E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing
Abstract
:1. Introduction
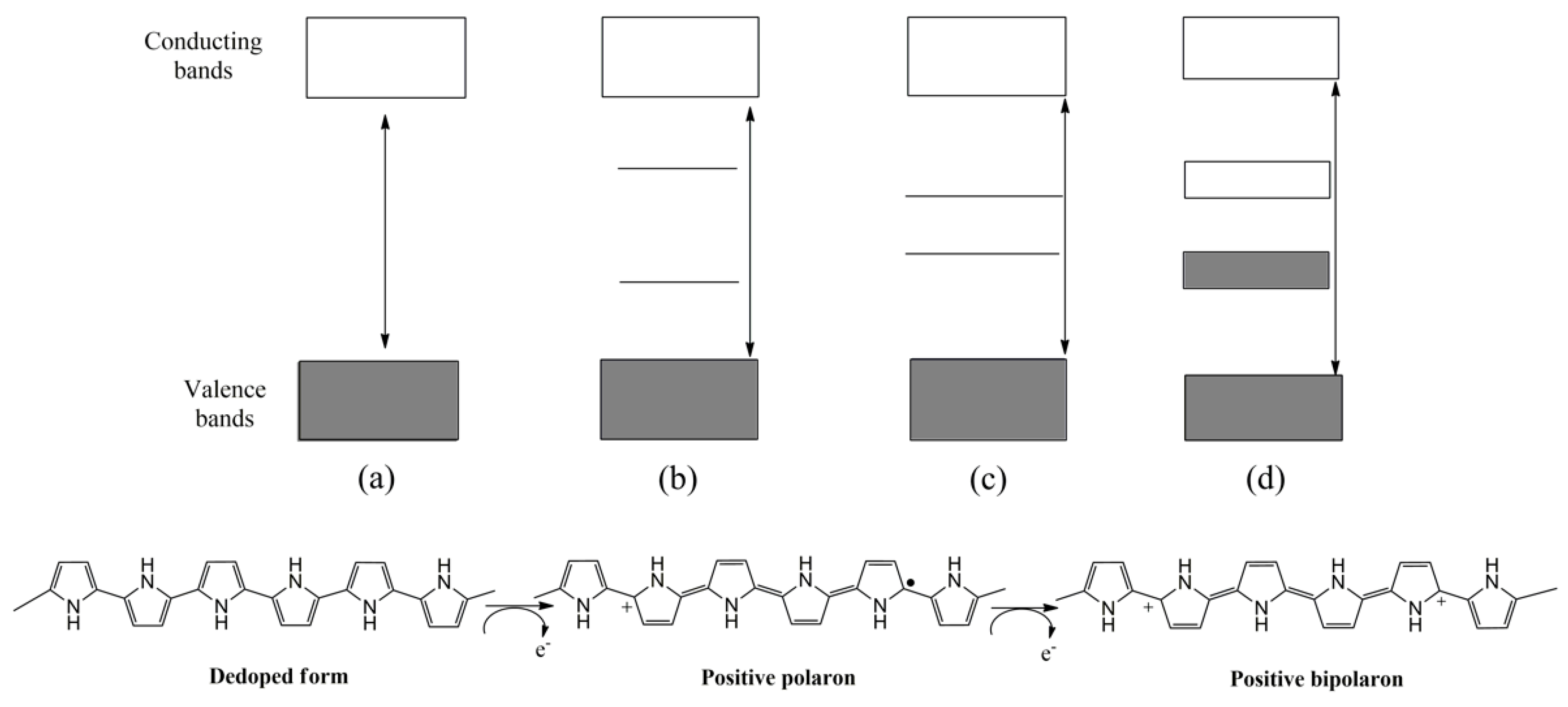
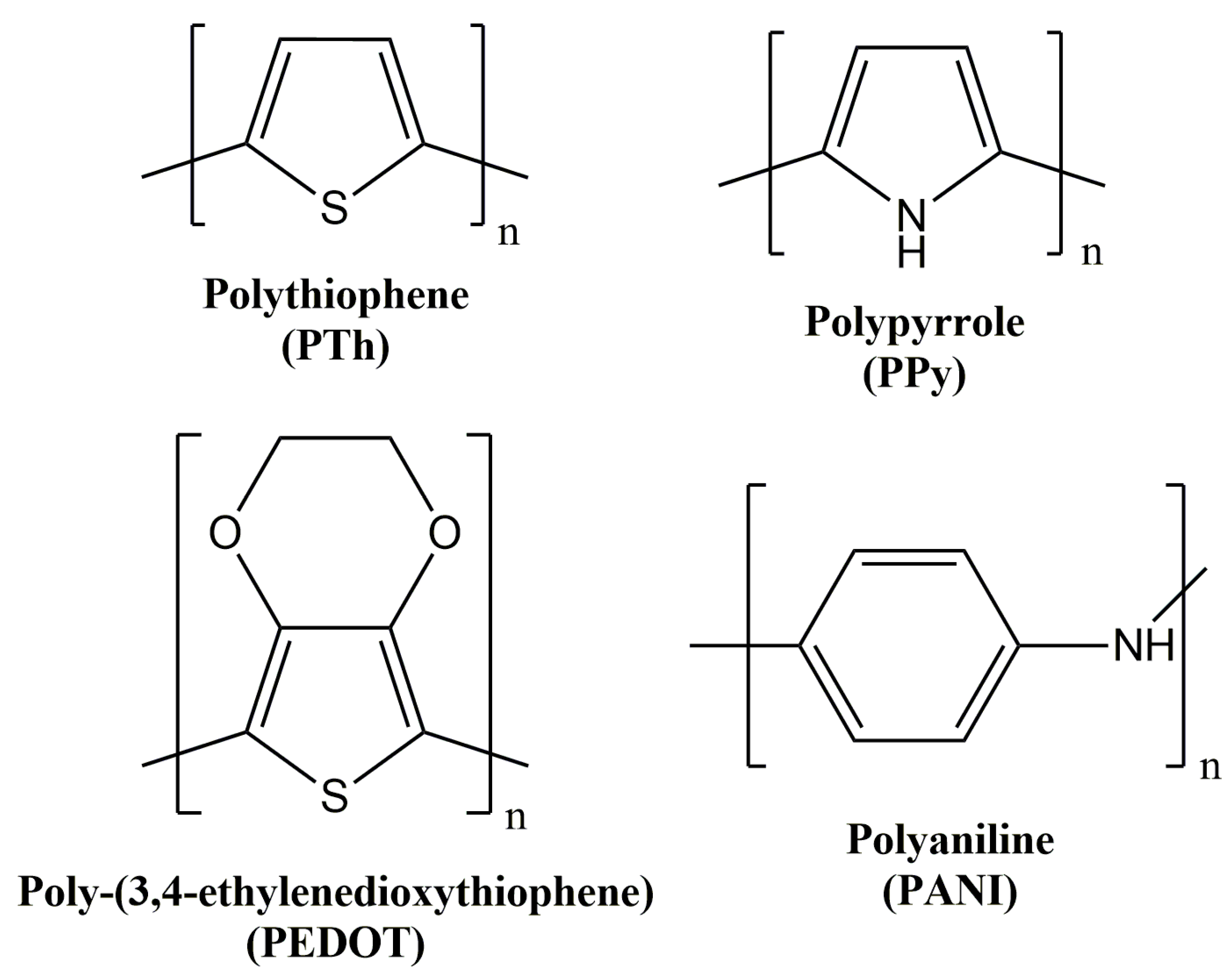
1.1. Polythiophene and Derivatives
1.2. Polyaniline
1.3. Polypyrrole
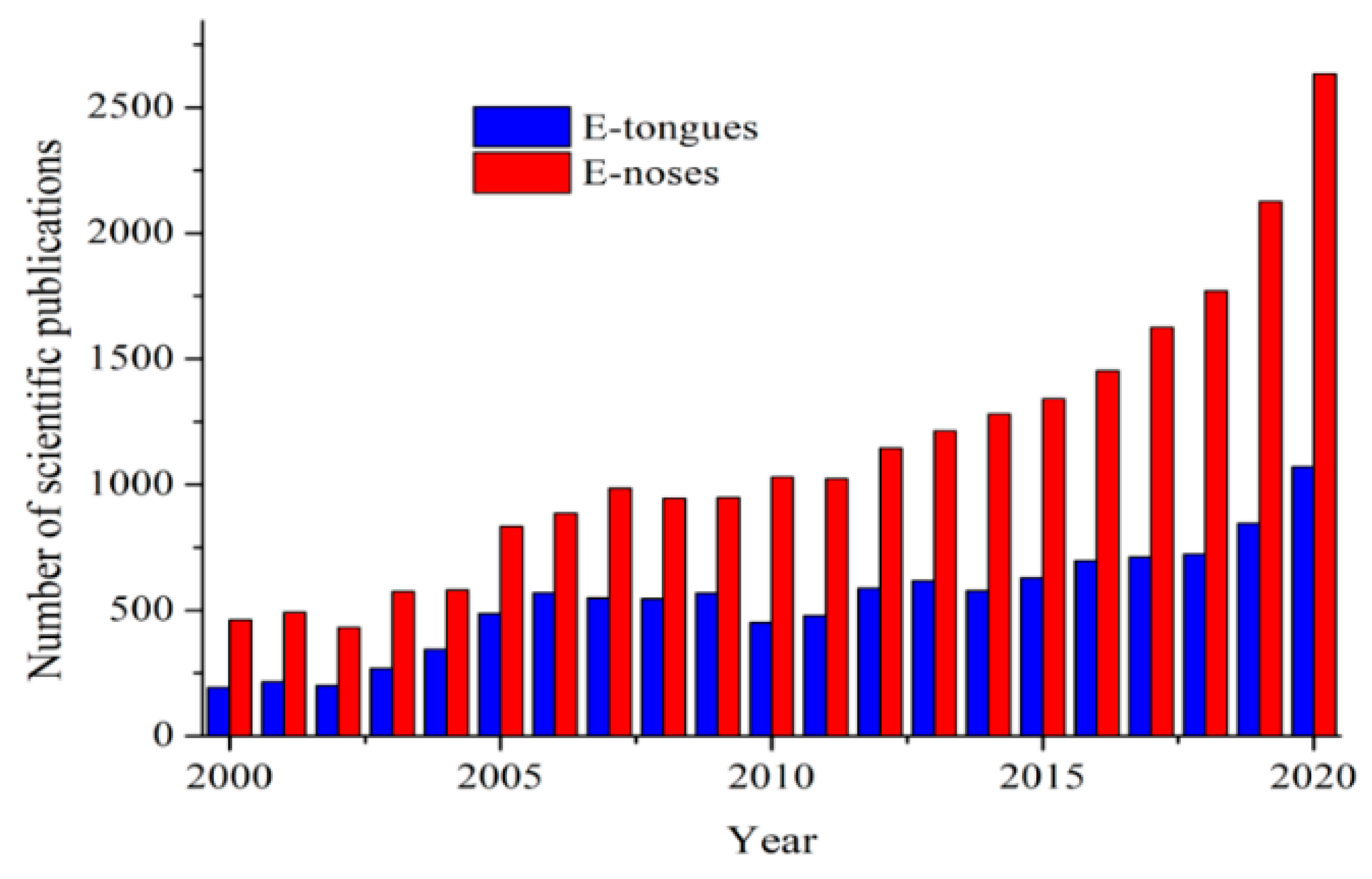
1.4. Electronic Systems: Electronic Tongues and Noses
1.4.1. Sensing Unit: Electrochemical Sensor Arrays
1.4.2. Processing of the Collected Data: Multivariate Methods
1.4.3. Novelty of the Work
2. Electronic Tongues (E-Tongues) Based on CPs
2.1. Sensing Unit: Electrochemical Sensors
2.2. Analytical Application of E-Tongues
3. Electronic Noses (E-Noses) Based on CPs
3.1. Sensing Unit: Chemiresistors
3.2. Analytical Application of E-Noses
4. Future Perspectives: Integration of E-Tongues and E-Noses in Commercial Systems
4.1. Commercial Prototypes of E-Tongues
4.2. Commercial Prototypes of E-Noses
4.3. Final Remarks: Challenges of Electrochemical/Gas Sensing Devices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hryniewicz, B.; Orth, E.; Vidotti, M. Enzymeless PEDOT-based electrochemical sensor for the detection of nitrophenols and organophosphates. Sens. Actuators B Chem. 2018, 257, 570–578. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting polymers as chemiresistive gas sensing materials: A review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, Y.; Xin, Q.; Jiang, C.; Song, A. Electrical control of the optical dielectric properties of PEDOT:PSS thin films. Opt. Mater. 2020, 108, 110435. [Google Scholar] [CrossRef]
- Ahad, I.Z.M.; Harun, S.W.; Gan, S.N.; Phang, S.W. Polyaniline (PAni) optical sensor in chloroform detection. Sens. Actuators B Chem. 2018, 261, 97–105. [Google Scholar] [CrossRef]
- Alqarni, S.A.; Hussein, M.A.; Ganash, A.A.; Khan, A. Composite material–based conducting polymers for electrochemical sensor applications: A mini review. BioNanoScience 2020, 10, 351–364. [Google Scholar] [CrossRef]
- Greco, F.; Zucca, A.; Taccola, S.; Menciassi, A.; Fujie, T.; Haniuda, H.; Takeoka, S.; Dario, P.; Mattoli, V. Ultra-Thin conductive free-standing PEDOT/PSS nanofilms. Soft Matter 2011, 7, 10642–10650. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Chen, S.; Zhou, Y.; Wei, Z.; Wang, H.; Zheng, Y.; Li, M.; Sun, K.; Li, Y. Unsubstituted polythiophene film deposited via In-Situ sequential solution polymerization for chemo-/electrochromism. Macromolecules 2020, 53, 4247–4254. [Google Scholar] [CrossRef]
- Chen, R.; Sun, K.; Zhang, Q.; Zhou, Y.; Li, M.; Sun, Y.; Wu, Z.; Wu, Y.; Li, X.; Xi, J.; et al. Sequential solution polymerization of poly(3,4-ethylenedioxythiophene) using V2O5 as oxidant for flexible touch sensors. iScience 2019, 12, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Tsakova, V.; Seeber, R. Conducting polymers in electrochemical sensing: Factors influencing the electroanalytical signal. Anal. Bioanal. Chem. 2016, 408, 7231–7241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Dai, K.; Liu, C.; Shen, C. Flexible conductive polymer composites for smart wearable strain sensors. SmartMat 2020, 1. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, C.; Zhang, S.; Gao, J.; Liu, M.; Xie, K.; Wang, S.; Sun, K.; Song, H. High performance electrochemical electrode based on polymeric composite film for sensing of dopamine and catechol. Sens. Actuators B Chem. 2018, 255, 1655–1662. [Google Scholar] [CrossRef]
- Mishra, A.K. Conducting polymers: Concepts and applications. J. At. Mol. Condens. Nano Phys. 2018, 5, 159–193. [Google Scholar] [CrossRef] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Tertis, M.; Florea, A.; Săndulescu, R.; Cristea, C. Carbon based electrodes modified with horseradish peroxidase immobilized in conducting polymers for acetaminophen analysis. Sensors 2013, 13, 4841–4854. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, H.B.; Çalışkan, S.; Kamaci, M.; Caliskan, A.; Yilmaz, H. l-Dopa synthesis catalyzed by tyrosinase immobilized in poly(ethyleneoxide) conducting polymers. Int. J. Biol. Macromol. 2013, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jugović, B.; Grgur, B.; Antov, M.; Knežević-Jugović, Z.; Stevanović, J.; Gvozdenović, M. Polypyrrole-Based enzyme electrode with immobilized glucose oxidase for electrochemical determination of glucose. Int. J. Electrochem. Sci. 2016, 11, 1152–1161. [Google Scholar]
