Removal of ECG Artifacts Affects Respiratory Muscle Fatigue Detection—A Simulation Study
Abstract
1. Introduction
2. Methods
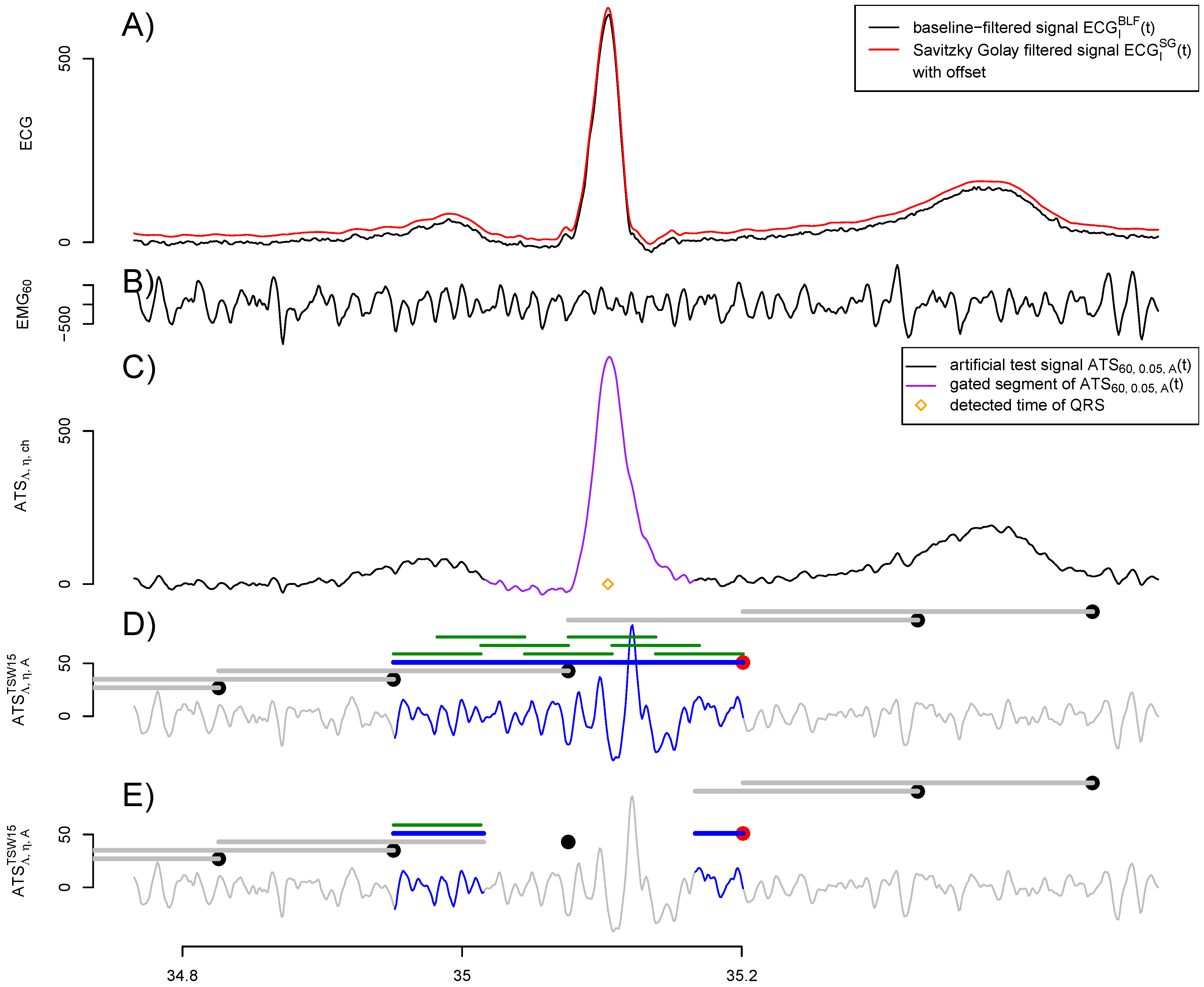
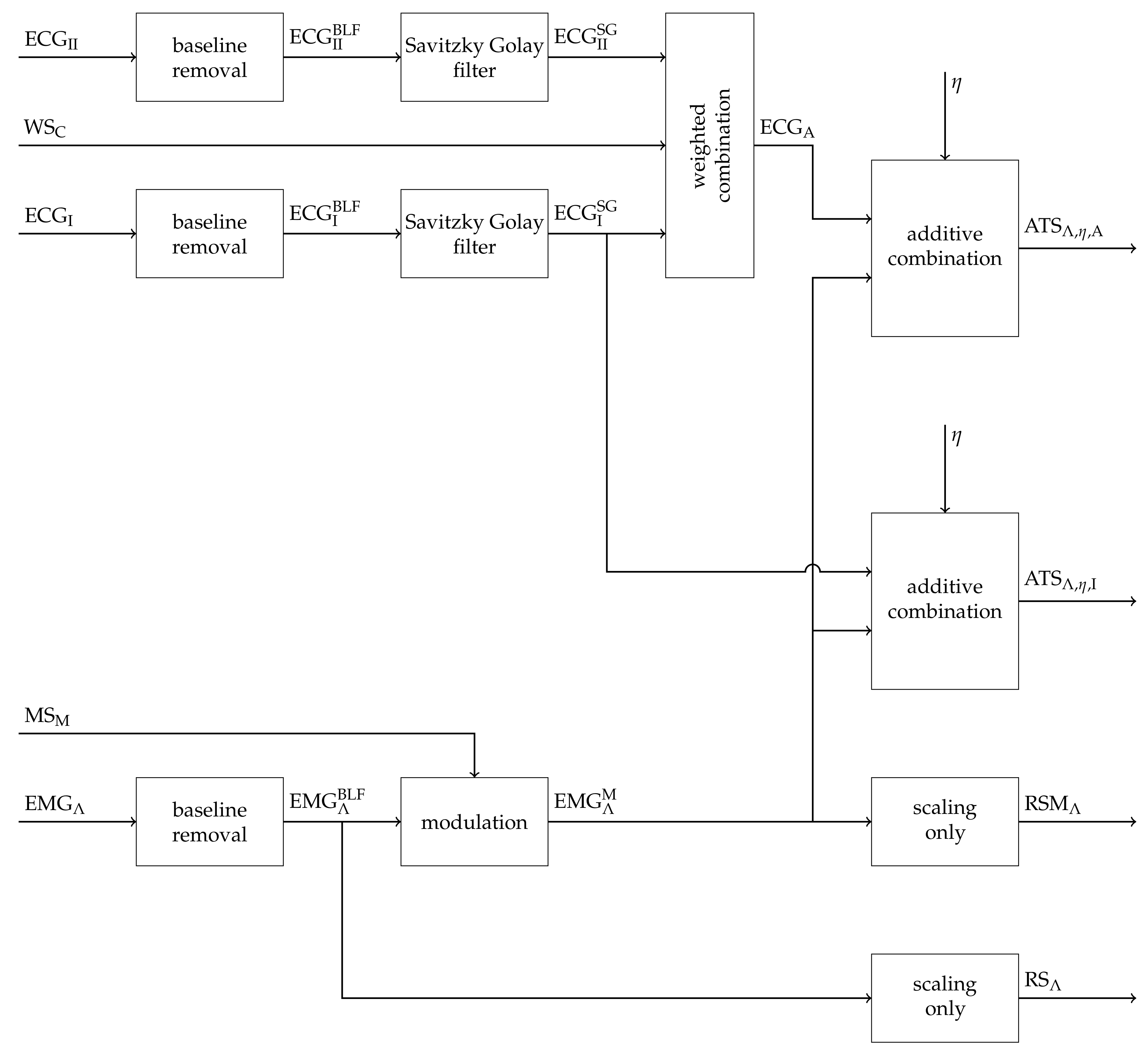
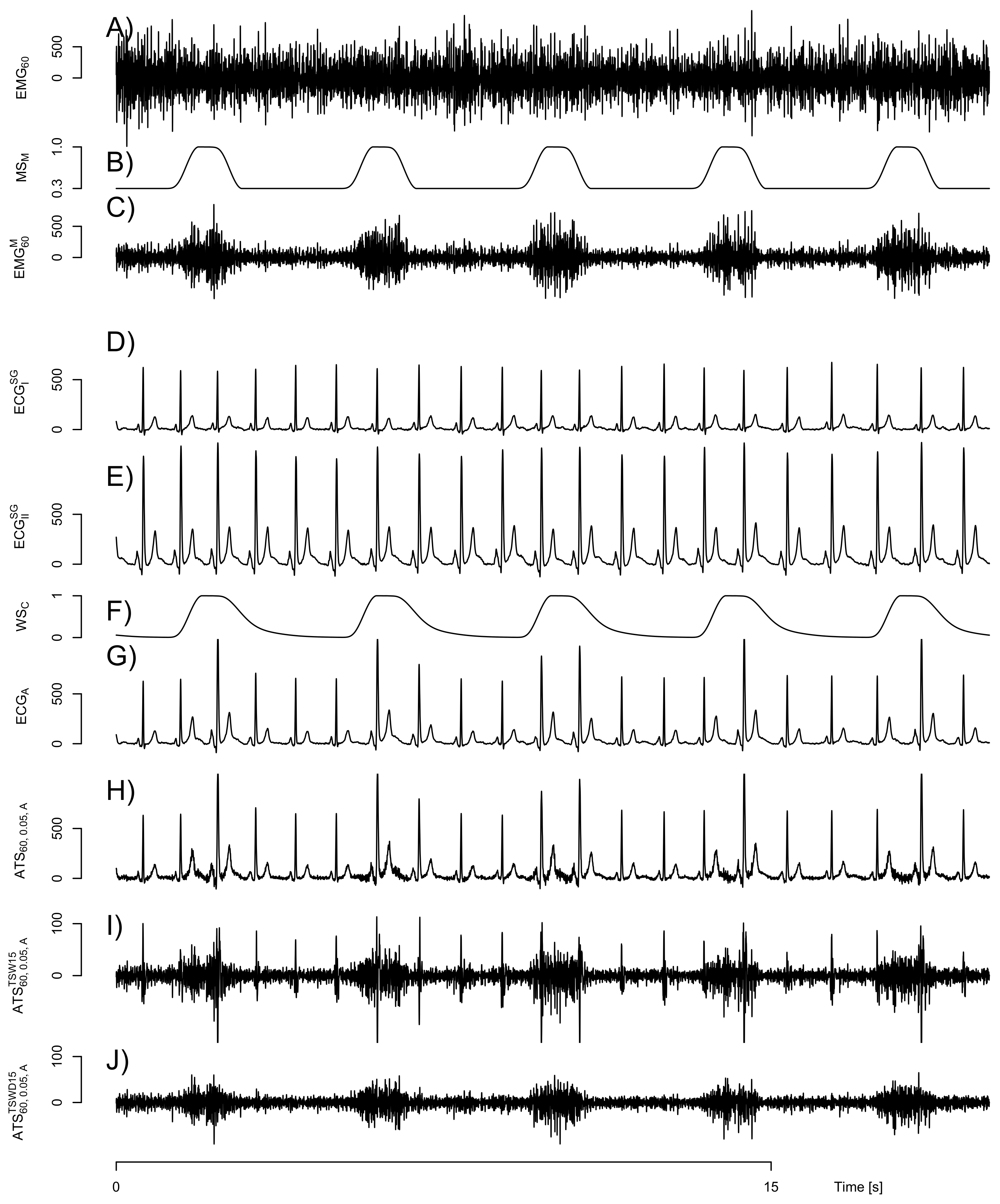
2.1. Construction of Artificial Signals
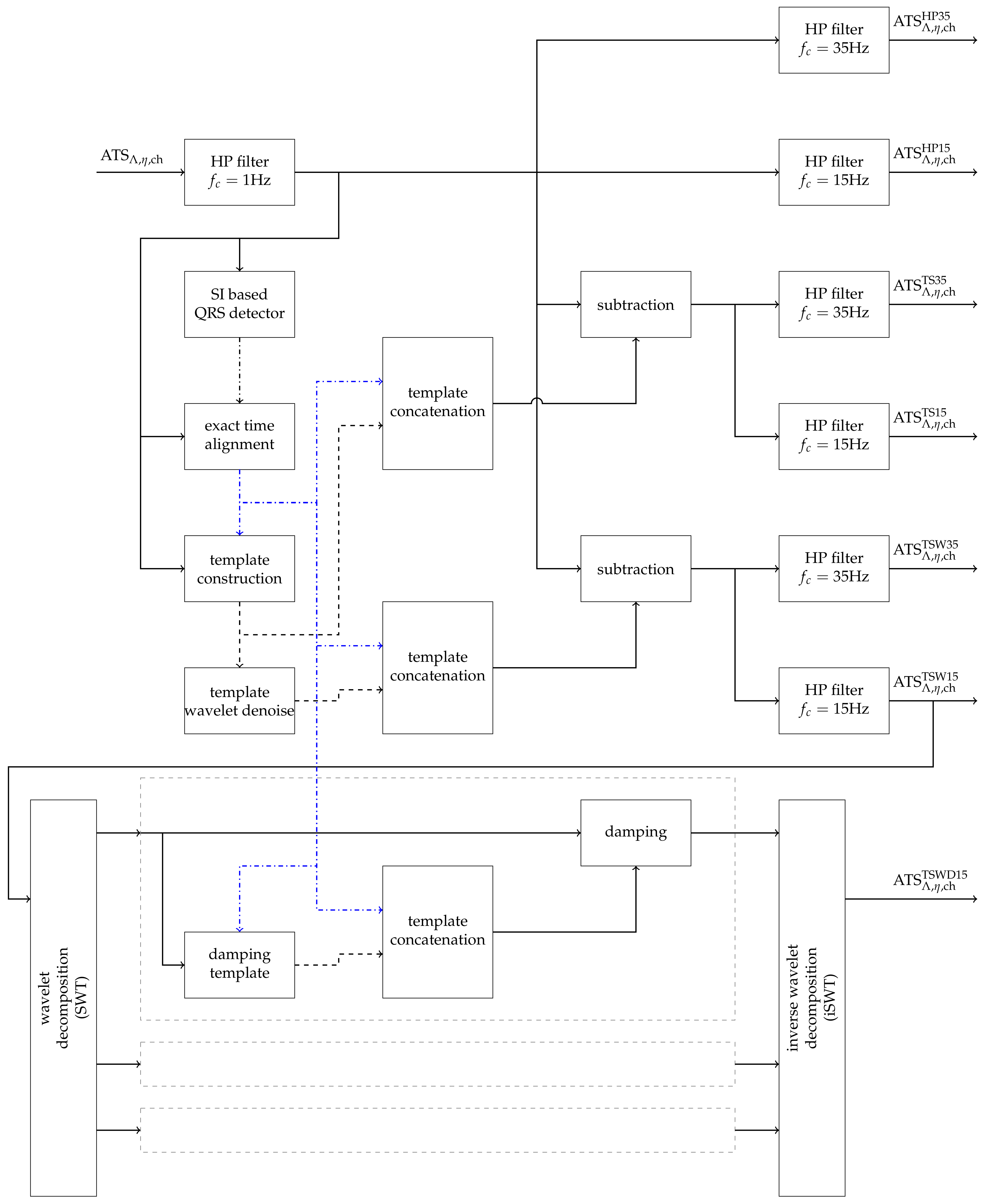
2.2. Removal of Cardiogenic Artifacts
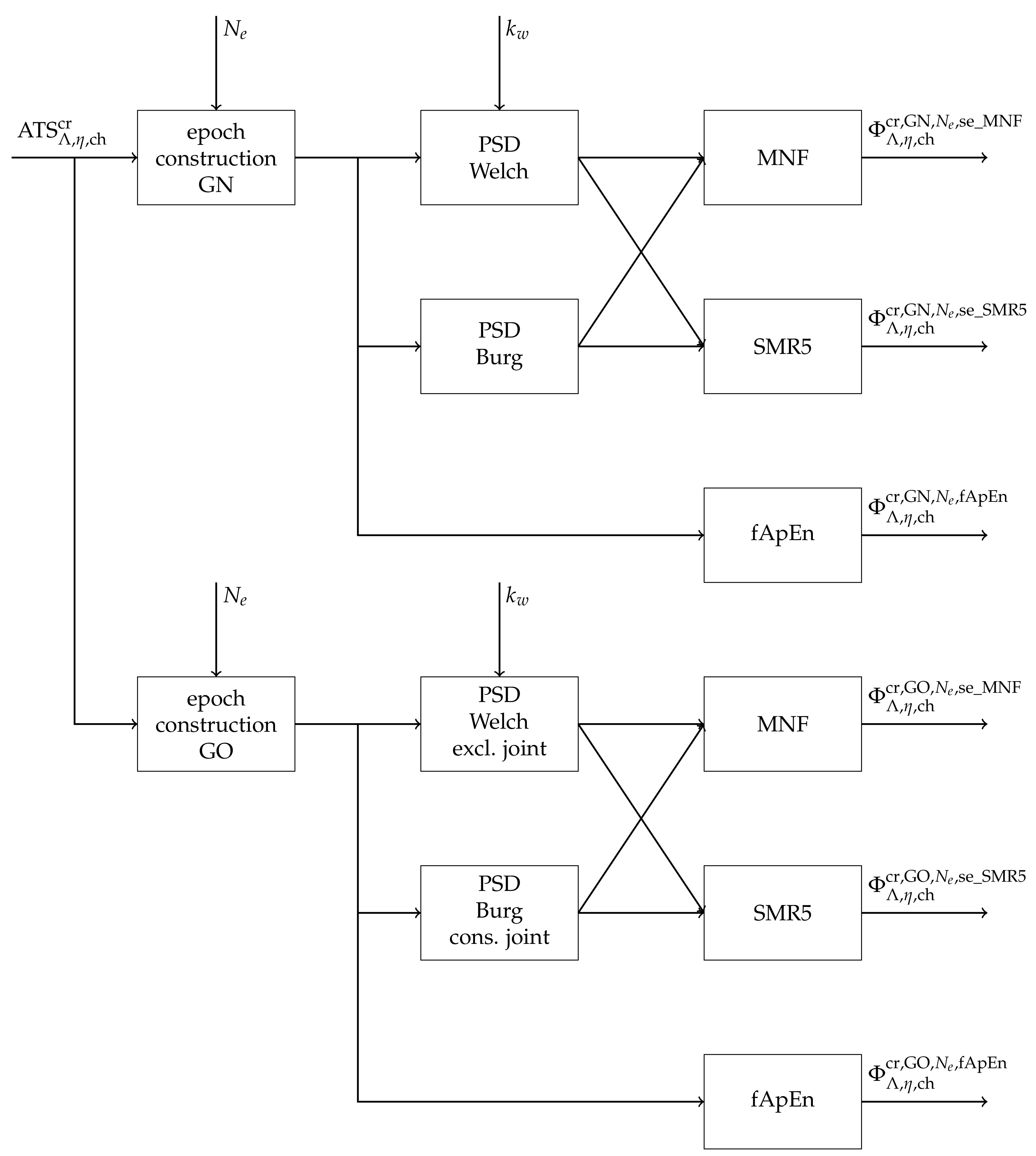
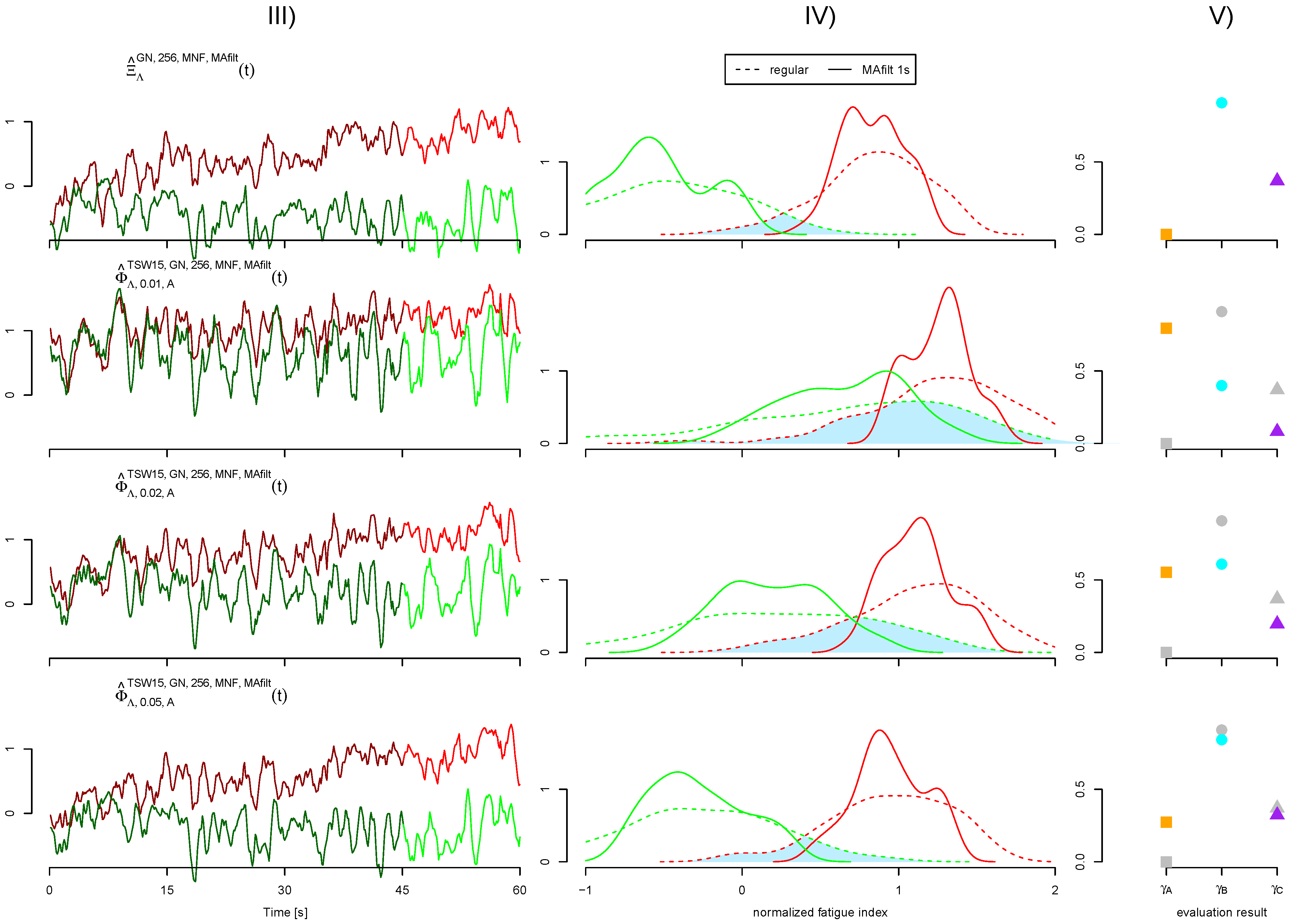
2.3. Fatigue Detection
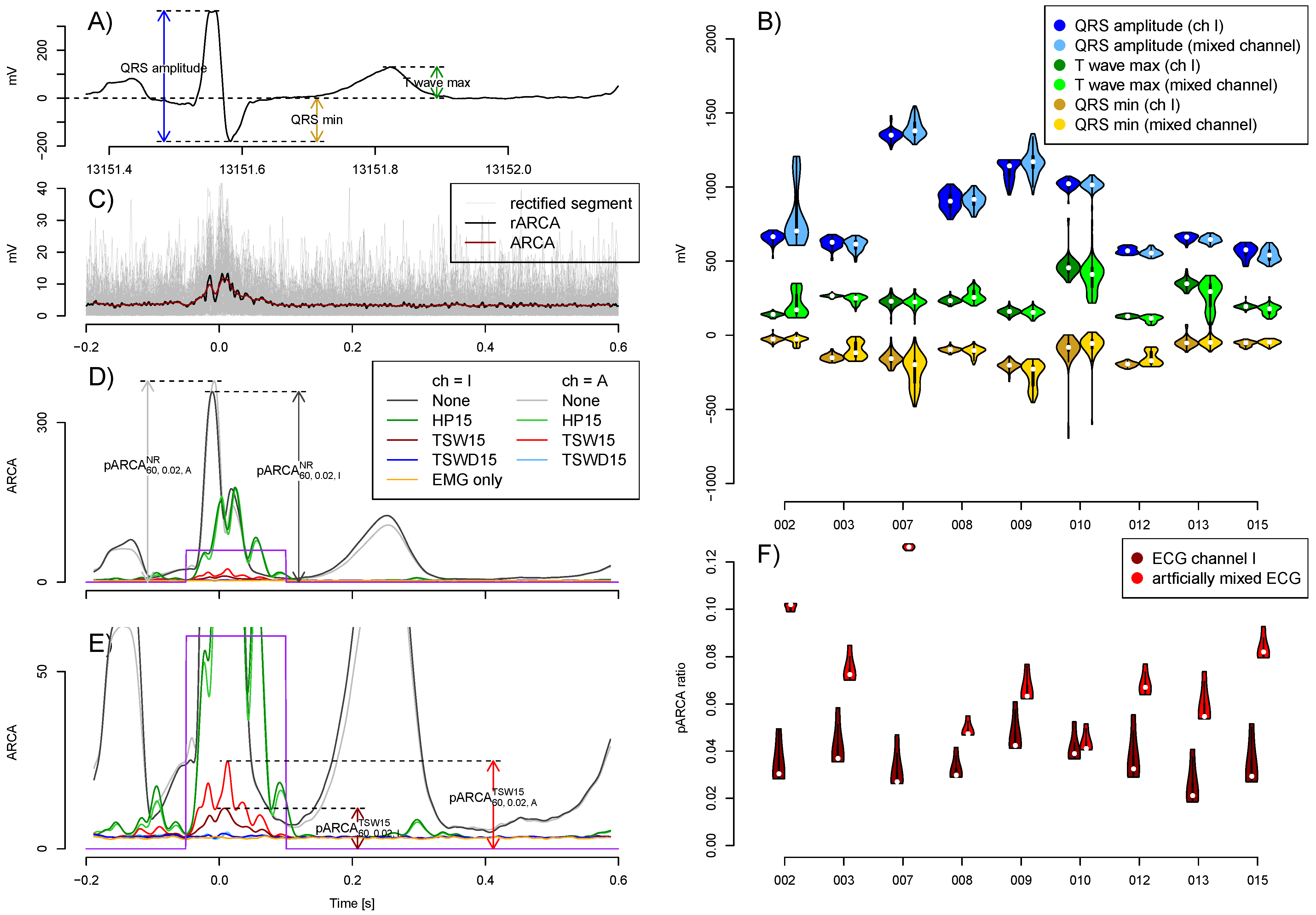
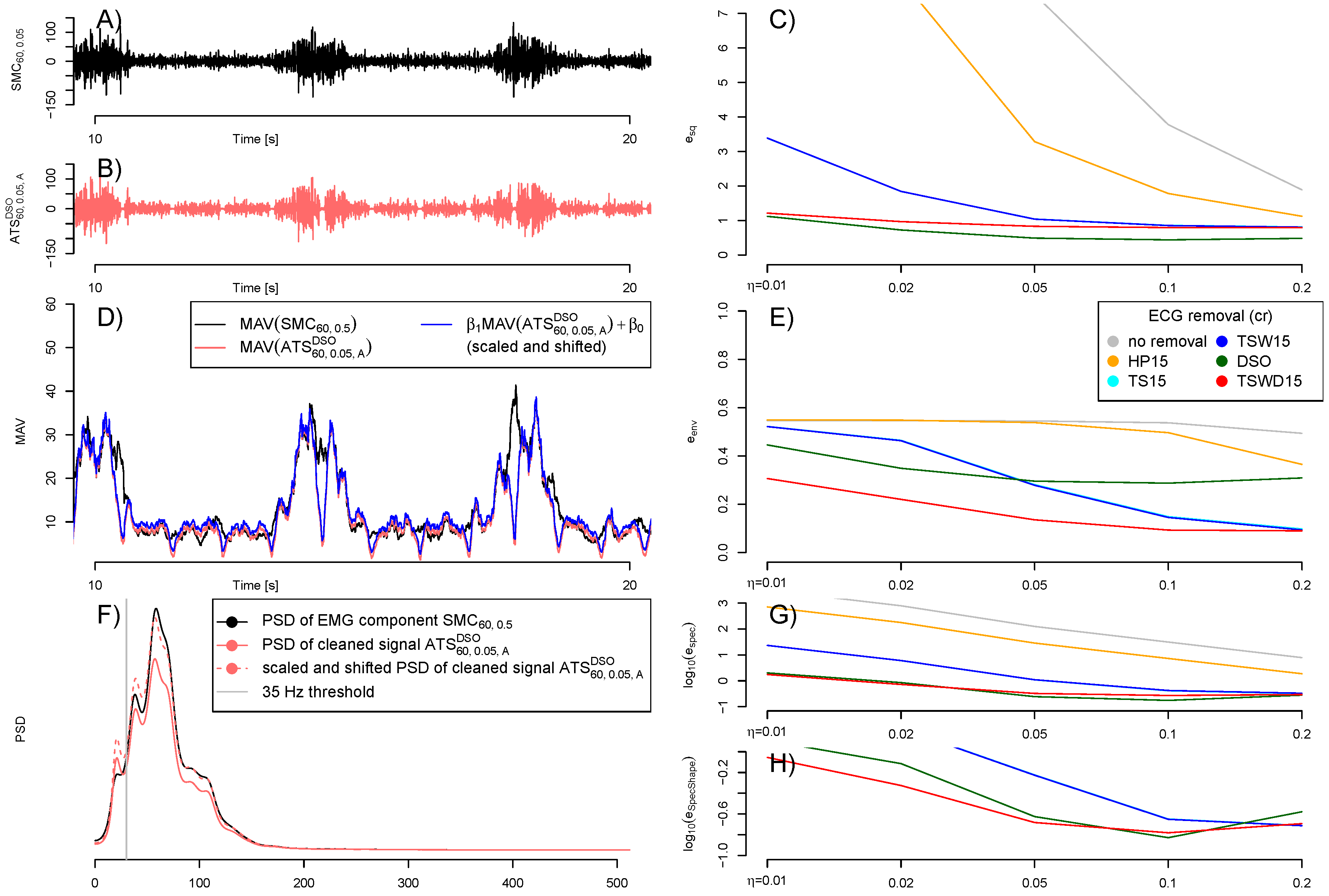
2.4. Evaluation of Quality
2.5. Statistical Analysis
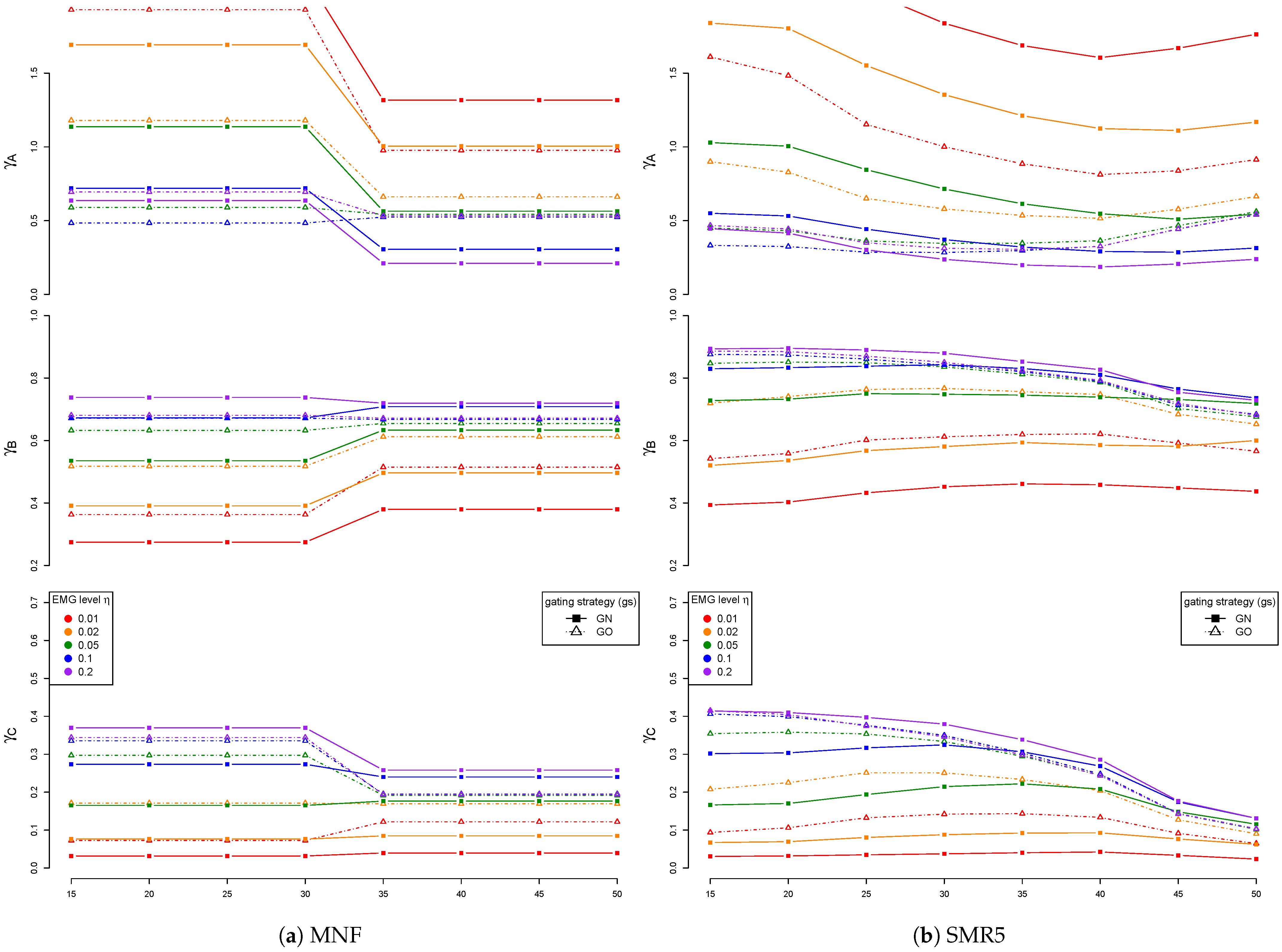
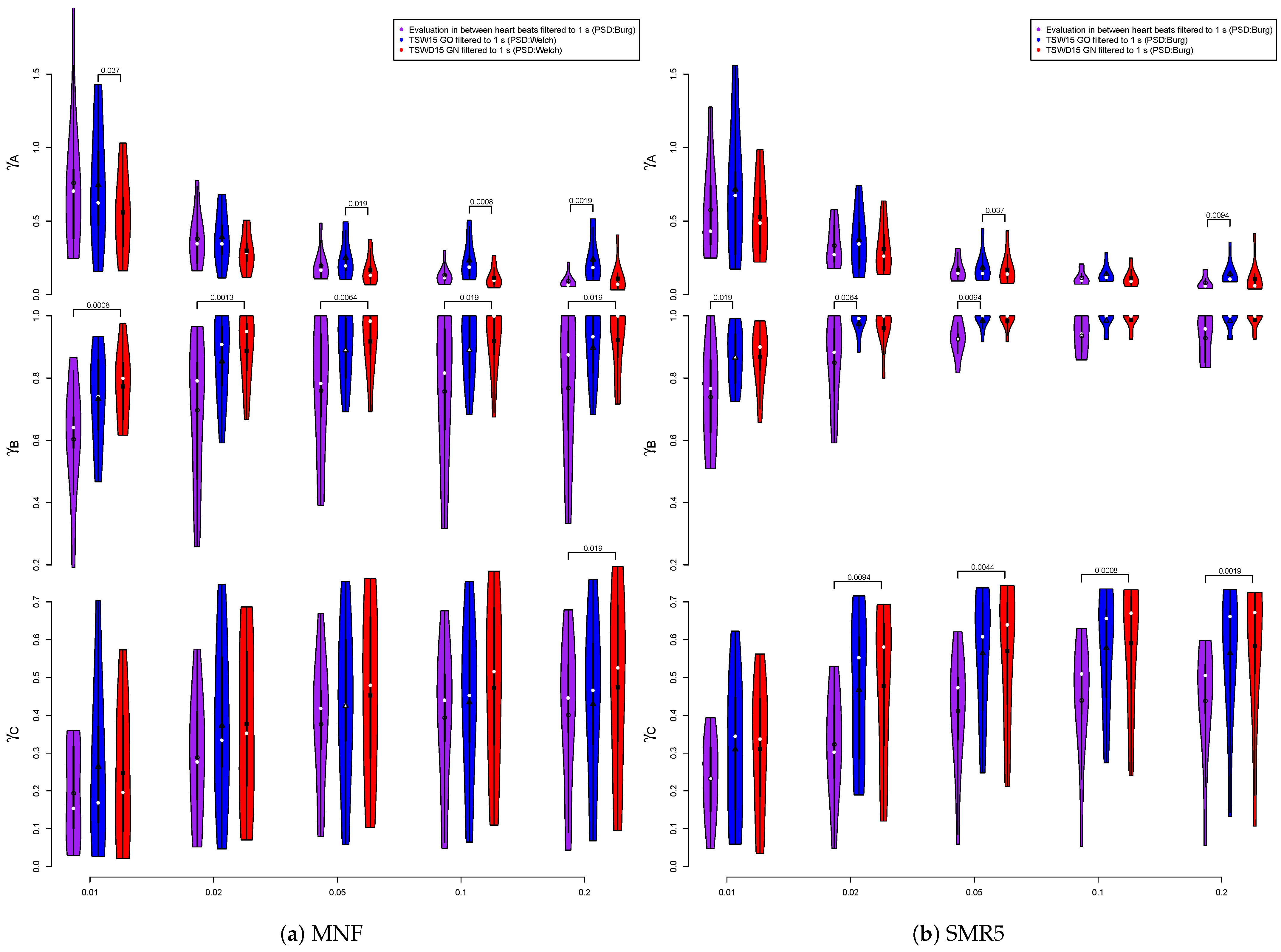
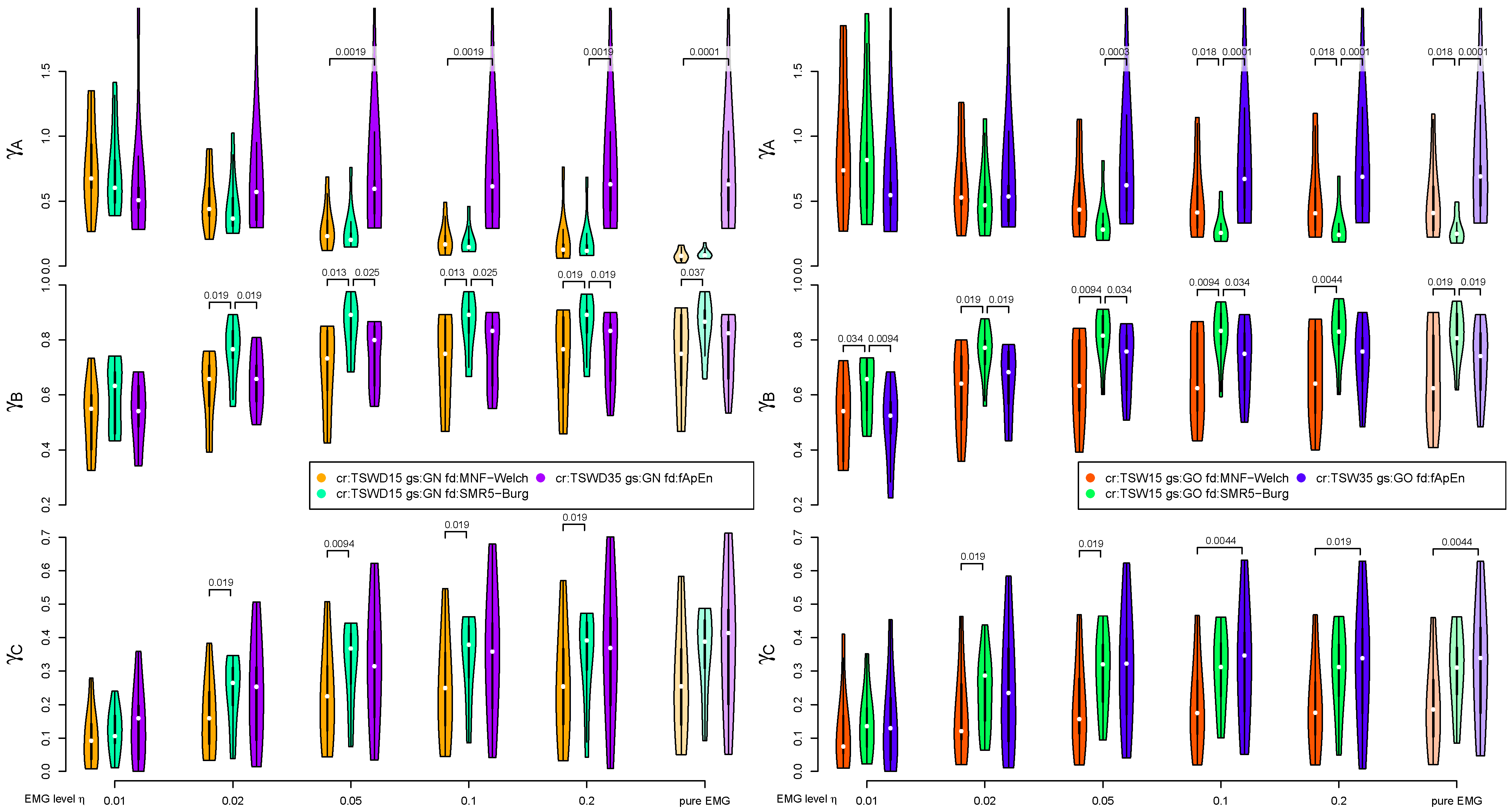
3. Results
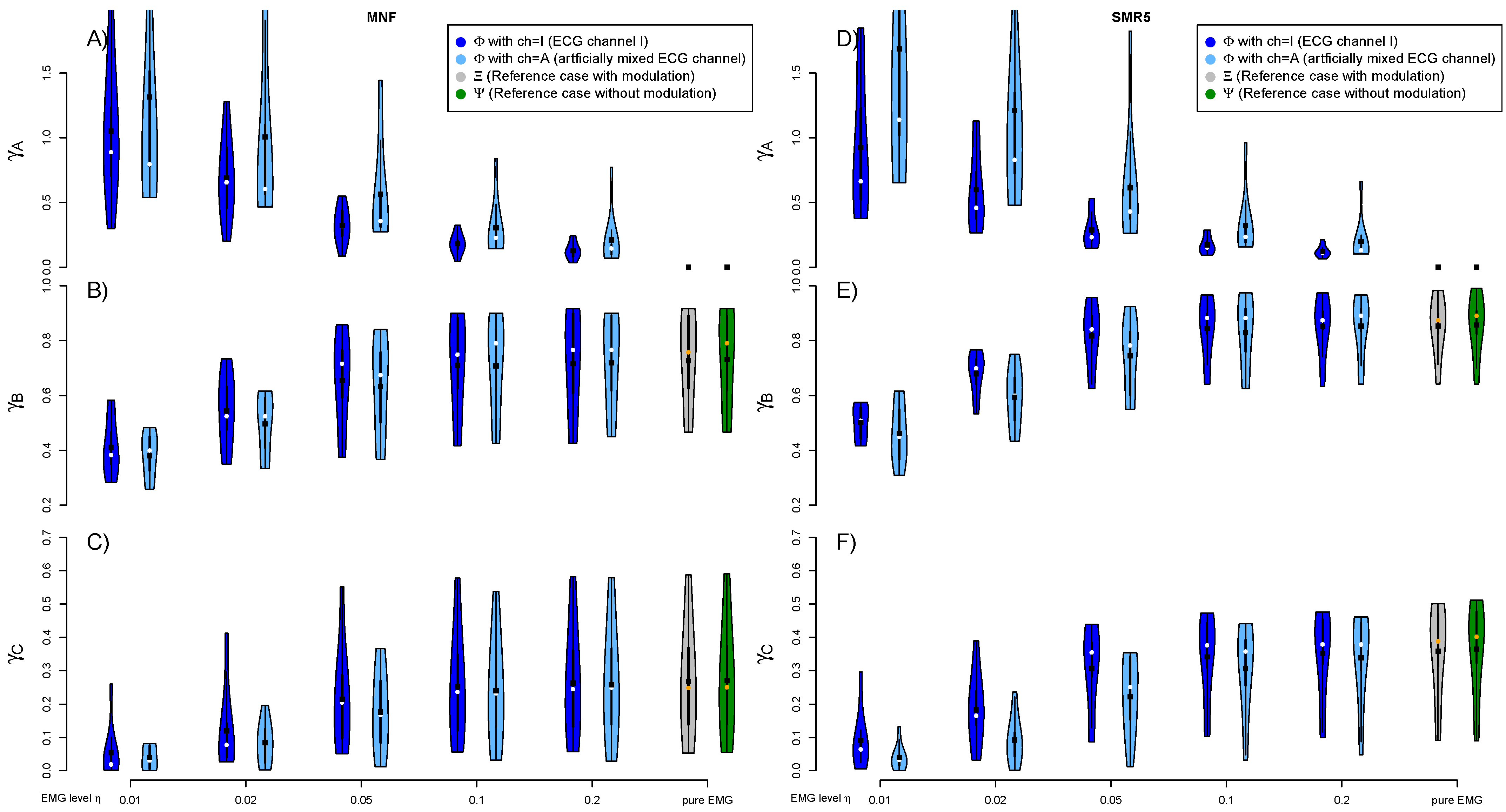
3.1. Quality of Artificial Signals
3.2. EMG Level and Artificially Mixed ECG Channel
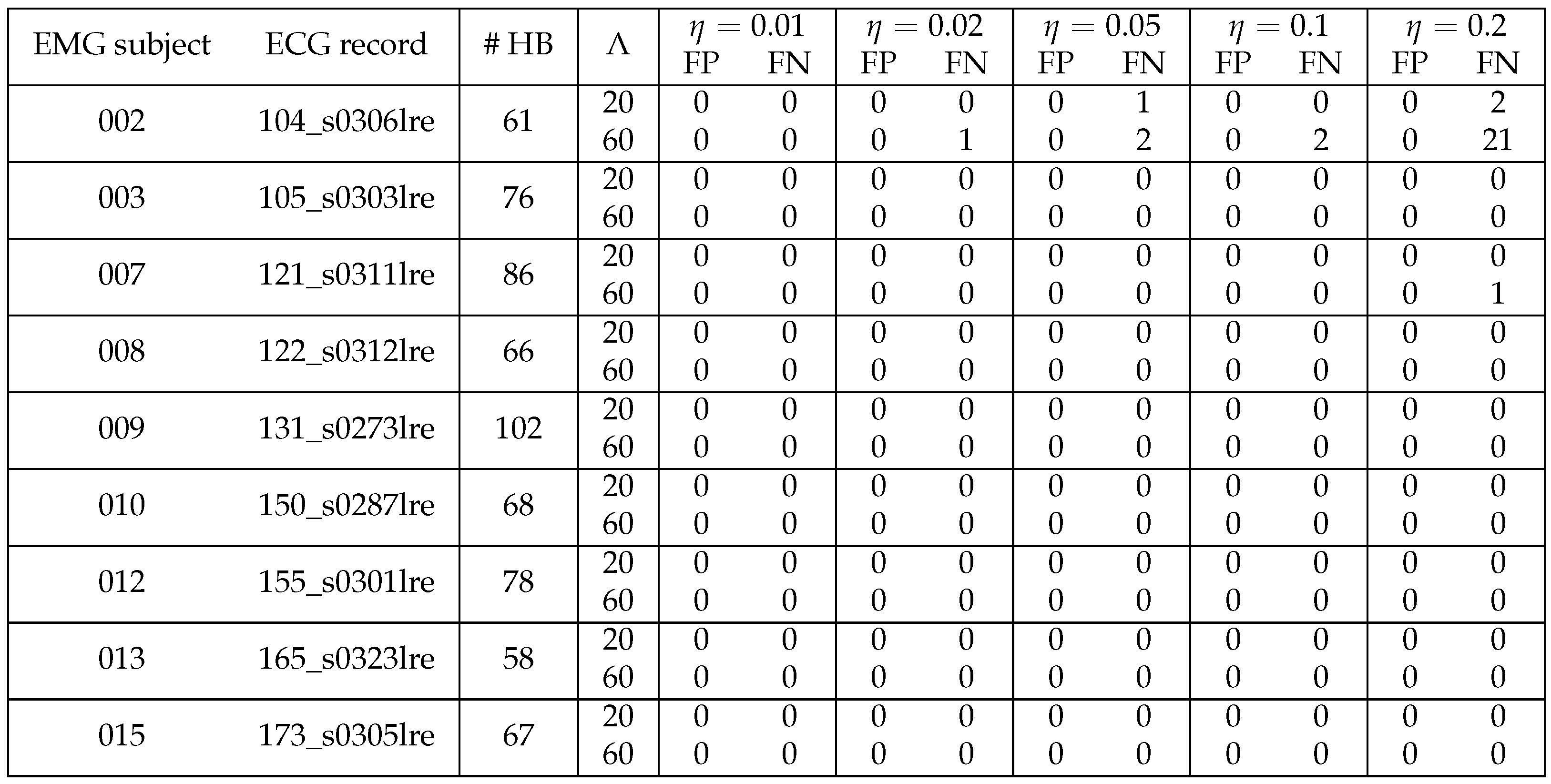
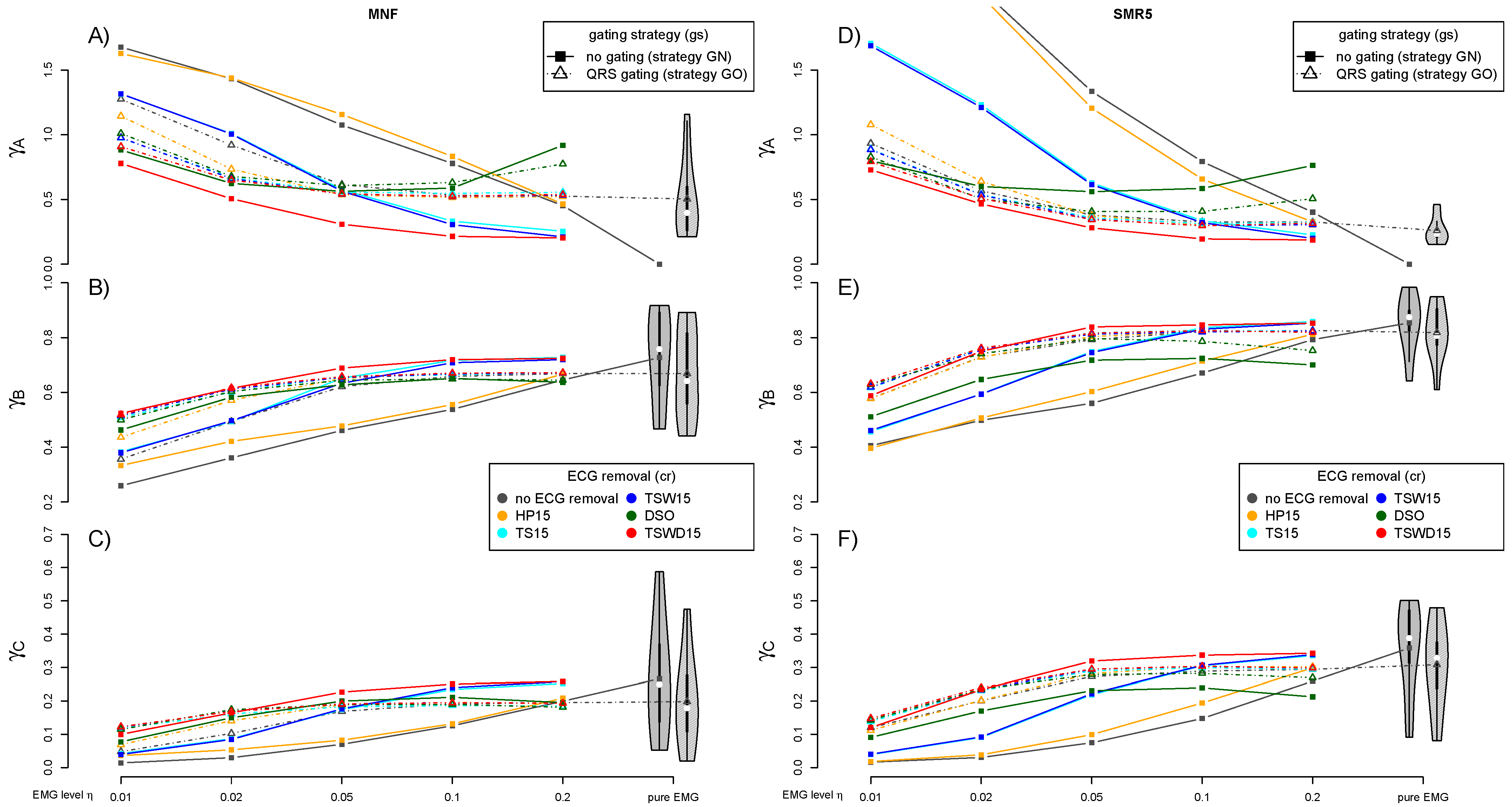
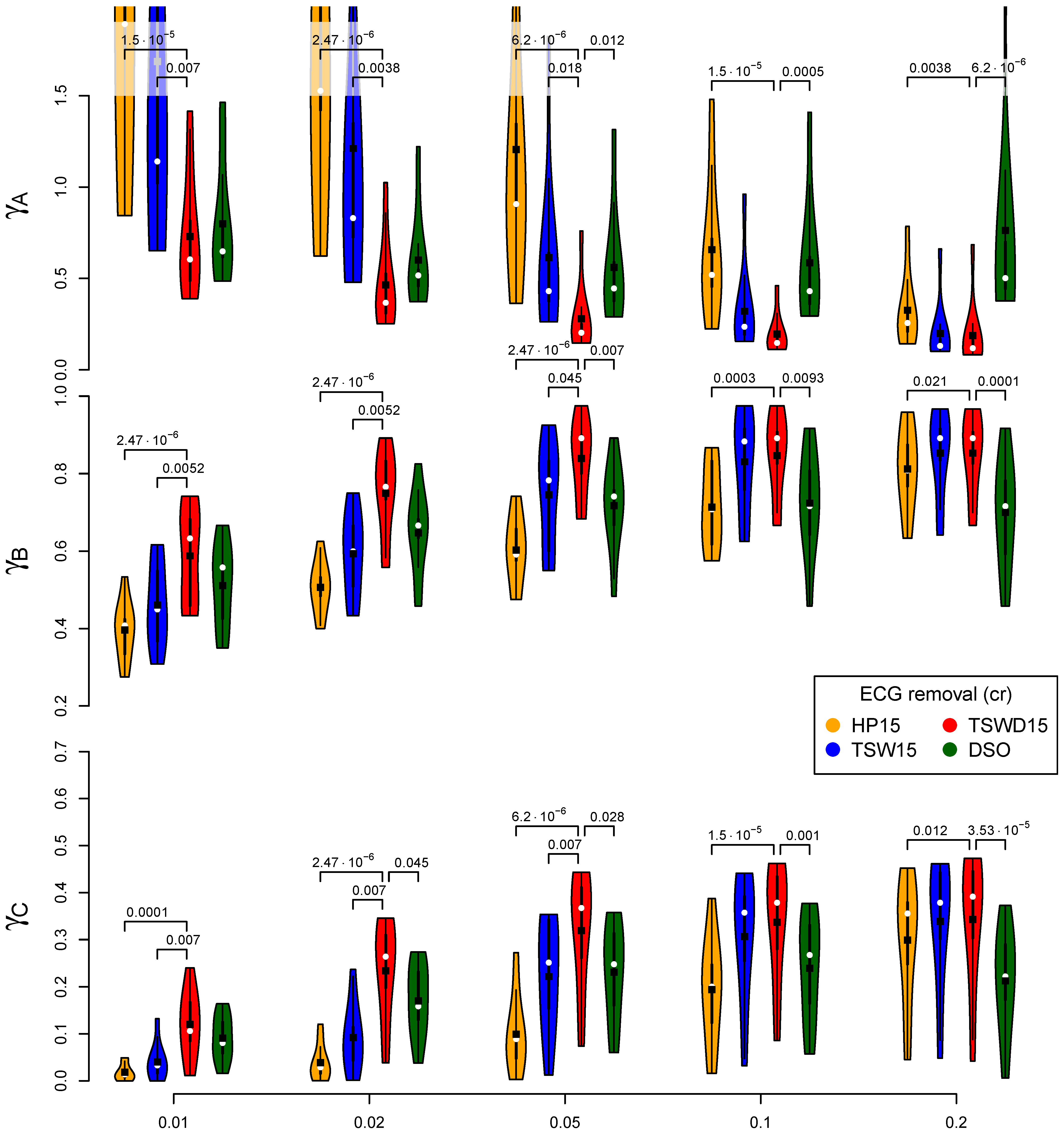
3.3. Performance of ECG Removal Algorithms
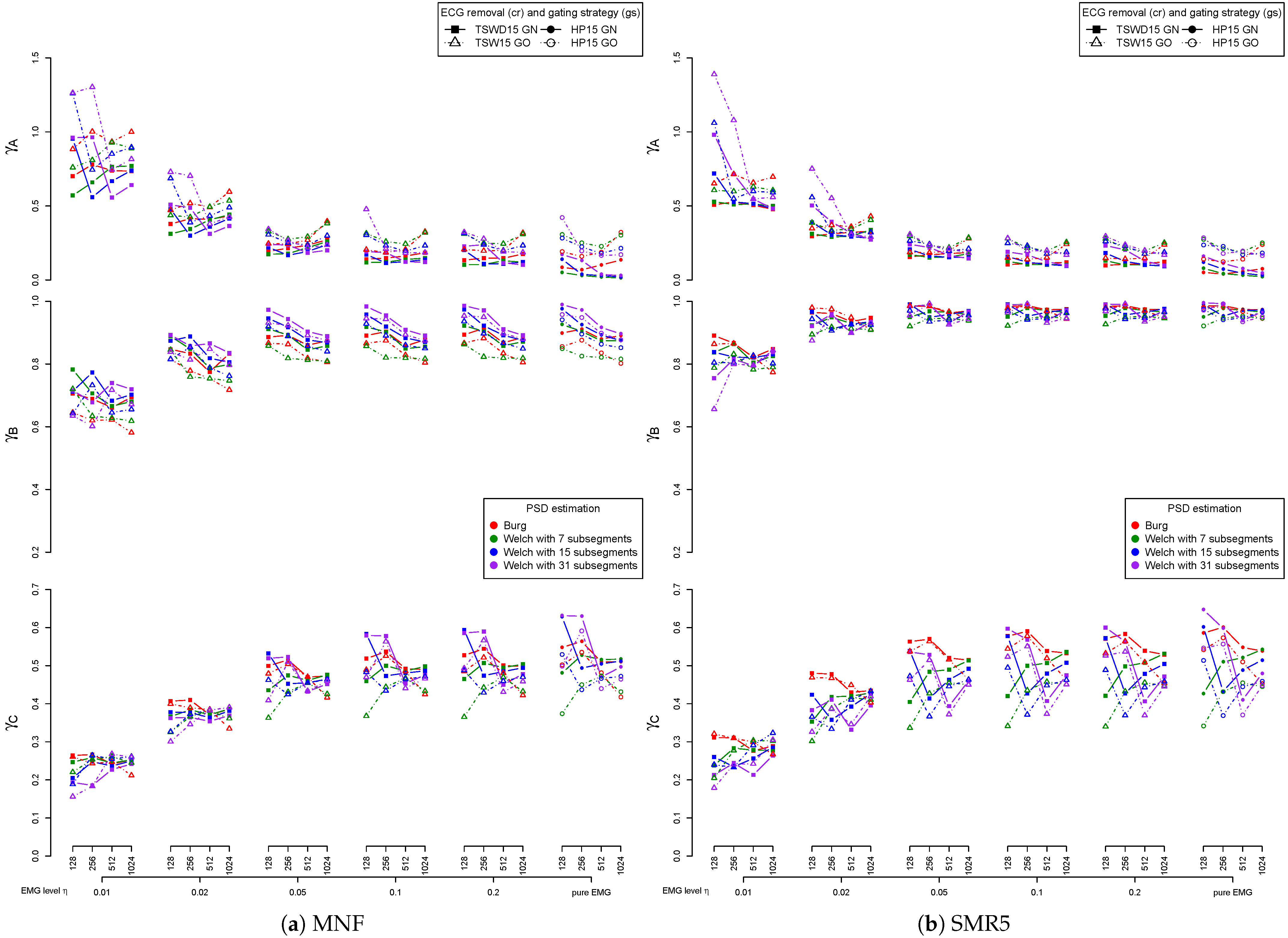
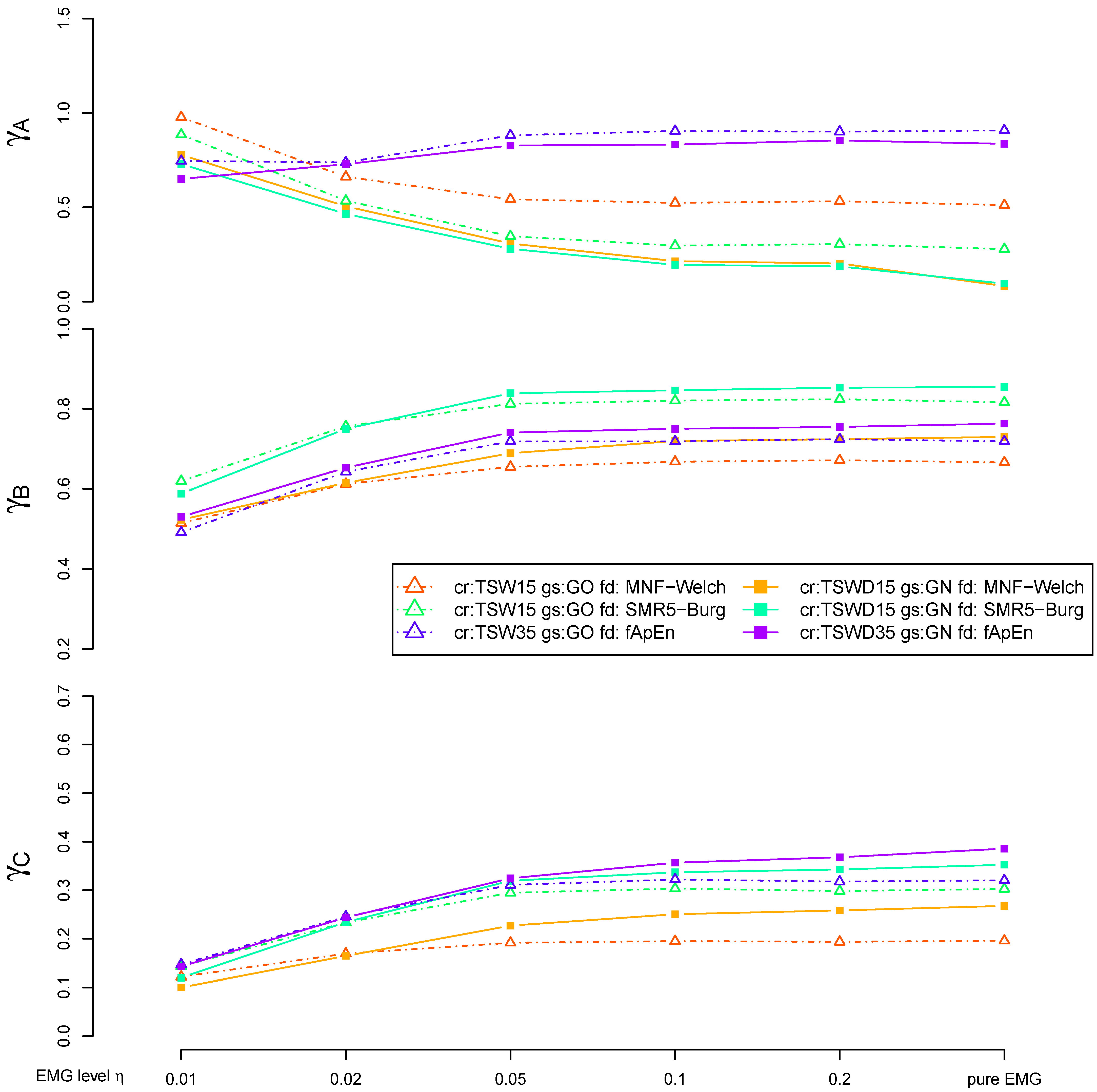
3.4. Detail Parameters for PSD-Based Fatigue Algorithms
