Matrix Pencil Method for Vital Sign Detection from Signals Acquired by Microwave Sensors
Abstract
:1. Introduction
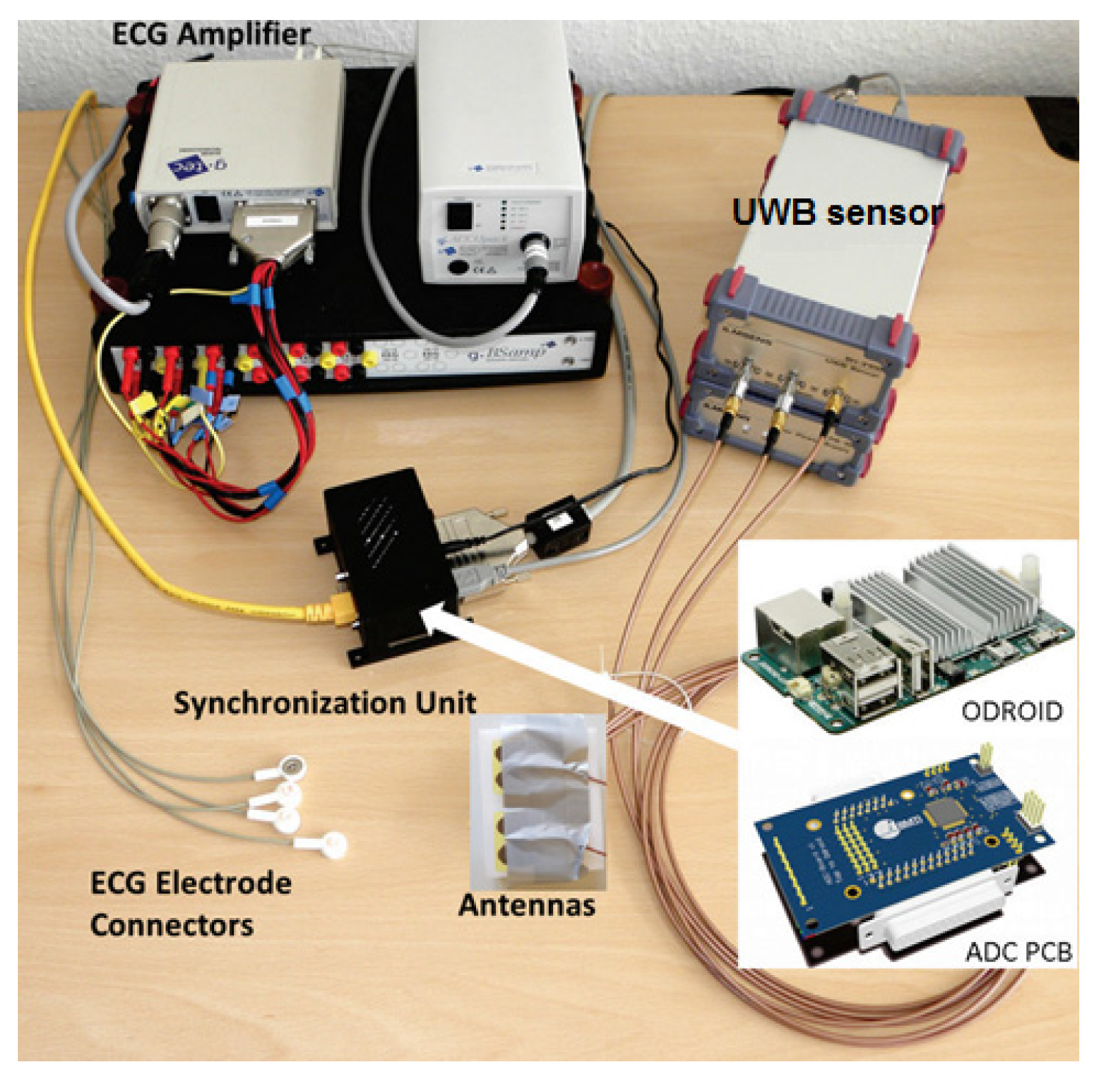
2. Data Acquisition Setup
2.1. CW Sensor
2.2. Ultra Wideband Sensor
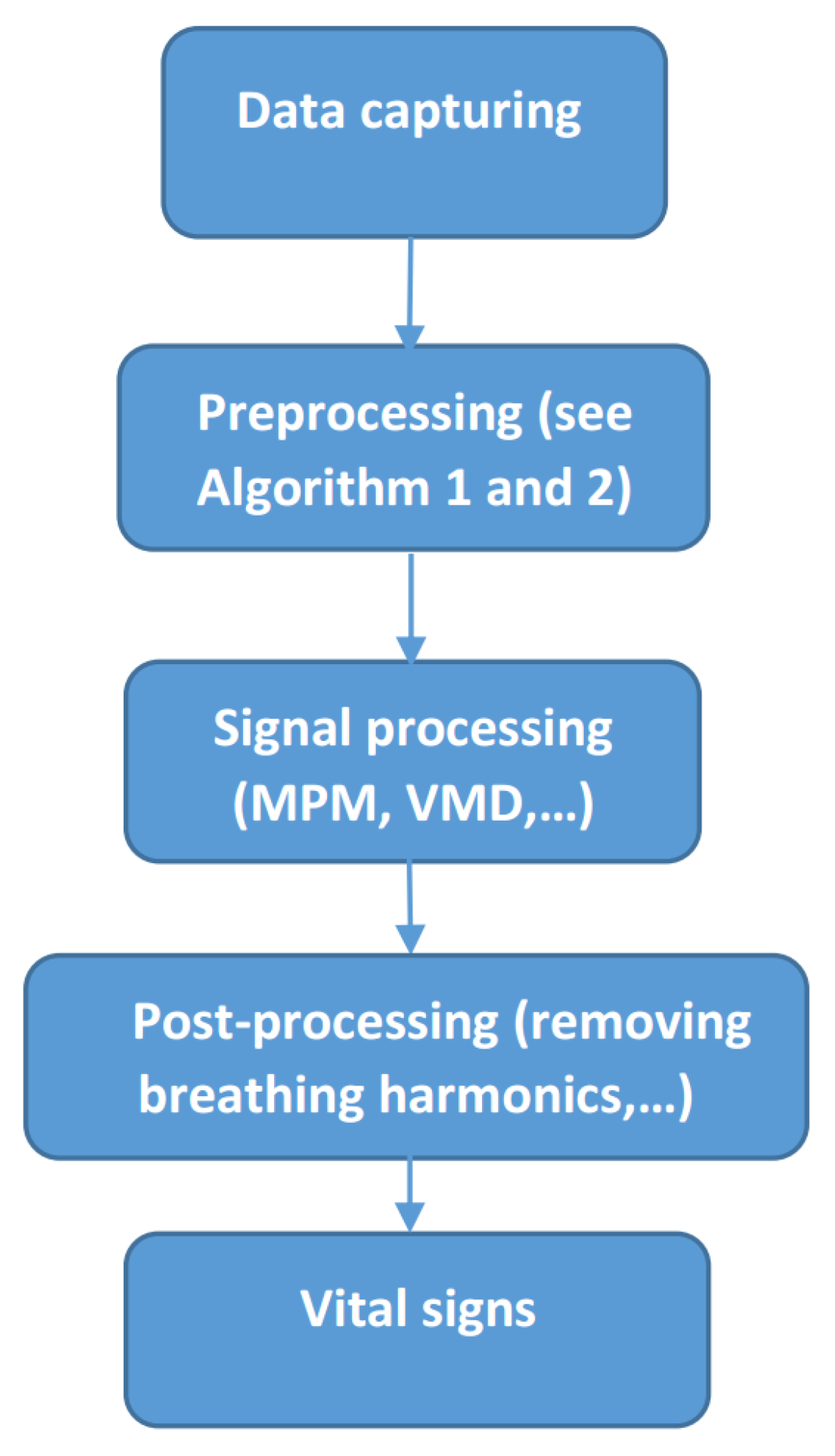
3. Materials and Methods
3.1. Continious Wave Sensor Signal Preprocessing
| Algorithm 1. CW Sensor Signal Preprocessing |
|
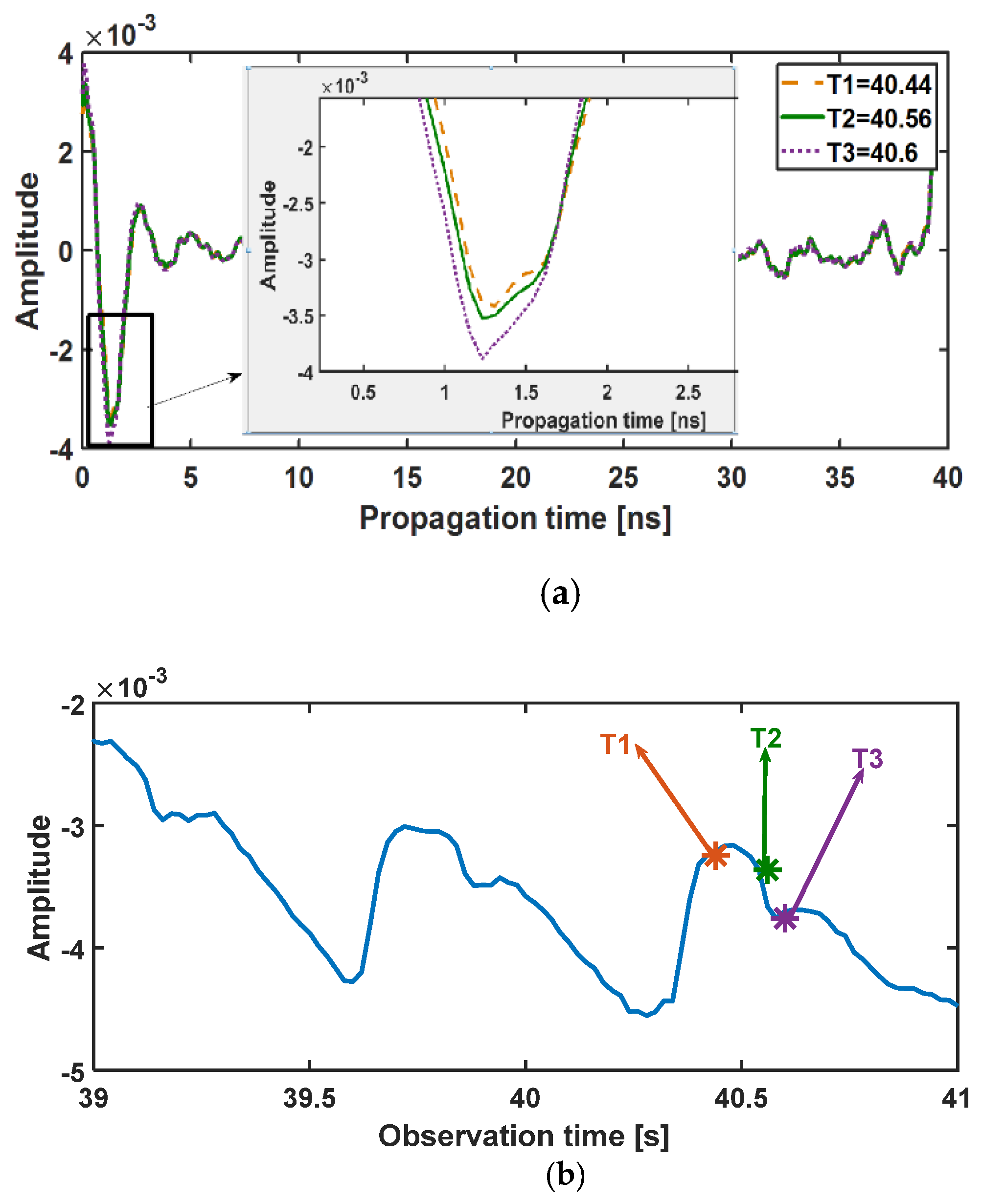
3.2. Ultra Wideband Sensor Signal Preprocessing
| Algorithm 2. UWB Sensor Signal Preprocessing |
|
1. Building the Radargram 2. Static clutter removal 3. Target range bin determination 4. Getting the reflected signal of target over the observation time |
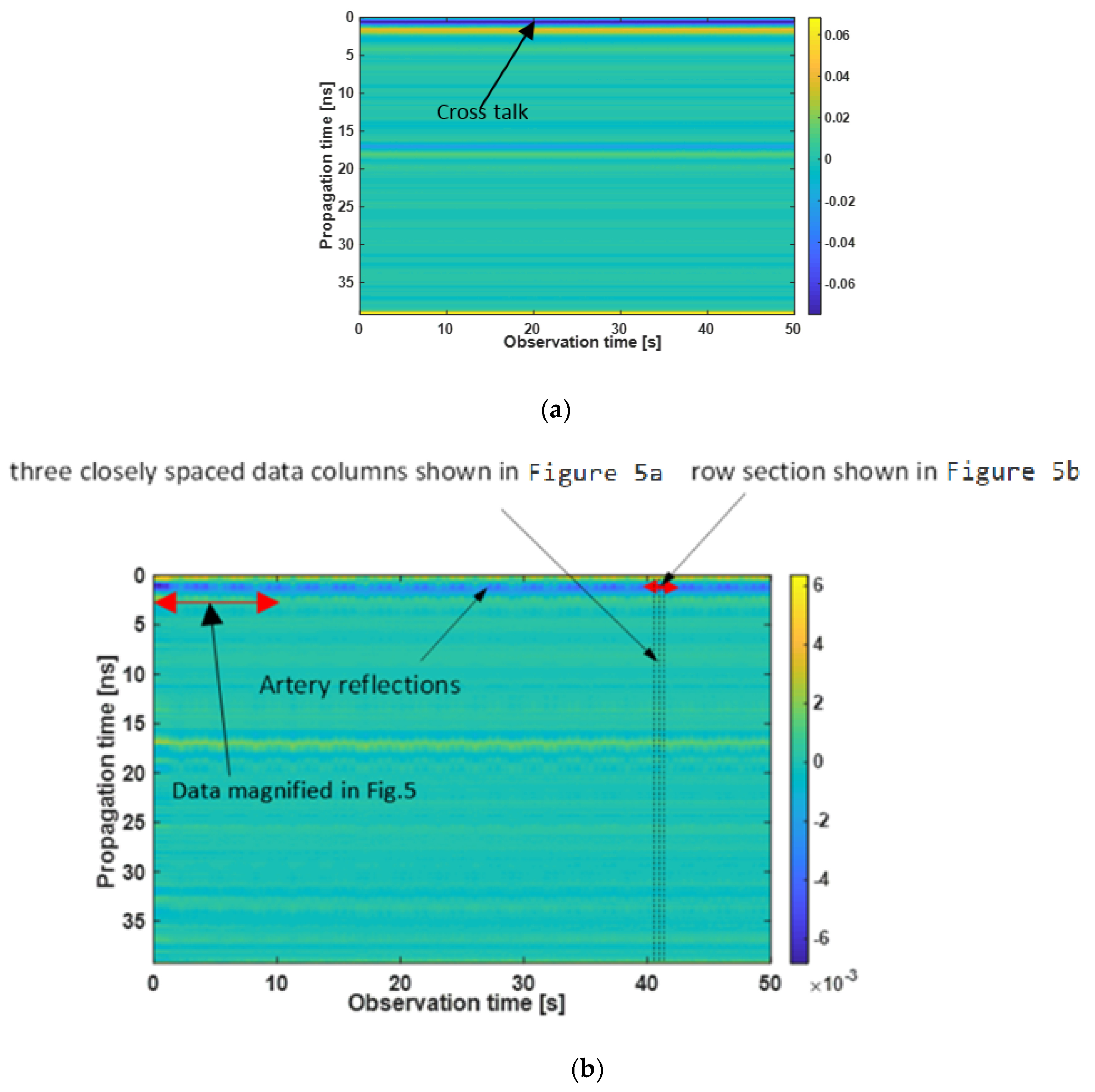
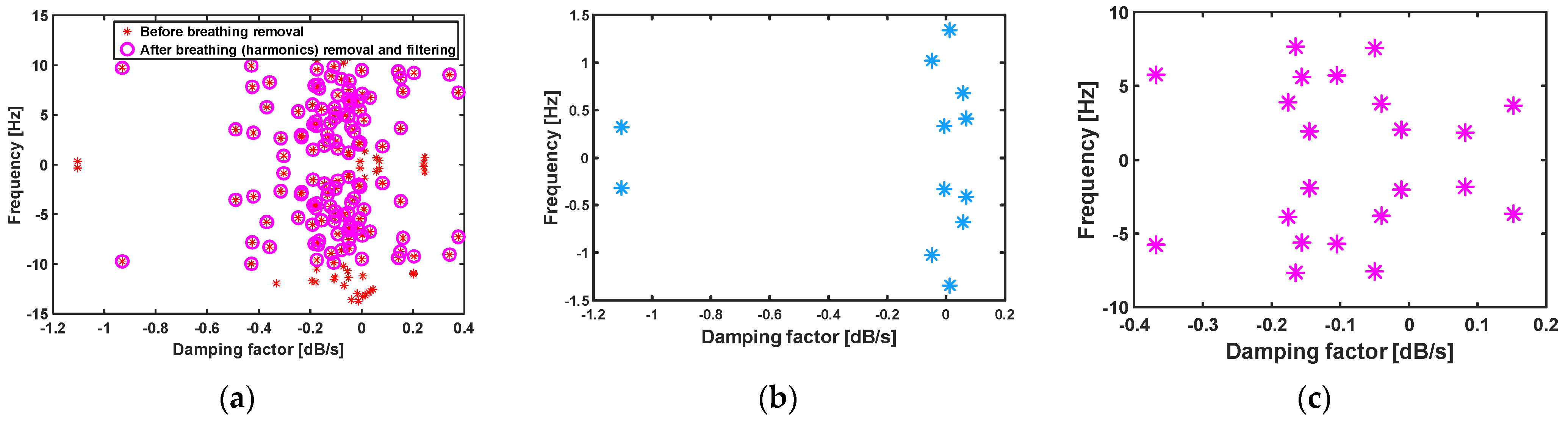
3.3. Matrix Pencil Method
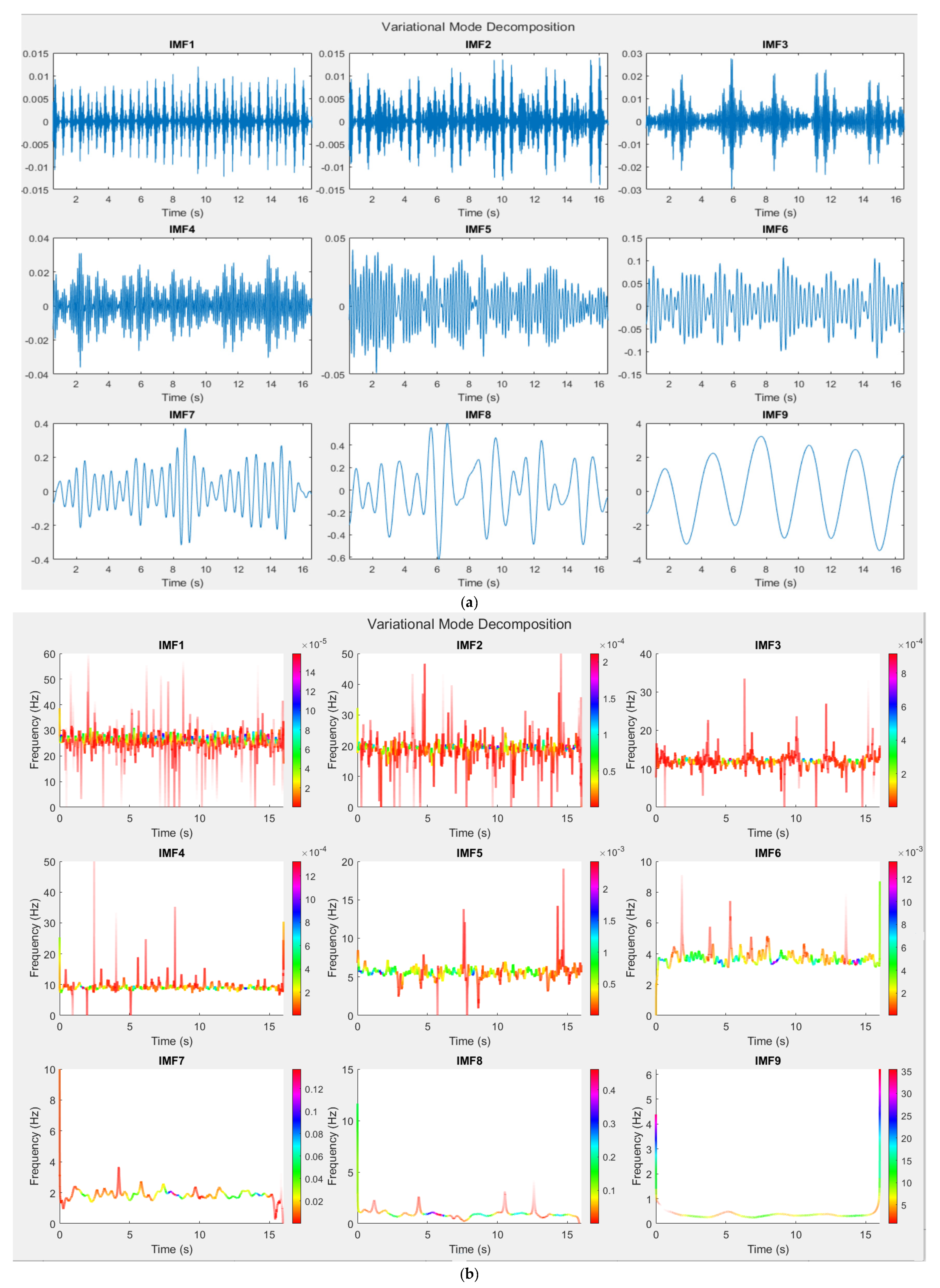
3.4. Variational Mode Decomposition Method
4. Results and Discussion
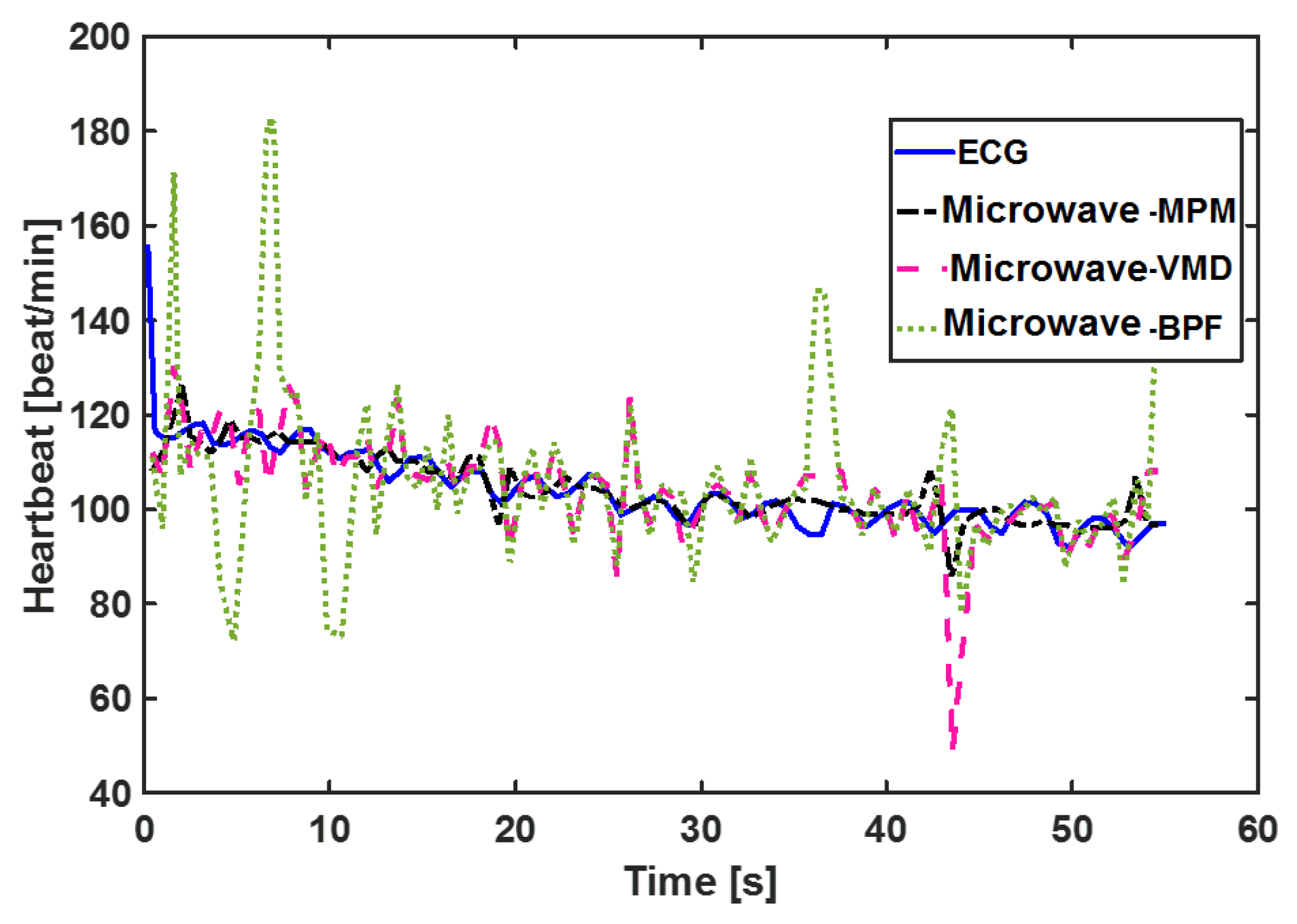
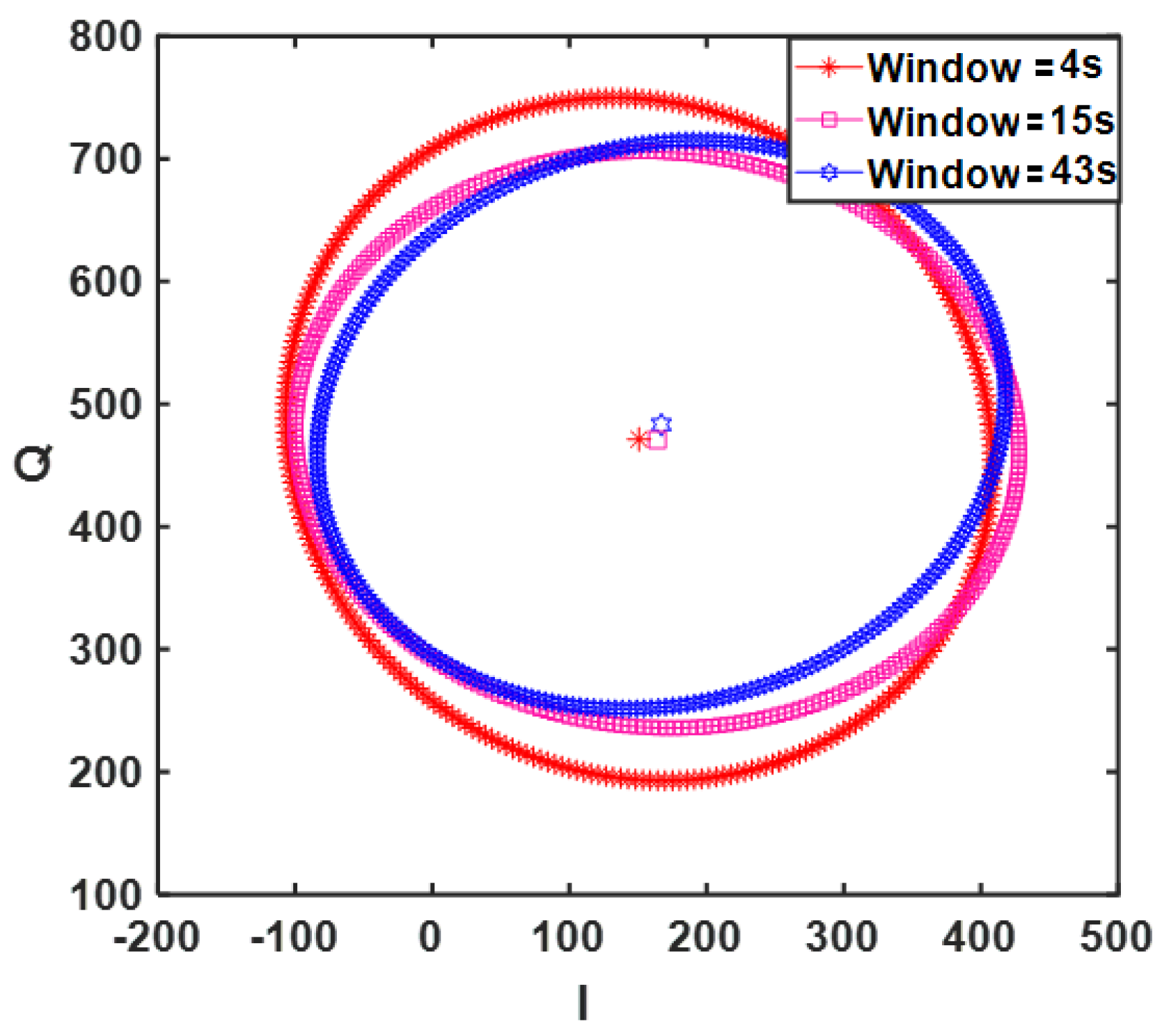
4.1. CW Sensor Results