- Pathiranage, T.M.S.K.; Dissanayake, D.S.; Niermann, C.N.; Ren, Y.; Biewer, M.C.; Stefan, M.C. Role of polythiophenes as electroactive materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3327–3346. [Google Scholar] [CrossRef] [Green Version]
- Groenendaal, L.; Zotti, G.; Aubert, P.-H.; Waybright, S.; Reynolds, J. Electrochemistry of poly(3,4-alkylenedioxythiophene) derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Zanardi, C.; Terzi, F.; Seeber, R. Polythiophenes and polythiophene-based composites in amperometric sensing. Anal. Bioanal. Chem. 2012, 405, 509–531. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, H.; Li, M.; Li, C.; Dai, H.; Sun, D.; Yang, B. Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sens. Actuators B Chem. 2018, 259, 433–442. [Google Scholar] [CrossRef]
- Guzmán, J.J.G.; Aguilera, L.C.; Milla, D.B.; Rodríguez, I.N.; Lete, C.; Palacios-Santander, J.M.; Lupu, S. Development of Sonogel-Carbon based biosensors using sinusoidal voltages and currents methods. Sens. Actuators B Chem. 2018, 255, 1525–1535. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C.; Lakard, B.; Hihn, J.-Y.; Del Campo, F.J. Development of amperometric biosensors based on nanostructured tyrosinase-conducting polymer composite electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [Green Version]
- Çetin, M.Z.; Camurlu, P. An amperometric glucose biosensor based on PEDOT nanofibers. RSC Adv. 2018, 8, 19724–19731. [Google Scholar] [CrossRef] [Green Version]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.; Lim, H. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Gao, X.; Wang, Z.; Wang, S. The electrochemical activity of polyaniline: An important issue on its use in electrochemical energy storage devices. Synth. Met. 2014, 187, 46–51. [Google Scholar] [CrossRef]
- Kashyap, R.; Kumar, R.; Kumar, M.; Tyagi, S.; Kumar, D. Polyaniline nanofibers based gas sensor for detection of volatile organic compounds at room temperature. Mater. Res. Express 2019, 6, 1150d3. [Google Scholar] [CrossRef]
- Kelly, F.M.; Meunier, L.; Cochrane, C.; Koncar, V. Polyaniline: Application as solid state electrochromic in a flexible textile display. Displays 2013, 34, 1–7. [Google Scholar] [CrossRef]
- Nate, Z.; Gill, A.A.; Chauhan, R.; Karpoormath, R. Polyaniline-cobalt oxide nanofibers for simultaneous electrochemical determination of antimalarial drugs: Primaquine and proguanil. Microchem. J. 2021, 160, 105709. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, W.; Yan, Y.; Wang, Y.; Zhang, J. Core-Shell self-doped polyaniline coated metal-organic-framework (SPAN@UIO-66-NH2) screen printed electrochemical sensor for Cd2+ ions. J. Electrochem. Soc. 2019, 166, B873–B880. [Google Scholar] [CrossRef]
- Sanchis, C.; Ghanem, M.; Salavagione, H.; Morallón, E.; Bartlett, P. The oxidation of ascorbate at copolymeric sulfonated poly(aniline) coated on glassy carbon electrodes. Bioelectrochemistry 2011, 80, 105–113. [Google Scholar] [CrossRef]
- Masdarolomoor, F.; Hajizadeh, S.; Chamjangali, M.A.; Innis, P.C. Novel approach to the synthesis of polyaniline possessing electroactivity at neutral pH. Synth. Met. 2019, 250, 121–130. [Google Scholar] [CrossRef]
- Ullah, H.; Shah, A.-u.-H.A.; Bilal, S.; Ayub, K. Doping and dedoping processes of polypyrrole: DFT study with hybrid functionals. J. Phys. Chem. C 2014, 118, 17819–17830. [Google Scholar] [CrossRef]
- Camurlu, P. Polypyrrole derivatives for electrochromic applications. RSC Adv. 2014, 4, 55832–55845. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Maksymiuk, K. Chemical reactivity of polypyrrole and its relevance to polypyrrole based electrochemical sensors. Electroanalysis 2006, 18, 1537–1551. [Google Scholar] [CrossRef]
- Cete, S.; Ozyurt, M.; Yildirim, E.; Akin, D. A novel biosensor with the use of polypyrrole–poly(sodium-4-styrenesulphonate) as a dopant in the determination of glucose. Chem. Pap. 2020, 74, 799–808. [Google Scholar] [CrossRef]
- Ayenimo, J.G.; Adeloju, S.B. Amperometric detection of glucose in fruit juices with polypyrrole-based biosensor with an integrated permselective layer for exclusion of interferences. Food Chem. 2017, 229, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, L.F.; Thesing, A.; Demingos, P.; de Albuquerque, C.D.; Rodrigues, R.S.; Brolo, A.G.; Santos, J.F. Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide. Sens. Actuators B Chem. 2021, 339, 129875. [Google Scholar] [CrossRef]