3.5. Detail Parameters for fApEn
3.6. Fatigue Algorithms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A




References
- Piper, H.E. Über die Ermüdung bei willkürlichen Muskelkontraktionen. Arch. Anat. Physiol. 1909, 4, 491–498. [Google Scholar]
- Lindström, L.; Kadefors, R.; Petersen, I. An electromyographic index for localized muscle fatigue. J. Appl. Physiol. 1977, 43, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Stulen, F.B.; De Luca, C.J. Muscle fatigue monitor: A non-invasive device for observing localized muscular fatigue. IEEE Trans. Biomed. Eng. 1982, 29, 760–768. [Google Scholar] [CrossRef]
- Roussos, C.; Macklem, P. Diaphragmatic fatigue in man. J. Appl. Physiol. 1977, 43, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Sieck, G.C.; Mazar, A.; Belman, M.J. Changes in diaphragmatic EMG spectra during hyperpneic loads. Respir. Physiol. 1985, 61, 137–152. [Google Scholar] [CrossRef]
- Kroworsch, B.P. Untersuchung des Frequenzspektrums des Oberflächen-Elektromyogramms des Zwerchfells zum frühzeitigen Nachweis einer Muskelermüdung. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Geschwister-Scholl-Platz 1, München, Germany, 2001. [Google Scholar]