| Method | Person 1 ID = 13-55-07 | Person 2 ID = 10-18-52 | Person 3 ID = 19-12-55 | Person 4 ID = 10-41-20 | Person 5 ID = 11-51-19 | Person 6 ID = 9-10-20 | Person 7 ID = 9-4-15 | Person 8 ID = 10-6-46 | Person 9 ID = 9-20-17 | Person 10 ID = 9-40-56 | Person 11 ID = 10-40-44 | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPM | 4.99 | 6.25 | 3.39 | 3.5 | 1.84 | 9.81 | 5.57 | 3.2 | 5.68 | 3.66 | 4.06 | 4.72 |
| VMD | 14.43 | 10.54 | 5.08 | 9.11 | 14.47 | 18.83 | 7.36 | 7.4 | 14.29 | 4.44 | 12.46 | 10.76 |
| BPF | 17.64 | 10.96 | 14.27 | 10.63 | 10.06 | 11.86 | 5.8 | 10.7 | 11.33 | 6.97 | 15.79 | 11.45 |
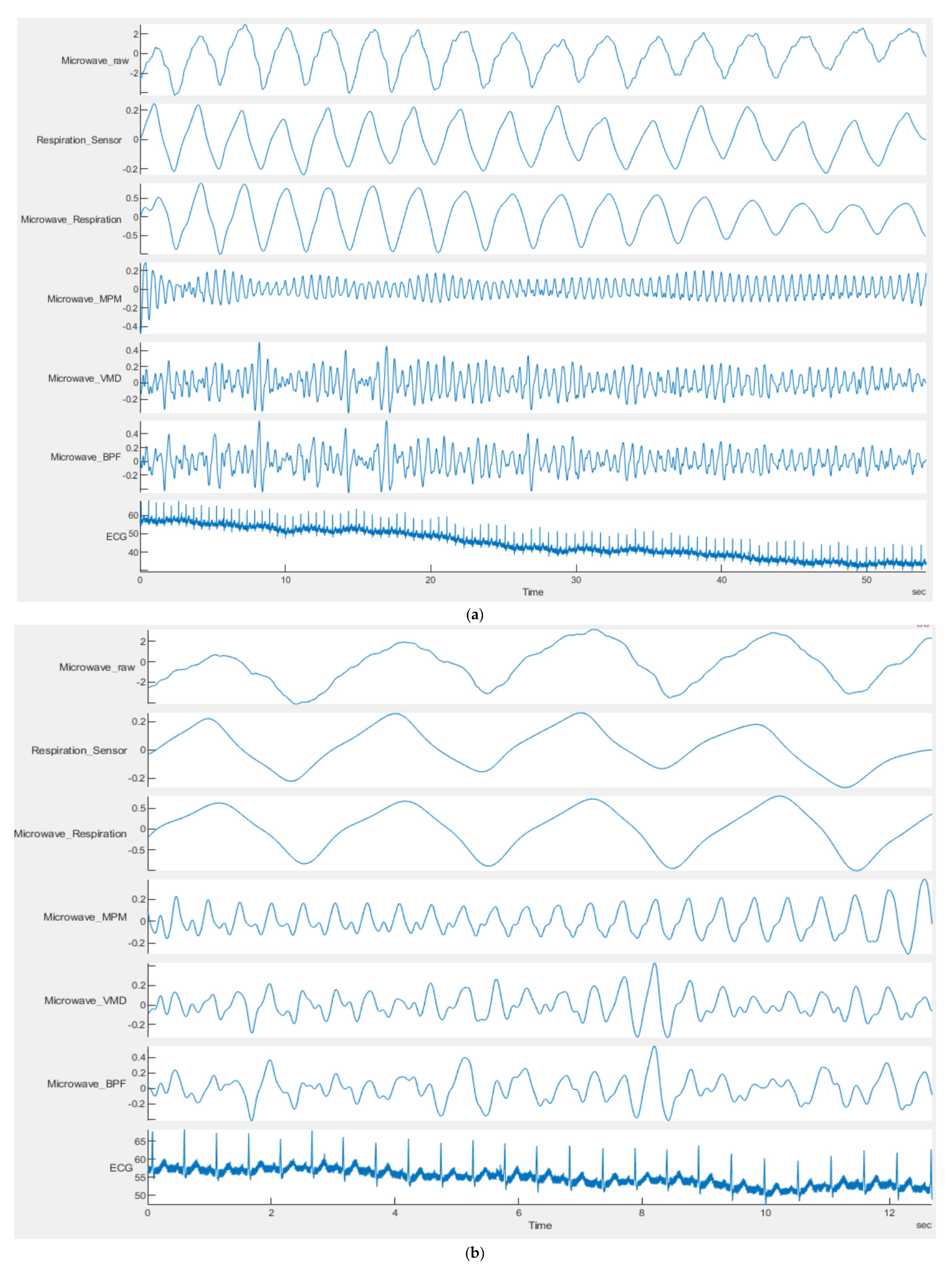
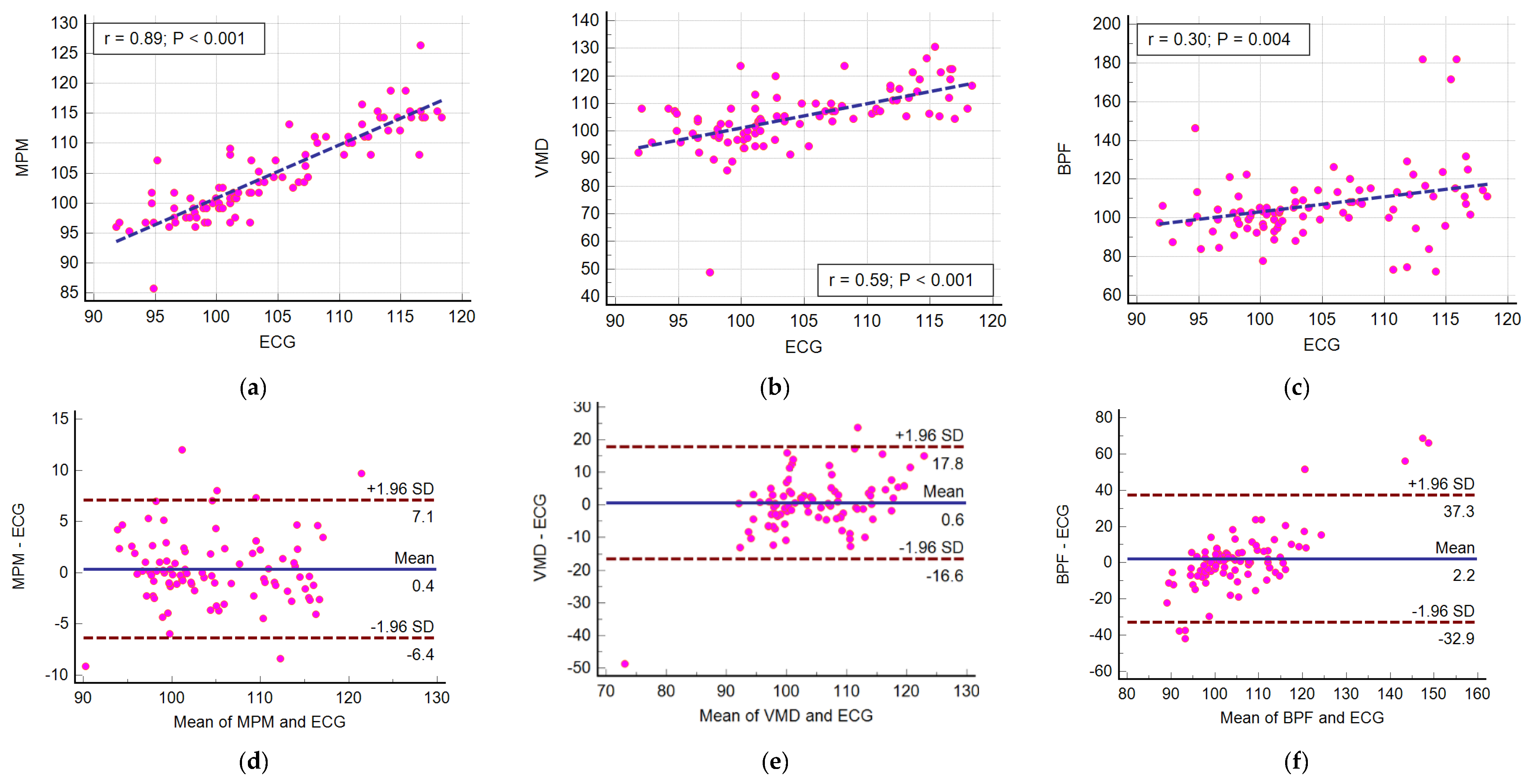
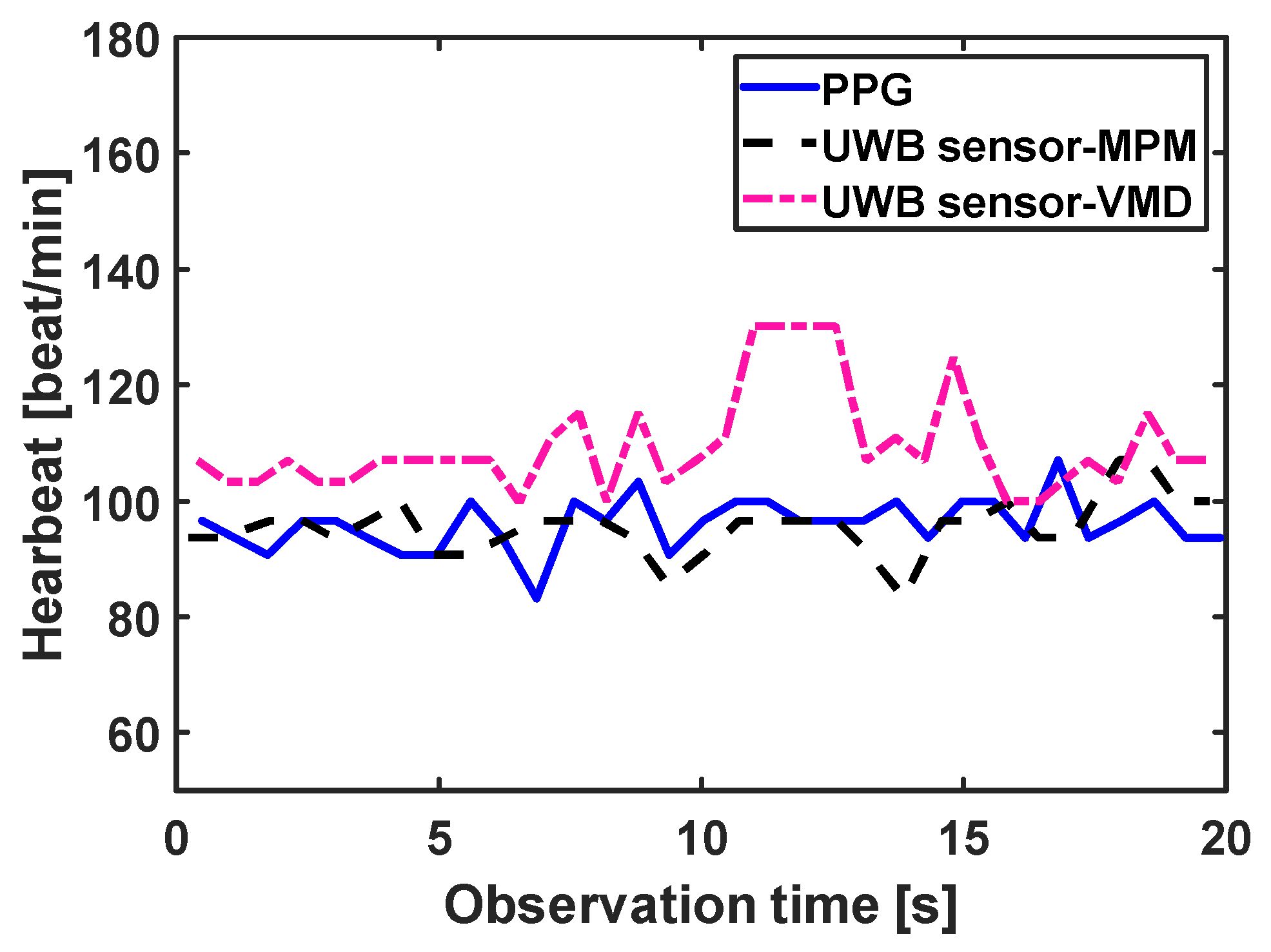
4.2. Ultra Wideband Sensor Results
| Method | RMSE (beat/min) |
|---|---|
| MPM | 2.59 |
| VMD | 2.57 |
| BPF | 3.44 |
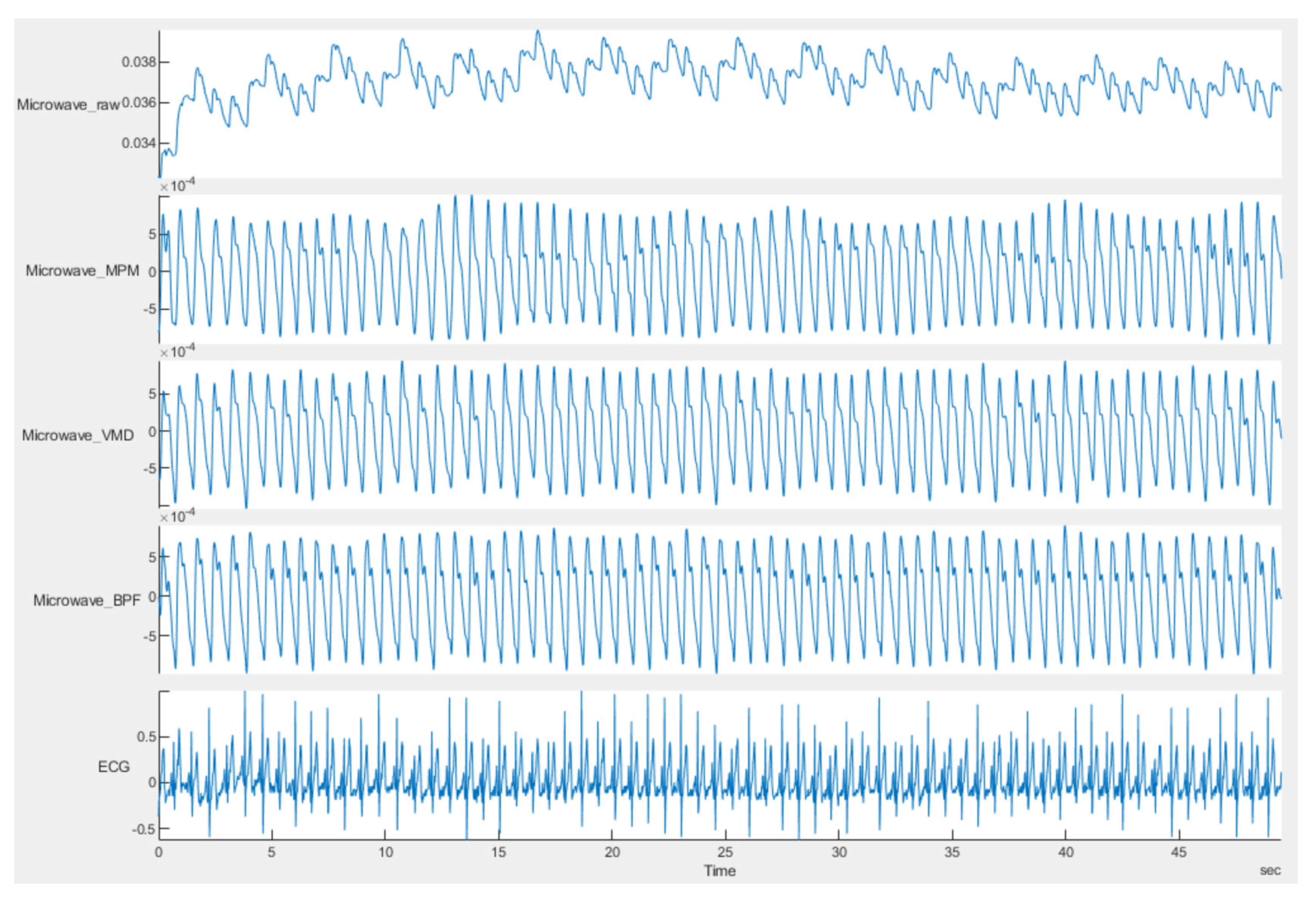


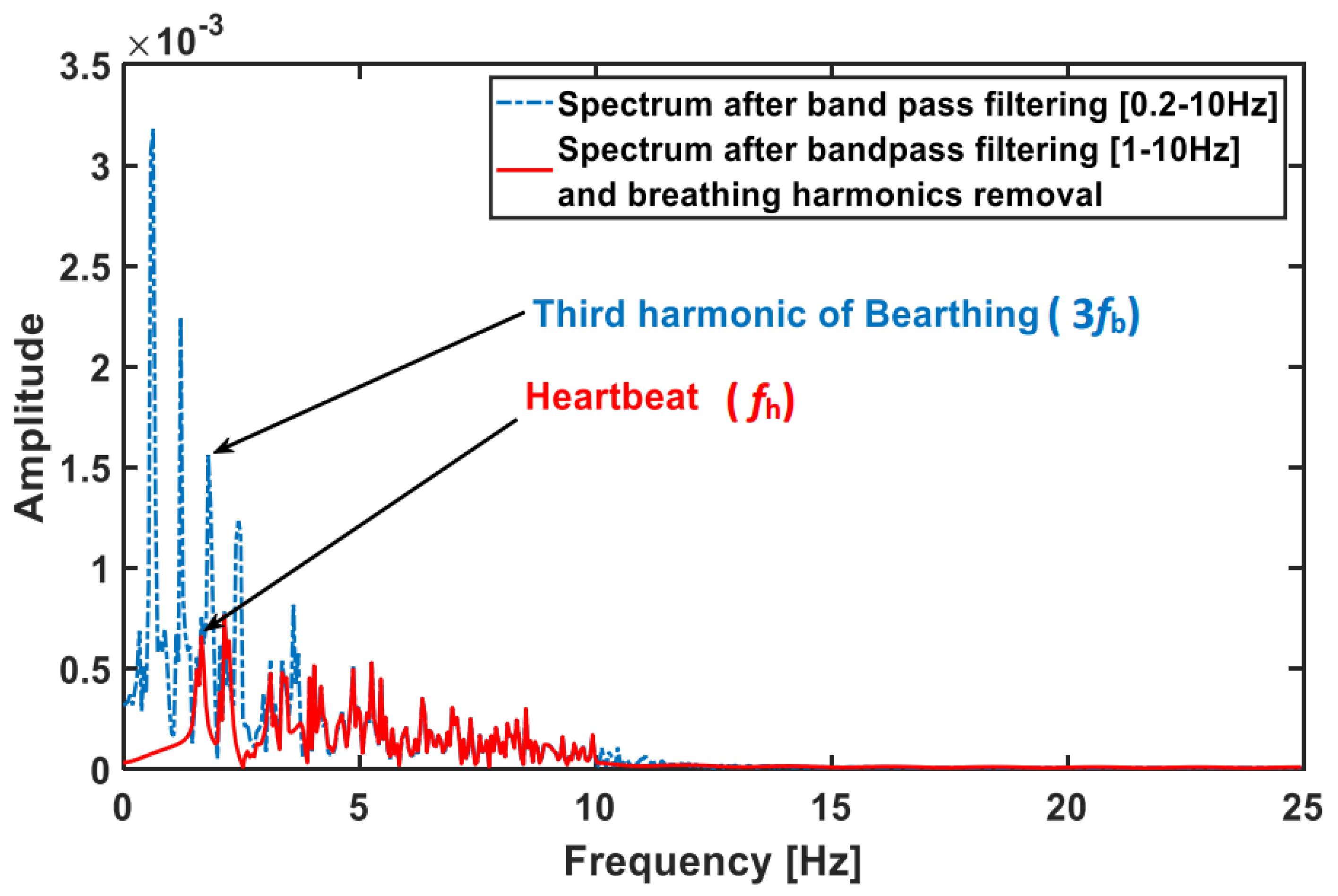
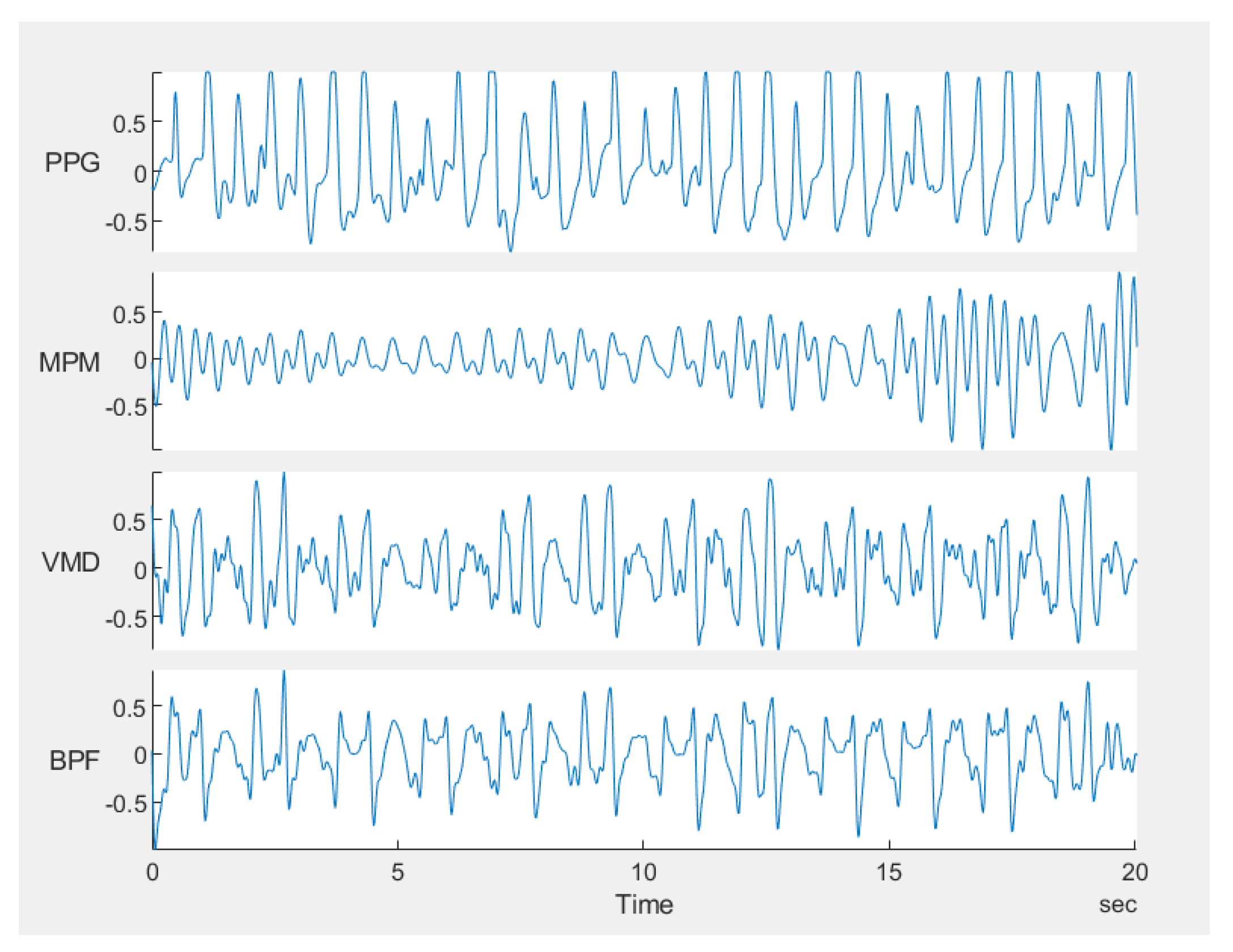
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, C.; Huang, T.Y.; Li, C.; Lin, J. Microwave and Millimeter-Wave Radars for Vital Sign Monitoring. In Radar for Indoor Monitoring Detection, Classification, and Assessment, 1st ed.; Amin, M., Ed.; CRC Press: New York, NY, USA, 2018; Chapter 9. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Ghaffar, A.; Khan, N.; Cho, S.H. An Overview of Signal Processing Techniques for Remote Health Monitoring Using Impulse Radio UWB Transceiver. Sensors 2020, 20, 2479. [Google Scholar] [CrossRef]
- Van, N.T.P.; Tnag, L.; Demir, V.; Hasan, S.F.; Minh, N.D.; Mukhopadhyay, S. Review-microwave radar sensing systems for search and rescue purposes. Sensors 2019, 29, 2879. [Google Scholar] [CrossRef] [Green Version]
- Lazaro, A.L.; Vilarino, R. Analysis of vital signs monitoring using an IR-UWB radar. Prog. Electromagn. Res. 2010, 100, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Tu, J.; Lin, J. Fast acquisition of heart rate in noncontact vital sign radar measurement using time-window-variation technique. IEEE Trans. Instrum. Meas. 2016, 65, 112–122. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Hong, H.; Xi, F.; Cai, W.; Zhu, X. Super-resolution spectral estimation in short-time non-contact vital sign measurement. Rev. Sci. Instrum. 2015, 86, 044708. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Yuan, Y. An EMD Based Breathing and Heartbeat Monitoring System. In Proceedings of the IEEE 2013 7th Asia Modell. Symp.(AMS 2013), Hong Kong, China, 23–25 July 2013; pp. 55–58. [Google Scholar] [CrossRef]