- De Morais, T.C.B.; Rodrigues, D.R.; de Carvalho Polari Souto, U.T.; Lemos, S.G. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef]
- Podrażka, M.; Bączyńska, E.; Kundys, M.; Jeleń, P.S.; Nery, E.W. Electronic tongue—A tool for all tastes? Biosensors 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Sobrino-Gregorio, L.; Bataller, R.; Soto, J.; Escriche, I. Monitoring honey adulteration with sugar syrups using an automatic pulse voltammetric electronic tongue. Food Control 2018, 91, 254–260. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Ayuso, J.; Álvarez, J.A.; Palma, M.; Barroso, C.G. An electronic nose based method for the discrimination of weathered petroleum-derived products. Sensors 2018, 18, 2180. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Łagód, G.; Duda, S.M.; Majerek, D.; Szutt, A.; Dołhańczuk-Śródka, A. Application of electronic nose for evaluation of wastewater treatment process effects at full-scale WWTP. Processes 2019, 7, 251. [Google Scholar] [CrossRef] [Green Version]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: A review. J. Nanostruct. Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Technol. Mater. 2021, 60, 504–521. [Google Scholar] [CrossRef]
- Dakshayini, B.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Lu, L. Electrochemical sensor based on poly(3,4-ethylenedioxy—Thiophene) doped with transition metals for detecting rutin in buck wheat tea. Int. J. Electrochem. Sci. 2018, 2126–2135. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting polymers in the fields of energy, environmental remediation, and chemical–chiral sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Vasantha, V.; Chen, S.-M. Electrocatalysis and simultaneous detection of dopamine and ascorbic acid using poly(3,4-ethylenedioxy)thiophene film modified electrodes. J. Electroanal. Chem. 2006, 592, 77–87. [Google Scholar] [CrossRef]
- Revin, S.B.; John, S.A. Simultaneous determination of two important dopamine metabolites at physiological pH by voltammetry. Anal. Methods 2012, 4, 348–352. [Google Scholar] [CrossRef]
- Revin, S.B.; John, A. Electrochemical sensor for neurotransmitters at physiological pH using a heterocyclic conducting polymer modified electrode. Analyst 2011, 137, 209–215. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Zhang, Q.; Li, F. Simultaneous electrochemical determination of dopamine and ascorbic acid using AuNPs@polyaniline core–shell nanocomposites modified electrode. Talanta 2012, 89, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.R.; Okajima, T.; Ohsaka, T. Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N,N-dimethylaniline)-modified electrodes. Bioelectrochemistry 2003, 59, 11–19. [Google Scholar] [CrossRef]
- Wan, J.; Si, Y.; Li, C.; Zhang, K. Bisphenol A electrochemical sensor based on multi-walled carbon nanotubes/polythiophene/Pt nanocomposites modified electrode. Anal. Methods 2016, 8, 3333–3338. [Google Scholar] [CrossRef]
- Ramachandran, T.; Dhayabaran, V.V. Utilization of a MnO2/polythiophene/rGO nanocomposite modified glassy carbon electrode as an electrochemical sensor for methyl parathion. J. Mater. Sci. Mater. Electron. 2019, 30, 12315–12327. [Google Scholar] [CrossRef]
- Saljooqi, A.; Shamspur, T.; Mostafavi, A. The electrochemical sensor based on graphene oxide nanosheets decorated by gold nanoparticles and polythiophene for nicotine sensing in biological samples and cigarette. J. Mater. Sci. Mater. Electron. 2020, 31, 5471–5477. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Bahrani, S.; Ramakrishna, S. Polythiophene silver bromide nanostructure as ultra-sensitive non-enzymatic electrochemical glucose biosensor. Eur. Polym. J. 2020, 138, 109959. [Google Scholar] [CrossRef]
- GunaVathana, S.D.; Thivya, P.; Wilson, J.; Peter, A.C. Sensitive voltammetric sensor based on silver dendrites decorated polythiophene nanocomposite: Selective determination of L-Tryptophan. J. Mol. Struct. 2020, 1205, 127649. [Google Scholar] [CrossRef]
