; Gosney, M.A. Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: Systematic review and meta-analyses. Br. Med. J. 2006, 334, 82. [Google Scholar] [CrossRef]
- Mecocci, P.; von Strauss, E.; Cherubini, A.; Ercolani, S.; Mariani, E.; Senin, U.; Winblad, B.; Fratiglioni, L. Cognitive Impairment Is the Major Risk Factor for Development of Geriatric Syndromes during Hospitalization: Results from the GIFA Study. Dement. Geriatr. Cogn. Disord. 2005, 20, 262–269. [Google Scholar] [CrossRef]
- Anstey, K.J.; Von Sanden, C.; Luszcz, M.A. An 8-Year Prospective Study of the Relationship between Cognitive Performance and Falling in Very Old Adults. J. Am. Geriatr. Soc. 2006, 54, 1169–1176. [Google Scholar] [CrossRef]
- Lin, C.-H.; Liao, K.-C.; Pu, S.-J.; Chen, Y.-C.; Liu, M.-S. Associated Factors for Falls among the Community-Dwelling Older People Assessed by Annual Geriatric Health Examinations. PLoS ONE 2011, 6, e18976. [Google Scholar] [CrossRef]
- Granger, C.V.; Dewis, L.S.; Peters, N.C.; Sherwood, C.C.; Barrett, J.E. Stroke rehabilitation: Analysis of repeated Barthel index measures. Arch. Phys. Med. Rehabil. 1979, 60, 14–17. [Google Scholar] [PubMed]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Review of fall risk assessment in geriatric populations using inertial sensors. J. Neuroeng. Rehabil. 2013, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Bet, P.; Castro, P.C.; Ponti, M. Fall detection and fall risk assessment in older person using wearable sensors: A systematic review. Int. J. Med. Inform. 2019, 130, 103946. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Sun, T.-L. Evaluation of postural stability based on a force plate and inertial sensor during static balance measurements. J. Physiol. Anthr. 2018, 37, 27. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale Entropy Analysis of Complex Physiologic Time Series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy maturity in premature infants Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Hear. Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Gu, Y.; Liang, Z.; Hagihira, S. Use of Multiple EEG Features and Artificial Neural Network to Monitor the Depth of Anesthesia. Sensors 2019, 19, 2499. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Niu, D.; Wang, K.; Sun, L.; Wu, J.; Xu, X. Short-term photovoltaic power generation forecasting based on random forest feature selection and CEEMD: A case study. Appl. Soft Comput. 2020, 93, 106389. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhang, Q.; Wu, L. Building Auto-Encoder Intrusion Detection System based on random forest feature selection. Comput. Secur. 2020, 95, 101851. [Google Scholar] [CrossRef]
- Zhong, Y.; He, J.; Chalise, P. Nested and repeated cross validation for classification model with high-dimensional data. Rev. Colomb. Estad. 2020, 43, 103–125. [Google Scholar] [CrossRef]