- Cohen, C.A.; Zagelbaum, G.; Gross, D.; Roussos, C.; Macklem, P.T. Clinical Manifestations of Inspiratory Muscle Fatigue. Am. J. Med. 1982, 73, 308–316. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Zakynthinos, S.; Roussos, C. Respiratory muscles and weaning failure. Eur. Respir. J. 1996, 9, 2383–2400. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Harf, A.; Lorino, H.; Lemaire, F. Inspiratory Pressure Support Prevents Diaphragmatic Fatigue during Weaning from Mechanical Ventilation. Am. Rev. Respir. Dis. 1989, 139, 513–521. [Google Scholar] [CrossRef]
- Sinderby, C.; Lindström, L.; Grassino, A.E. Automatic assessment of electromyogram quality. J. Appl. Physiol. 1995, 79, 1803–1815. [Google Scholar] [CrossRef]
- Ortega, I.C.M.; Valdivieso, A.M.H.; Lopez, J.F.A.; Ángel Mañanas Villanueva, M.; Lopez, L.H.A. Assessment of weaning indexes based on diaphragm activity in mechanically ventilated subjects after cardiovascular surgery. A pilot study. Rev. Bras. Ter. Intensiva 2017, 29, 213–221. [Google Scholar] [CrossRef]
- Bloch, R. Subtraction of electrocardiographic signal from respiratory electromyogram. J. Appl. Physiol. 1983, 55, 619–623. [Google Scholar] [CrossRef]