- Song, Z.; Sheng, G.; Cui, Y.; Li, M.; Song, Z.; Ding, C.; Luo, X. Low fouling electrochemical sensing in complex biological media by using the ionic liquid-doped conducting polymer PEDOT: Application to voltammetric determination of dopamine. Microchim. Acta 2019, 186, 220. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, J.; Zuo, Y.; Li, Y.; Zhang, J.; Zhou, Y.; Duan, X.; Lu, L.; Jia, H.; Xu, Q.; et al. Three-dimensional PEDOT composite based electrochemical sensor for sensitive detection of chlorophenol. J. Electroanal. Chem. 2019, 837, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Chen, Z.; Song, Z.; Luo, X. Electrochemical determination of paracetamol based on Au@graphene core-shell nanoparticles doped conducting polymer PEDOT nanocomposite. Sens. Actuators B Chem. 2018, 260, 778–785. [Google Scholar] [CrossRef]
- Wu, L.-N.; Zhong, J.-P.; Waqas, M.; Jiang, Z.; Fan, Y.-J.; Sun, Y.; Li, J.; Chen, W. Controllable synthesis of six corner star-like Cu2O/PEDOT-MWCNT composites and their performance toward electrochemical glucose sensing. Electrochim. Acta 2019, 318, 837–846. [Google Scholar] [CrossRef]
- Bottari, D.; Pigani, L.; Zanardi, C.; Terzi, F.; Paţurcă, S.V.; Grigorescu, S.D.; Matei, C.; Lete, C.; Lupu, S. Electrochemical sensing of caffeic acid using gold nanoparticles embedded in poly(3,4-ethylenedioxythiophene) layer by sinusoidal voltage procedure. Chemosensors 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, J.M.; Milla, D.B. Assessment of the polyphenol indices and antioxidant capacity for beers and wines using a tyrosinase-based biosensor prepared by sinusoidal current method. Sensors 2018, 19, 66. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Jamal, R.; Zhang, R.; Zhang, W.; Yu, Z.; Yan, Y.; Liu, Y.; Abdiryim, T. Electrochemical synthesis of multilayered PEDOT/PEDOT-SH/Au nanocomposites for electrochemical sensing of nitrite. Microchim. Acta 2020, 187, 248. [Google Scholar] [CrossRef] [PubMed]
- Motshakeri, M.; Phillips, A.R.J.; Travas-Sejdic, J.; Kilmartin, P.A. Electrochemical study of gold microelectrodes modified with PEDOT to quantify uric acid in milk samples. Electroanalysis 2020, 32, 2101–2111. [Google Scholar] [CrossRef]
- Zhang, B.; El Jaouhari, A.; Wu, X.; Liu, W.; Zhu, J.; Liu, X. Synthesis and characterization of PEDOT-MC decorated AgNPs for voltammetric detection of rutin in real samples. J. Electroanal. Chem. 2020, 877, 114632. [Google Scholar] [CrossRef]
- Lete, C.; Marin, M.; Anghel, E.M.; Preda, L.; Matei, C.; Lupu, S. Sinusoidal voltage electrodeposition of PEDOT-Prussian blue nanoparticles composite and its application to amperometric sensing of H2O2 in human blood. Mater. Sci. Eng. C 2019, 102, 661–669. [Google Scholar] [CrossRef]
- Yassin, M.A.; Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. Exfoliated nanosheets of Co3O4 webbed with polyaniline nanofibers: A novel composite electrode material for enzymeless glucose sensing application. J. Ind. Eng. Chem. 2019, 73, 106–117. [Google Scholar] [CrossRef]
- Saeb, E.; Asadpour-Zeynali, K. Facile synthesis of TiO2@PANI@Au nanocomposite as an electrochemical sensor for determination of hydrazine. Microchem. J. 2021, 160, 105603. [Google Scholar] [CrossRef]
- Duan, C.; Zheng, J. Porous coralloid Polyaniline/SnO2-based enzyme-free sensor for sensitive and selective detection of nitrite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 271–277. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.; Swamy, B.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine in real samples using polyaniline/NiO, ZnO, and Fe3O4 nanocomposites on glassy carbon electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Basirun, W.; Sookhakian, M.; Woi, P.M.; Zalnezhad, E.; Hazarkhani, H.; Alias, Y. Synthesis and characterization of α-Fe2O3/polyaniline nanotube composite as electrochemical sensor for uric acid detection. Adv. Powder Technol. 2019, 30, 384–392. [Google Scholar] [CrossRef]
- Kailasaa, S.; Rani, B.G.; Reddy, M.S.B.; Jayarambabu, N.; Munindra, P.; Sharma, S.; Rao, K.V. NiO nanoparticles -decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Mater. Chem. Phys. 2020, 242, 122524. [Google Scholar] [CrossRef]
- Naghib, S.M.; Behzad, F.; Rahmanian, M.; Zare, Y.; Rhee, K.Y. A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination. Nanotechnol. Rev. 2020, 9, 760–767. [Google Scholar] [CrossRef]