- Daines, K.J.F.; Baddour, N.; Burger, H.; Bavec, A.; Lemaire, E.D. Fall risk classification for people with lower extremity amputations using random forests and smartphone sensor features from a 6-minute walk test. PLoS ONE 2021, 16, e0247574. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Riedl, M.; Müller, A.; Wessel, N. Practical considerations of permutation entropy: A tutorial review. Eur. Phys. J. Spéc. Top. 2013, 222, 249–262. [Google Scholar] [CrossRef]
| Number | Mean ± STD | ||
|---|---|---|---|
| Gender | Female | 49 | 77 ± 6.60 |
| Male | 16 | 73 ± 6.00 | |
| Age | 65–70 | 12 | 69 ± 1.72 |
| 71–75 | 25 | 73 ± 1.53 | |
| 76–80 | 12 | 78 ± 1.48 | |
| 80+ | 16 | 85 ± 4.60 |
| BBS | TUG | TUG + BBS | SPMSQ | TUG + SPMSQ | BBS + SPMSQ | |
|---|---|---|---|---|---|---|
| Healthy | 61 | 57 | 64 | 68 | 69 | 70 |
| Fall Risk | 13 | 17 | 10 | 6 | 5 | 4 |
| Feature Type | Feature Name | Direction |
|---|---|---|
| Statistic Features | Mean (MEAN) (1–3) | ML,V,AP |
| Standard deviation (STD) (4–6) | ML,V,AP | |
| Maximum value (MAX) (7–9) | ML,V,AP | |
| Minimum value (MIN) (10–12) | ML,V,AP | |
| Zero-crossing rate (ZCR) (13–15) | ML,V,AP | |
| Multi Scale Entropy Features | Mean (MSEM) (16–18) | ML,V,AP |
| Standard Deviation (MSTD) (19–21) | ML,V,AP | |
| Complexity Index (CI) (22–24) | ML,V,AP | |
| Permutation Entropy Features | Entropy (PE) (25–27) | ML,V,AP |
| Top Features | BBS | TUG | SPMSQ | TUG + BBS | TUG + SPMSQ | BBS + SPMSQ |
|---|---|---|---|---|---|---|
| STD (AP) (0.087) | MAX (V) (0.098) | STD (V) (0.078) | CI (ML) (0.079) | STD (V) (0.099) | STD (AP) (0.086) | |
| ZCR (ML) (0.078) | STD (ML) (0.096) | STD (AP) (0.074) | MSEM (ML) (0.078) | MAX (V) (0.067) | STD (V) (0.081) | |
| MIN (V) (0.071) | CI (ML) (0.093) | MAX (ML) (0.061) | ZCR (ML) (0.076) | MAX (ML) (065) | MAX (ML) (0.077) | |
| MSEM (ML) (0.062) | MSEM (ML) (0.085) | STD (ML) (0.049) | STD (AP) (0.068) | MIN (V) (0.063) | MIN (V) (0.067) | |
| TOP 5 | CI (ML) (0.060) | STD (V) (0.071) | MAX (V) (0.045) | MAX (V) (0.067) | STD (ML) (0.062) | ZCR (ML) (0.063) |
| STD (V) (0.058) | MIN (AP) (0.05) | MIN (V) (0.045) | STD (V) (0.065) | CI (ML) (0.061) | PE (AP) (0.062) | |
| MAX (V) (0.052) | ZCR (ML) (0.048) | MSEM (AP) (0.044) | MAX (ML) (0.063) | MSEM (ML) (0.054) | STD (ML) (0.053) | |
| STD (ML) (0.048) | MAX (ML) (0.046) | CI (AP) (0.043) | STD (ML) (0.044) | STD (AP) (0.050) | CI (ML) (0.045) | |
| MSTD (AP) (0.044) | MIN (V) (0.039) | MAX (AP) (0.043) | MIN (AP) (0.040) | PE (ML) (0.046) | MAX (V) (0.041) | |
| TOP 10 | MAX (ML) (0.040) | MIN (ML) (0.037) | MSEM (ML) (0.043) | MIN (V) (0.038) | ZCR (ML) (0.042) | MSEM (ML) (0.038) |
| MEAN (V) (0.034) | STD (AP) (0.035) | CI (ML) (0.040) | CI (V) (0.034) | ZCR (AP) (0.040) | MAX (AP) (0.035) | |
| CI (V) (0.034) | CI (V) (0.027) | MSTD (ML) (0.036) | MSEM (V) (0.034) | MAX (AP) (0.035) | MEAN (V) (0.035) | |
| MIN (AP) (0.034) | MSEM (AP) (0.025) | MSTD (AP) (0.035) | MAX (AP) (0.033) | PE (AP) (0.031) | CI (AP) (0.034) | |
| MSEM (V) (0.03) | MSEM (V) (0.024) | ZCR (AP) (0.034) | CI (AP) (0.030) | MIN (AP) (0.029) | MEAN (ML) (0.028) | |