- Strobach, P.; Abraham-Fuchs, K.; Harer, W. Event-synchronous cancellation of the heart interference in biomedical signals. IEEE Trans. Biomed. Eng. 1994, 41, 343–350. [Google Scholar] [CrossRef]
- Ungureanu, M.; Wolf, W. Basic Aspects Concerning the Event-Synchronous Interference Canceller. IEEE Trans. Biomed. Eng. 2006, 53, 2240–2247. [Google Scholar] [CrossRef]
- Petersen, E.; Sauer, J.; Graßhoff, J.; Rostalski, P. Removing Cardiac Artifacts From Single-Channel Respiratory Electromyograms. IEEE Access 2020, 8, 30905–30917. [Google Scholar] [CrossRef]
- Kahl, L.; Eger, M.; Hofmann, U.G. Effects of sampling rate on automated fatigue recognition in surface EMG signals. Curr. Dir. Biomed. Eng. 2015, 1, 80–84. [Google Scholar] [CrossRef]
- Kahl, L.; Hofmann, U.G. Comparison of algorithms to quantify muscle fatigue in upper limb muscles based on sEMG signals. Med. Eng. Phys. 2016, 38, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.; Agostini, E. Dynamics of Breathing. In Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts, Section 3: Respiration, Volume I; Fenn, W.O., Rahn, H., Eds.; Chapter 14; American Physiological Society: Washington, DC, USA, 1964. [Google Scholar]