- Tavousi, A.; Ahmadi, E.; Mohammadi-Behzad, L.; Riahifar, V.; Maghemi, F. Sensitive electrochemical sensor using polypyrrole-coated Fe3O4 core-shell nanoparticles/multiwall carbon nanotubes modified graphite electrode for atorvastatin analysis. Microchem. J. 2020, 158, 105159. [Google Scholar] [CrossRef]
- Kannan, A.; Radhakrishnan, S. Fabrication of an electrochemical sensor based on gold nanoparticles functionalized polypyrrole nanotubes for the highly sensitive detection of l-dopa. Mater. Today Commun. 2020, 25, 101330. [Google Scholar] [CrossRef]
- Adeosun, W.A.; Asiri, A.M.; Marwani, H.M.; Rahman, M. Enzymeless electrocatalytic detection of uric acid using polydopamine/polypyrrole copolymeric film. ChemistrySelect 2020, 5, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Arabali, V.; Malekmohammadi, S.; Karimi, F. Surface amplification of pencil graphite electrode using CuO nanoparticle/polypyrrole nanocomposite; a powerful electrochemical strategy for determination of tramadol. Microchem. J. 2020, 158, 105179. [Google Scholar] [CrossRef]
- Mengarda, P.; Dias, F.A.L.; Peixoto, J.V.; Osiecki, R.; Bergamini, M.F.; Marcolino-Junior, L.H. Determination of lactate levels in biological fluids using a disposable ion-selective potentiometric sensor based on polypyrrole films. Sens. Actuators B Chem. 2019, 296, 126663. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, J. Voltammetric determination of hydrogen peroxide using AuCu nanoparticles attached on polypyrrole-modified 2D metal-organic framework nanosheets. Microchim. Acta 2020, 187, 389. [Google Scholar] [CrossRef]
- Robak, J.; Burnat, B.; Leniart, A.; Kisielewska, A.; Brycht, M.; Skrzypek, S. The effect of carbon material on the electroanalytical determination of 4-chloro-3-methylphenol using the sol-gel derived carbon ceramic electrodes. Sens. Actuators B Chem. 2016, 236, 318–325. [Google Scholar] [CrossRef]
- Darmokoesoemo, H.; Widayanti, N.; Khasanah, M.; Kusuma, H. Analysis of uric acid using carbon paste electrodes modified by molecularly imprinted polymer as potentiometry sensor. Rasayan J. Chem. 2017, 10, 54–58. [Google Scholar] [CrossRef]
- Abrishamkar, M.; Kiani, F. Electrocatalytic oxidation of hydrogen peroxide on the surface of nano zeolite modified carbon paste electrode. Int. J. Hydrogen Energy 2017, 42, 23826–23831. [Google Scholar] [CrossRef]
- Li, D.; Liu, M.; Zhan, Y.; Su, Q.; Zhang, Y.; Zhang, D. Electrodeposited poly(3,4-ethylenedioxythiophene) doped with graphene oxide for the simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2020, 187, 94. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, S.; Huang, J.; Ma, Y.; Zeng, Q.; Wang, M.; Wang, L. Simultaneous detection of nitrophenol isomers using an easy-to-fabricate thiophene-based microporous polymer film modified electrode. Microchem. J. 2020, 153, 104465. [Google Scholar] [CrossRef]
- Prathap, M.A.; Srivastava, R. Tailoring properties of polyaniline for simultaneous determination of a quaternary mixture of ascorbic acid, dopamine, uric acid, and tryptophan. Sens. Actuators B Chem. 2013, 177, 239–250. [Google Scholar] [CrossRef]
- Prathap, M.A.; Satpati, B.; Srivastava, R. Facile preparation of polyaniline/MnO2 nanofibers and its electrochemical application in the simultaneous determination of catechol, hydroquinone, and resorcinol. Sens. Actuators B Chem. 2013, 186, 67–77. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold nanoparticle decorated polypyrrole/graphene oxide nanosheets as a modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. New J. Chem. 2020, 44, 4916–4926. [Google Scholar] [CrossRef]
- Wang, M.; Cui, M.; Liu, W.; Liu, X. Highly dispersed conductive polypyrrole hydrogels as sensitive sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2019, 832, 174–181. [Google Scholar] [CrossRef]
- Gautam, V.; Srivastava, A.; Singh, K.P.; Yadav, V.L. Preparation and characterization of polyaniline, multiwall carbon nanotubes, and starch bionanocomposite material for potential bioanalytical applications. Polym. Compos. 2017, 38, 496–506. [Google Scholar] [CrossRef]