- Madhavi, K.V.; Ram, M.R.; Krishna, E.H.; Reddy, K.N.; Redd, K.A. Estimation of respiratory rate from principal components of photoplethysmographic signals. In Proceedings of the IEEE EMBS Conf Biomed. Eng. Sci. (IECBES 2010), Kuala Lumpur, Malaysia, 30 November–2 December 2010; pp. 311–314. [Google Scholar] [CrossRef]
- Mostafa, M.; Chamaani, S.; Sachs, J. Applying singular value decomposition for clutter reduction in heartbeat estimation using M-sequence UWB Radar. In Proceedings of the Int. Radar Symp, Brisbane, Australia, 27–31 August 2018; pp. 1–10. [Google Scholar] [CrossRef]
- Mostafa, M.; Chamaani, S.; Sachs, J. Singular Spectrum Analysis based algorithm for vitality monitoring using M-sequence UWB Sensor. IEEE Sens. J. 2019, 20, 1748. [Google Scholar] [CrossRef]
- Regev, N.; Wulich, D. Remote sensing of vital signs using an ultra-wide- band radar. Int. J. Remote Sens. 2019, 40, 6596–6606. [Google Scholar] [CrossRef]
- Duda, K.; Zielin’ski, T.P. Efficacy of the Frequency and Damping Estimation of a Real-Value Sinusoid. IEEE Instrum. Meas. Mag. 2013, 16, 48–58. [Google Scholar] [CrossRef]
- Hua, Y.; Sarkar, T.J. Matrix Pencil Method for Estimating Parameters of exponentially damped sinusoids in noise. IEEE Trans. Acoust. Speech Sig. Proc. 1990, 38, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, T.K.; Pereira, O. Using the Matrix Pencil Method to Estimate the Parameters of a Sum of Complex Exponentials. IEEE Ant. Propagat. Mag. 1995, 37, 48–55. [Google Scholar] [CrossRef] [Green Version]
- del Rıo, J.F.; Sarkar, T.K. Comparison between the Matrix Pencil Method and the Fourier Transform Technique for High-Resolution Spectral Estimation. Sci. Direct. Digit Signal Process. 1996, 6, 108–125. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.F.; Rodrigo, L.D.S.; Guillén, E.L.; Ascariz, J.M.R.; Jiménez, J.M.M.; Boquete, L. Coding Prony’s method in MATLAB and applying it to biomedical signal filtering. BMC Bioinf. 2018, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, U.C.; Murthy, I.S.N. ECG component delineation by Prony’s method. Signal Process. 