- Larsen, R. Beatmung: Grundlagen und Praxis; Springer: Berlin, Germany, 1997. [Google Scholar]
- Bousseljot, R.; Kreiseler, D.; Schnabel, A. Nutzung der EKG-Signaldatenbank CARDIODAT der PTB über das Internet. Biomed. Tech. Biomed. Eng. 1995, 317–318. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Awal, M.A.; Mostafa, S.; Ahmad, M. Performance Analysis of Savitzky-Golay Smoothing Filter Using ECG Signal. Int. J. Comput. Inf. Technol. 2011, 1, 24. [Google Scholar]
- Samann, F.; Schanze, T. An efficient ECG Denoising method using Discrete Wavelet with Savitzky-Golay filter. Curr. Dir. Biomed. Eng. 2019, 5, 385–387. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Bellani, G.; Bronco, A.; Marocco, S.A.; Pozzi, M.; Sala, V.; Eronia, N.; Villa, G.; Foti, G.; Tagliabue, G.; Eger, M.; et al. Measurement of Diaphragmatic Electrical Activity by Surface Electromyography in Intubated Subjects and Its Relationship with Inspiratory Effort. Respir. Care 2018, 63, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- So, C.S. Praktische EKG-Deutung; Thieme: Stuttgart, Germany, 1998. [Google Scholar]