- Gautam, V.; Singh, K.P.; Yadav, V.L. Polyaniline/MWCNTs/starch modified carbon paste electrode for non-enzymatic detection of cholesterol: Application to real sample (cow milk). Anal. Bioanal. Chem. 2018, 410, 2173–2181. [Google Scholar] [CrossRef]
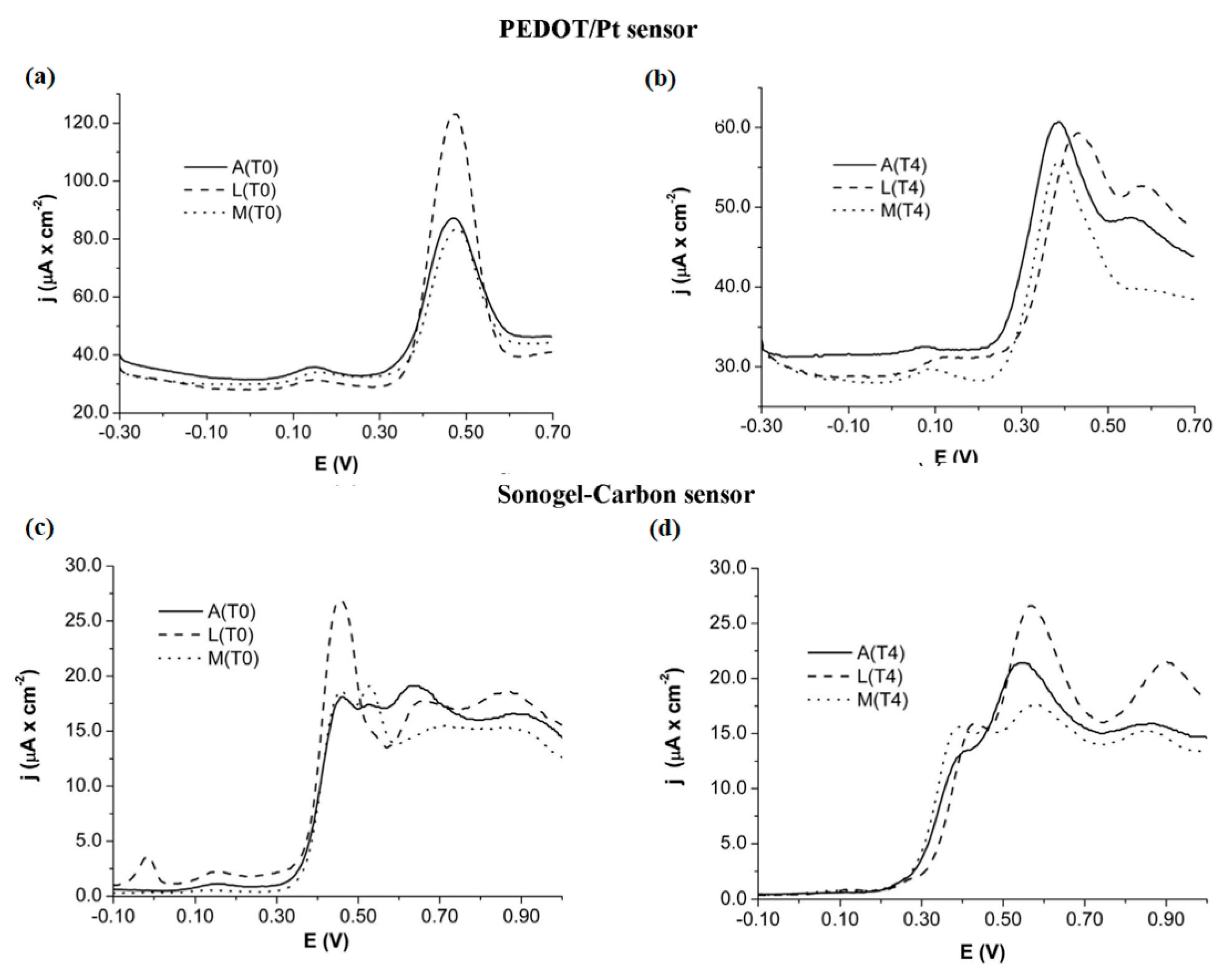
- López-Iglesias, D.; García-Guzmán, J.J.; Bellido-Milla, D.; Naranjo-Rodríguez, I.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. The sonogel-carbon-PEDOT Material: An innovative bulk material for sensor devices. J. Electrochem. Soc. 2018, 165, B906–B915. [Google Scholar] [CrossRef]
- López-Iglesias, D.; García-Guzmán, J.J.; Zanardi, C.; Palacios-Santander, J.M.; Cubillana-Aguilera, L.; Pigani, L. Fast electroanalytical determination of Cannabidiol and Cannabinol in aqueous solution using Sonogel-carbon-PEDOT devices. J. Electroanal. Chem. 2020, 878, 114591. [Google Scholar] [CrossRef]
- Ha, D.; Sun, Q.; Su, K.; Wan, H.; Li, H.; Xu, N.; Sun, F.; Zhuang, L.; Hu, N.; Wang, P. Recent achievements in electronic tongue and bioelectronic tongue as taste sensors. Sens. Actuators B Chem. 2015, 207, 1136–1146. [Google Scholar] [CrossRef]
- Del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ràfols, C.; Serrano, N.; Ariño, C.; Esteban, M.; Díaz-Cruz, J.M. Voltammetric electronic tongues in food analysis. Sensors 2019, 19, 4261. [Google Scholar] [CrossRef] [Green Version]
- Kirsanov, D.; Correa, D.S.; Gaal, G.; Riul, A.; Braunger, M.L.; Shimizu, F.M.; Oliveira, O.N.; Liang, T.; Wan, H.; Wang, P.; et al. Electronic tongues for inedible media. Sensors 2019, 19, 5113. [Google Scholar] [CrossRef] [Green Version]
- Riul, A.; Malmegrim, R.; Fonseca, F.; Mattoso, L. An artificial taste sensor based on conducting polymers. Biosens. Bioelectron. 2003, 18, 1365–1369. [Google Scholar] [CrossRef]
- Riul, A., Jr.; dos Santos, D.S., Jr.; Wohnrath, K.; Di Tommazo, R.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N., Jr.; Taylor, D.M.; Mattoso, L.H.C. Artificial taste sensor: Efficient combination of sensors made from langmuir−blodgett films of conducting polymers and a ruthenium complex and self-assembled films of an azobenzene-containing polymer. Langmuir 2002, 18, 239–245. [Google Scholar] [CrossRef]
- Riul, A.; Soto, A.G.; Mello, S.; Bone, S.; Taylor, D.; Mattoso, L. An electronic tongue using polypyrrole and polyaniline. Synth. Met. 2003, 132, 109–116. [Google Scholar] [CrossRef]
- Arrieta, A.; Apetrei, C.; Rodriguez-Mendez, M.L.; de Saja, J. Voltammetric sensor array based on conducting polymer-modified electrodes for the discrimination of liquids. Electrochim. Acta 2004, 49, 4543–4551. [Google Scholar] [CrossRef]
- Lvova, L.; Legin, A.; Vlasov, Y.; Cha, G.S.; Nam, H. Multicomponent analysis of Korean green tea by means of disposable all-solid-state potentiometric electronic tongue microsystem. Sens. Actuators B Chem. 2003, 95, 391–399. [Google Scholar] [CrossRef]
- Pigani, L.; Simone, G.V.; Foca, G.; Ulrici, A.; Masino, F.; Aguilera, L.C.; Calvini, R.; Seeber, R. Prediction of parameters related to grape ripening by multivariate calibration of voltammetric signals acquired by an electronic tongue. Talanta 2018, 178, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Pigani, L.; Culetu, A.; Ulrici, A.; Foca, G.; Vignali, M.; Seeber, R. Pedot modified electrodes in amperometric sensing for analysis of red wine samples. Food Chem. 2011, 129, 226–233. [Google Scholar] [CrossRef]
- Martina, V.; Ionescu, K.; Pigani, L.; Terzi, F.; Ulrici, A.; Zanardi, C.; Seeber, R. Development of an electronic tongue based on a PEDOT-modified voltammetric sensor. Anal. Bioanal. Chem. 2007, 387, 2101–2110. [Google Scholar] [CrossRef]
- Scagion, V.P.; Mercante, L.; Sakamoto, K.Y.; Oliveira, J.; Fonseca, F.J.; Mattoso, L.H.C.; Ferreira, M.D.; Correa, D.S. An electronic tongue based on conducting electrospun nanofibers for detecting tetracycline in milk samples. RSC Adv. 2016, 6, 103740–103746. [Google Scholar] [CrossRef]
- Yu, Y.; Joshi, P.C.; Wu, J.; Hu, A. Laser-Induced carbon-based smart flexible sensor array for multiflavors detection. ACS Appl. Mater. Interfaces 2018, 10, 34005–34012. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M. Analysis of red wines using an electronic tongue and infrared spectroscopy. Correlations with phenolic content and color parameters. LWT 2020, 118, 108785. [Google Scholar] [CrossRef]