| TOP 15 | MEAN (ML) (0.029) | CI (AP) (0.024) | ZCR (ML) (0.033) | MSEM (AP) (0.029) | MSTD (ML) (0.026) | ZCR (V) (0.028) |
| Clinical Test | Top 15 Features | Top 10 Features | Top 5 Features |
|---|---|---|---|
| BBS | 0.828 | 0.856 | 0.866 |
| TUG | 0.899 | 0.913 | 0.921 |
| TUG + BBS | 0.883 | 0.910 | 0.922 |
| SPMSQ | 0.722 | 0.747 | 0.680 |
| TUG + SPMSQ | 0.814 | 0.808 | 0.783 |
| BBS + SPMSQ | 0.781 | 0.801 | 0.774 |
| With MSE Features | Without MSE Features | |||||
|---|---|---|---|---|---|---|
| Clinical Test | Top 15 | Top 10 | Top 5 | Top 15 | Top 10 | Top 5 |
| BBS | 0.828 | 0.856 | 0.866 | 0.785 | 0.793 | 0.782 |
| Tug | 0.899 | 0.913 | 0.921 | 0.881 | 0.875 | 0.866 |
| TUG + BBS | 0.883 | 0.910 | 0.922 | 0.799 | 0.812 | 0.860 |
| With MSE Features | Without MSE Features | ||||||
|---|---|---|---|---|---|---|---|
| Clinical Test | Top 15 | Top 10 | Top 5 | Top 15 | Top 10 | Top 5 | |
| BBS | Precision | 0.829 | 0.855 | 0.83 | 0.7562 | 0.7875 | 0.809 |
| Recall | 0.841 | 0.862 | 0.846 | 0.816 | 0.832 | 0.826 | |
| TUG | Precision | 0.869 | 0.861 | 0.866 | 0.841 | 0.848 | 0.861 |
| Recall | 0.863 | 0.861 | 0.861 | 0.842 | 0.845 | 0.861 | |
| BBS + TUG | Precision | 0.843 | 0.88 | 0.913 | 0.814 | 0.855 | 0.87 |
| Recall | 0.869 | 0.899 | 0.915 | 0.864 | 0.877 | 0.891 | |
| With MSE | Without MSE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Test | Top 15 | Top 10 | Top 5 | BBS | TUG | BBS + TUG | Top 15 | Top 10 | Top 5 | BBS | TUG | BBS + TUG |
| KS | 0.332 | 0.409 | 0.421 | 0.312 | 0.255 | 0.297 | 0.370 | 0.332 | 0.401 | 0.331 | 0.234 | 0.338 |
| p-value | 0.782 | 0.571 | 0.537 | 0.852 | 0.967 | 0.893 | 0.677 | 0.783 | 0.591 | 0.787 | 0.985 | 0.764 |
| Top 15 | Top 10 | Top 5 | BBS | TUG | TUG + BBS | |
|---|---|---|---|---|---|---|
| t-Test | 0.064 | 0.031 | 0.008 | 0.017 | 0.037 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, T.; Lee, C.-H.; Huang, C.-H.; Sun, T.-L. Random Forest for Automatic Feature Importance Estimation and Selection for Explainable Postural Stability of a Multi-Factor Clinical Test. Sensors 2021, 21, 5930. https://doi.org/10.3390/s21175930
Mendoza T, Lee C-H, Huang C-H, Sun T-L. Random Forest for Automatic Feature Importance Estimation and Selection for Explainable Postural Stability of a Multi-Factor Clinical Test. Sensors. 2021; 21(17):5930. https://doi.org/10.3390/s21175930
Chicago/Turabian StyleMendoza, Tomas, Chia-Hsuan Lee, Chien-Hua Huang, and Tien-Lung Sun. 2021. "Random Forest for Automatic Feature Importance Estimation and Selection for Explainable Postural Stability of a Multi-Factor Clinical Test" Sensors 21, no. 17: 5930. https://doi.org/10.3390/s21175930
APA StyleMendoza, T., Lee, C.-H., Huang, C.-H., & Sun, T.-L. (2021). Random Forest for Automatic Feature Importance Estimation and Selection for Explainable Postural Stability of a Multi-Factor Clinical Test. Sensors, 21(17), 5930. https://doi.org/10.3390/s21175930