1993, 31, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Akbarpour, A.; Chamaani, S. Estimation of target circumferential size by backscattered wave. IET Microw. Antennas Propag. 2019, 13, 2159–2165. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Ewins, D.J.; Robb, D.A. Non-stationary analysis and noise filtering using a technique extended from the original Prony method. Mech. Syst. Signal. Process. 2003, 17, 533–549. [Google Scholar] [CrossRef]
- Adve, R.S.; Sarkar, T.K.; Pereira-Filho, O.M.C.; Rao, S.M. Extrapolation of Time-Domain Responses from Three-Dimensional Conducting Objects Utilizing the Matrix Pencil Technique. IEEE Trans. Ant. Propagat. 1997, 45, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Aliouche, K.A.; Benazzouz, D. Split array of antenna sensors and matrix pencil method for azimuth and elevation angles estimation. IET Signal Proc. 2017, 11, 687–694. [Google Scholar] [CrossRef]
- Tuglar, O.; Ergin, A.A. Matrix pencil method for estimating radar cross section of moving targets with near-field measurements. Microw. Opt. Technol. Lett. 2016, 58, 471–476. [Google Scholar] [CrossRef]
- Tulgar, O.; Ergin, A.A. Matrix pencil method for chirp ISAR imaging with near-field measurements. Microw. Opt. Technol. Lett. 2015, 57, 1237–1242. [Google Scholar] [CrossRef]
- Ruan, M.; Cheng, Y.; Zhang, T.; Wang, A.; Xue, H. Improved Prony method for high-frequency-resolution harmonic and interharmonic analysis. In Proceedings of the 2019 IEEE 2nd International Conference on Electronics Technology (ICET), Chengdu, China, 10–13 May 2019. [Google Scholar]
- McSwiggan, D.; Littler, T. A Wavelet-Prony Method for Modeling of Fixed-Speed Wind Farm Low-Frequency Power Pulsations. In Proceedings of the International Conference on Intelligent Computing for Sustainable Energy and Environment, ICSEE 2010, Wuxi, China, 17–20 September 2010. [Google Scholar]
- Shi, K.; Schellenberger, S.; Will, C.; Steigleder, T.; Michler, F.; Fuchs, J.; Weigel, R.; Ostgathe, C.; Koeplin, A. A dataset of radar-recorded heart sounds and vital signs including synchronised reference sensor signals. Sci. Data 2020, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- How to Take a Respiratory Rate in First Aid. Available online: https://www.firstaidforfree.com/how-to-take-a-respiratory-rate-in-first-aid/ (accessed on 10 November 2020).