- Geană, E.-I.; Ciucure, C.T.; Artem, V.; Apetrei, C. Wine varietal discrimination and classification using a voltammetric sensor array based on modified screen-printed electrodes in conjunction with chemometric analysis. Microchem. J. 2020, 159, 105451. [Google Scholar] [CrossRef]
- Parra, V.; Arrieta, Á.A.; Escudero, J.F.; Rodríguez-Méndez, M.L.; De Saja, J.A. Electronic tongue based on chemically modified electrodes and voltammetry for the detection of adulterations in wines. Sens. Actuators B Chem. 2006, 118, 448–453. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Application of voltammetric e-tongue for the detection of ammonia and putrescine in beef products. Sens. Actuators B Chem. 2016, 234, 371–379. [Google Scholar] [CrossRef]
- Arrieta, Á.A.; Rodriguez-Mendez, M.L.; de Saja, J.A.; Blanco, C.A.; Nimubona, D. Prediction of bitterness and alcoholic strength in beer using an electronic tongue. Food Chem. 2010, 123, 642–646. [Google Scholar] [CrossRef]
- Apetrei, C. Novel method based on polypyrrole-modified sensors and emulsions for the evaluation of bitterness in extra virgin olive oils. Food Res. Int. 2012, 48, 673–680. [Google Scholar] [CrossRef]
- Geană, E.-I.; Artem, V.; Apetrei, C. Discrimination and classification of wines based on polypyrrole modified screen-printed carbon electrodes coupled with multivariate data analysis. J. Food Compos. Anal. 2021, 96, 103704. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Moreno-Barón, L.; Pividori, M.I.; Alegret, S.; del Valle, M. A voltammetric electronic tongue made of modified epoxy-graphite electrodes for the qualitative analysis of wine. Microchim. Acta 2010, 169, 261–268. [Google Scholar] [CrossRef]
- Cetó, X.; Capdevila, J.; Puig-Pujol, A.; Del Valle, M. Cava wine authentication employing a voltammetric electronic tongue. Electroanalysis 2014, 26, 1504–1512. [Google Scholar] [CrossRef] [Green Version]
- Cetó, X.; Gutiérrez, J.M.; Gutiérrez, M.; Céspedes, F.; Capdevila, J.; Mínguez, S.; Jimenez-Jorquera, C.; Del Valle, M. Determination of total polyphenol index in wines employing a voltammetric electronic tongue. Anal. Chim. Acta 2012, 732, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Yang, Y.; Xiao, X.; Zhang, W.; Wang, J. Fabrication of conducting polymer/noble metal nanocomposite modified electrodes for glucose, ascorbic acid and tyrosine detection and its application to identify the marked ages of rice wines. Sens. Actuators B Chem. 2018, 255, 895–906. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Zhang, W.; Wei, Z. Application of the voltammetric electronic tongue based on nanocomposite modified electrodes for identifying rice wines of different geographical origins. Anal. Chim. Acta 2019, 1050, 60–70. [Google Scholar] [CrossRef]
- Pioggia, G.; Di Francesco, F.; Marchetti, A.; Ferro, M.; Ahluwalia, A. A composite sensor array impedentiometric electronic tongue: Part I. Characterization. Biosens. Bioelectron. 2007, 22, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Pioggia, G.; Di Francesco, F.; Marchetti, A.; Ferro, M.; Leardi, R.; Ahluwalia, A. A composite sensor array impedentiometric electronic tongue: Part II. Discrimination of basic tastes. Biosens. Bioelectron. 2007, 22, 2624–2628. [Google Scholar] [CrossRef]
- Braga, G.S.; Paterno, L.G.; Fonseca, F.J. Performance of an electronic tongue during monitoring 2-methylisoborneol and geosmin in water samples. Sens. Actuators B Chem. 2012, 171–172, 181–189. [Google Scholar] [CrossRef]
- Carvalho, E.R.; Lopes, W.T.; Silva, D. Evaluation of tap water based on sensor array and conducting nanostructured polymers. Nanotechnol. J. Water Environ. Nanotechnol 2016, 1, 116–123. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Mercante, L.; Mattoso, L.H.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Scagion, V.P.; Grassi, V.; Correa, D.; Mattoso, L.H. Modification of electrospun nylon nanofibers using layer-by-layer films for application in flow injection electronic tongue: Detection of paraoxon pesticide in corn crop. Sens. Actuators B Chem. 2012, 171–172, 249–255. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Amperometric biosensor based on polypyrrole and tyrosinase for the detection of tyramine in food samples. Sens. Actuators B Chem. 2013, 178, 40–46. [Google Scholar] [CrossRef]