- Kahl, L.; Hofmann, U.G. Removal of ECG artifacts from EMG signals with different artifact magnitudes by template subtraction. Curr. Dir. Biomed. Eng. 2019, 5, 357–359. [Google Scholar] [CrossRef]
- Drake, J.D.; Callaghan, J.P. Elimination of electrocardiogram contamination from electromyogram signals: An evaluation of currently used removal techniques. J. Electromyogr. Kinesiol. 2006, 16, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, S. Proposing Combined Approaches to Remove ECG Artifacts from Surface EMG Signals. Ph.D. Thesis, Mälardalen University, Västerås, Sweden, 2015. [Google Scholar]
- Guméry, P.Y.; Fontecave-Jallon, J.; Aïthocine, E.; Meignen, S.; Heyer, L.; Baconnier, P. Modified structural intensity for singularity localization in noisy signals: Application to coherent averaging for event-synchronous ECG interference cancellation in diaphragmatic EMG signals. Int. J. Adapt. Control Signal Process. 2009, 24, 424–433. [Google Scholar] [CrossRef]
- Mallat, S. A Wavelet Tour of Signal Processing: The Sparse Way; Academic Press: Burlington, MA, USA, 2009. [Google Scholar]
- Abbaspour, S.; Fallah, A. A Combination Method for Electrocardiogram Rejection from Surface Electromyogram. Open Biomed. Eng. J. 2014, 8, 13–19. [Google Scholar] [CrossRef]