- Dragomiretskiy, K.; Zosso, D. Variational mode decomposition. IEEE Trans. Signal Process. 2014, 62, 531–544. [Google Scholar] [CrossRef]
- He, W.; Ye, Y.; Li, Y.; Xu, H.; Lu, L.; Huang, W.; Sun, M. Variational Mode Decomposition-Based Heart Rate Estimation Using Wrist-Type Photoplethysmography during Physical Exercise. In Proceedings of the 2018 24th International Conference on Pattern Recognition (ICPR), Beijing, China, 20–24 August 2018. [Google Scholar]
- Mishra, M.; Banerjee, S.; Thomas, D.C.; Dutta, S.; Mukherjee, A. Detection of third heart sound using variational mode decomposition. IEEE Trans. Instrum. Meas. 2018, 67, 1713–1721. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Dong, X.; Xing, G.; Peng, Z.; Zhang, W. Warped Variational Mode Decomposition with Application to Vibration Signals of Varying-Speed Rotating Machineries. IEEE Trans. Instrum. Meas. 2019, 68, 2755–22767. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, T.; Hu, H.; Hu, Y. Chatter frequency identification and amplitude tracking using short-time difference spectrum analysis. IEEE Trans. Instrum. Meas. 2020, 69, 9844–9852. [Google Scholar] [CrossRef]
- Hong, K.; Wang, L.; Xu, S. A Variational Mode Decomposition Approach for Degradation Assessment of Power Transformer Windings. IEEE Trans. Instrum. Meas. 2019, 68, 1221–1229. [Google Scholar] [CrossRef]
- Maji, U.; Pal, S. Empirical mode decomposition vs. variational mode decomposition on ECG signal processing: A comparative study. In Proceedings of the 2016 International Conference on Advances in Computing, Communications and Informatics, Jaipur, India, 21–24 September 2016; pp. 1129–1134. [Google Scholar] [CrossRef]
- Smruthy, A.; Suchetha, M. Real-Time Classification of Healthy and Apnea Subjects Using ECG Signals with Variational Mode Decomposition. IEEE Sens. J. 2017, 17, 3092–3099. [Google Scholar] [CrossRef]
- Yan, J.; Hong, H.; Zhao, H.; Li, Y.; Gu, C.; Zhu, X. Through-wall multiple targets vital signs tracking based on VMD algorithm. Sensors 2016, 16, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.; Yang, X.; Li, L.; Zhang, L.; Kitsuwan, N. HEAR: Approach for Heartbeat Monitoring with Body Movement Compensation by IR-UWB Radar. Sensors 2018, 18, 77. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Luo, Z.; Huang, J.; Yan, S. A comparative study of four kinds of adaptive decomposition algorithms and their applications. Sensors 2018, 18, 2120. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Lauteslager, T.; Member, S.; Fellow, T.S.L. UWB radar for non-contact heart rate variability monitoring and mental state classification. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Berlin, Germany, 23–27 July 2019; pp. 6578–6582. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Hong, H.; Sun, L.; Li, Y.; Li, C.; Zhu, X. Noncontact physiological dynamics detection using low-power digital-IF Doppler radar. IEEE Trans. Instrum. Meas. 2017, 66, 1780–1788. [Google Scholar] [CrossRef]
- Cho, H.; Park, Y. Detection of heart rate through a wall using UWB. J. Healthc. Eng. 2018, 1–8. [Google Scholar] [CrossRef]
- Kazemi, S.; Ghorbani, A.; Amindavar, H.; Morgan, D.R. Vital-sign extraction using bootstrap-based generalized warblet transform in heart and respiration monitoring radar system. IEEE Trans. Instrum. Meas. 2016, 65, 255–263. [Google Scholar] [CrossRef]
- Helbig, M.; Zender, J.; Ley, S. Simultaneous electrical and mechanical heart activity registration by means of synchronized ECG and M-sequence UWB Sensor. In Proceedings of the 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Sachs, J.; Ley, S.; Just, T.; Chamaani, S.; Helbig, M. Differential ultra-wideband microwave imaging: Principle application challenges. Sensors 2018, 18, 2136. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Gao, X.; Yavari, E.; Zakrzewski, M.; Cao, X.H.; Lubecke, V.M.; Boric-Lubecke, O. Data-based quadrature imbalance compensation for a CW doppler radar system. IEEE Trans. Microw. Theory Tech. 2013, 61, 1718–1724. [Google Scholar] [CrossRef]
- Mabrouk, M.; Rajan, S.; Bolic, M.; Batkin, I.; Dajani, H.R.; Groza, V.Z. Detection of human targets behind the wall based on singular value decomposition and skewness variations. In Proceedings of the 2014 IEEE Radar Conference, Cincinnati, OH, USA, 19–23 May 2014; pp. 1466–1470. [Google Scholar] [CrossRef]
- Shen, L.; Kim, D.; Lee, J.; Kim, H.; Park, P.; Yu, H.K. Human detection based on the excess kurtosis in the non-stationary clutter enviornment using UWB impulse radar. In Proceedings of the 2011 3rd International Asia-Pacific Conference on Synthetic Aperture Radar (APSAR), Seoul, Korea, 26–30 September 2011; pp. 1–4. [Google Scholar]
- Hua, Y.; Sarkar, T.K. Matrix pencil and system poles. Signal Process. 1990, 21, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Rezaiesarlak, R.; Manteghi, M. Short-Time Matrix Pencil Method for Chipless RFID Detection Applications. IEEE Trans. Antennas Propag. 2013, 61, 2801–2806. [Google Scholar] [CrossRef]
- Will, C.; Shi, K.; Weigel, R.; Koelpin, A. Advanced Template Matching Algorithm for Instantaneous Heartbeat Detection using Continuous Wave Radar Systems. In Proceedings of the 2017 First IEEE MTT-S International Microwave Bio Conference (IMBIOC), Gothenburg, Sweden, 15–17 May 2017; p. 3. [Google Scholar] [CrossRef]
- Sakamoto, T.; Imasaka, R.; Taki, H.; Sato, T. Feature-Based Correlation and Topological Similarity for Interbeat Interval Estimation Using Ultrawideband Radar. IEEE Trans. Biomed. Eng. 2016, 63, 747–757. [Google Scholar] [CrossRef] [PubMed]
- MedCalc Statistical Software version 19.2 (MedCalc Software Ltd., Ostend, Belgium. Available online: https://www.medcalc.org (accessed on 14 February 2019).
- Özdemir, C. Inverse Synthetic Aperture Radar Imaging with MATLAB Algorithms, 1st ed.; JOHN WILEY & SONS: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sharafi, A.; Baboli, M.; Eshghi, M.; Ahmadian, A. Respiration-rate estimation of a moving target using impulse-based ultra wideband radars. Australas. Phys. Eng. Sci. Med. 2012, 35, 31–39. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamaani, S.; Akbarpour, A.; Helbig, M.; Sachs, J. Matrix Pencil Method for Vital Sign Detection from Signals Acquired by Microwave Sensors. Sensors 2021, 21, 5735. https://doi.org/10.3390/s21175735
Chamaani S, Akbarpour A, Helbig M, Sachs J. Matrix Pencil Method for Vital Sign Detection from Signals Acquired by Microwave Sensors. Sensors. 2021; 21(17):5735. https://doi.org/10.3390/s21175735
Chicago/Turabian StyleChamaani, Somayyeh, Alireza Akbarpour, Marko Helbig, and Jürgen Sachs. 2021. "Matrix Pencil Method for Vital Sign Detection from Signals Acquired by Microwave Sensors" Sensors 21, no. 17: 5735. https://doi.org/10.3390/s21175735
APA StyleChamaani, S., Akbarpour, A., Helbig, M., & Sachs, J. (2021). Matrix Pencil Method for Vital Sign Detection from Signals Acquired by Microwave Sensors. Sensors, 21(17), 5735. https://doi.org/10.3390/s21175735