- Deng, Y.; Wolf, W.; Schnell, R.; Appel, U. New aspects to event-synchronous cancellation of ECG interference: An application of the method in diaphragmatic EMG signals. IEEE Trans. Biomed. Eng. 2000, 47, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Farina, D.; Filligoi, G. Single-Channel Techniques for Information Extraction from the Surface EMG Signal. In Electromyography: Physiology, Engineering, and Noninvasive Applications; Merletti, R., Parker, P.A., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]
- Dimitrov, G.V.; Arabadzhiev, T.I.; Mileva, K.N.; Bowtell, J.L.; Crichton, N.; Dimitrova, N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc. 2006, 11, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.B.; Guo, J.Y.; Zheng, Y.P. Fuzzy approximate entropy analysis of chaotic and natural complex systems: Detecting muscle fatigue using electromyography signals. Ann. Biomed. Eng. 2010, 38, 1483–1496. [Google Scholar] [CrossRef]
- Kammeyer, K.D.; Kroschel, K. Digitale Signalverarbeitung, 7th ed.; Vieweg + Teubner: Wiesbaden, Germany, 2009. [Google Scholar]
- Sachs, L. Angewandte Statistik; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R; SAGE Publ.: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Derrac, J.; García, S.; Molina, D.; Herrera, F. A practical tutorial on the use of nonparametric statistical tests as a methodology for comparing evolutionary and swarm intelligence algorithms. Swarm Evol. Comput. 2011, 1, 3–18. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahl, L.; Hofmann, U.G. Removal of ECG Artifacts Affects Respiratory Muscle Fatigue Detection—A Simulation Study. Sensors 2021, 21, 5663. https://doi.org/10.3390/s21165663
Kahl L, Hofmann UG. Removal of ECG Artifacts Affects Respiratory Muscle Fatigue Detection—A Simulation Study. Sensors. 2021; 21(16):5663. https://doi.org/10.3390/s21165663
Chicago/Turabian StyleKahl, Lorenz, and Ulrich G. Hofmann. 2021. "Removal of ECG Artifacts Affects Respiratory Muscle Fatigue Detection—A Simulation Study" Sensors 21, no. 16: 5663. https://doi.org/10.3390/s21165663
APA StyleKahl, L., & Hofmann, U. G. (2021). Removal of ECG Artifacts Affects Respiratory Muscle Fatigue Detection—A Simulation Study. Sensors, 21(16), 5663. https://doi.org/10.3390/s21165663