- Kochana, J.; Hnida, K.; Sulka, G.; Knihnicki, P.; Kozak, J.; Gilowska, A. Application of polypyrrole nanowires for the development of a tyrosinase biosensor. Chem. Pap. 2015, 69, 1130–1135. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M. Analysis of musts and wines by means of a bio-electronic tongue based on tyrosinase and glucose oxidase using polypyrrole/gold nanoparticles as the electron mediator. Food Chem. 2019, 289, 751–756. [Google Scholar] [CrossRef]
- Mao, H.; Liu, X.; Chao, D.; Cui, L.; Li, Y.; Zhang, W.; Wang, C. Preparation of unique PEDOT nanorods with a couple of cuspate tips by reverse interfacial polymerization and their electrocatalytic application to detect nitrite. J. Mater. Chem. 2010, 20, 10277–10284. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, J.; Zhu, X.; Lu, L.; Duan, X.; Hu, D.; Dong, L.; Sun, H.; Gao, Y.; Wu, Y. Poly(3,4-ethylenedioxythiophene) nanorods grown on graphene oxide sheets as electrochemical sensing platform for rutin. J. Electroanal. Chem. 2015, 739, 66–72. [Google Scholar] [CrossRef]
- Wiziack, N.K.L.; Paterno, L.G.; Fonseca, F.J.; Mattoso, L.H.C. Effect of film thickness and different electrode geometries on the performance of chemical sensors made of nanostructured conducting polymer films. Sens. Actuators B Chem. 2007, 122, 484–492. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Pirsa, S. Chemiresistive gas sensors based on conducting polymers. In Materials Science and Engineering: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2017; pp. 543–574. [Google Scholar]
- Lakard, B.; Carquigny, S.; Segut, O.; Patois, T.; Lakard, S. Gas sensors based on electrodeposited polymers. Metals 2015, 5, 1371–1386. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical gas sensors based on conducting polymer hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Wu, W.; Wang, B.; Segev-Bar, M.; Dou, W.; Niu, F.; Horev, Y.D.; Deng, Y.; Plotkin, M.; Huynh, T.-P.; Jeries, R.; et al. Free-Standing and eco-friendly polyaniline thin films for multifunctional sensing of physical and chemical stimuli. Adv. Funct. Mater. 2017, 27, 27. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, H.; Liao, Z.; Horev, Y.D.; Panes-Ruiz, L.A.; Petkov, P.S.; Zhang, Z.; Shivhare, R.; Zhang, P.; Liu, K.; et al. Engineering crystalline quasi-two-dimensional polyaniline thin film with enhanced electrical and chemiresistive sensing performances. Nat. Commun. 2019, 10, 4225. [Google Scholar] [CrossRef] [Green Version]
- Duc, C.; Boukhenane, M.-L.; Wojkiewicz, J.-L.; Redon, N. Hydrogen sulfide detection by sensors based on conductive polymers: A review. Front. Mater. 2020, 7, 215. [Google Scholar] [CrossRef]
- Mikhaylov, S.; Ogurtsov, N.A.; Redon, N.; Coddeville, P.; Wojkiewicz, J.-L.; Pud, A.A. The PANI-DBSA content and dispersing solvent as influencing parameters in sensing performances of TiO2/PANI-DBSA hybrid nanocomposites to ammonia. RSC Adv. 2016, 6, 82625–82634. [Google Scholar] [CrossRef]
- Hong, K.H.; Oh, K.W.; Kang, T.J. Polyaniline-nylon 6 composite fabric for ammonia gas sensor. J. Appl. Polym. Sci. 2004, 92, 37–42. [Google Scholar] [CrossRef]
- Hu, H.; Trejo, M.; Nicho, M.; Saniger, J.; García-Valenzuela, A. Adsorption kinetics of optochemical NH3 gas sensing with semiconductor polyaniline films. Sens. Actuators B Chem. 2002, 82, 14–23. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Wang, S.; Guo, X.; Xia, H.; Wang, Y.; Zhang, S.; Huang, W.; Wu, S. Gas sensing properties of SnO2 hollow spheres/polythiophene inorganic–organic hybrids. Sens. Actuators B Chem. 2010, 146, 8–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Bunes, B.R.; Wu, N.; Ansari, A.; Rajabali, S.; Zang, L. Sensing methamphetamine with chemiresistive sensors based on polythiophene-blended single-walled carbon nanotubes. Sens. Actuators B Chem. 2018, 255, 1814–1818. [Google Scholar] [CrossRef]
- Lv, D.; Chen, W.; Shen, W.; Peng, M.; Zhang, X.; Wang, R.; Xu, L.; Xu, W.; Song, W.; Tan, R. Enhanced flexible room temperature ammonia sensor based on PEDOT: PSS thin film with FeCl3 additives prepared by inkjet printing. Sens. Actuators B Chem. 2019, 298, 126890. [Google Scholar] [CrossRef]
- Ram, J.; Singh, R.G.; Singh, F.; Kumar, V.; Chauhan, V.; Gupta, R.; Kumar, U.; Yadav, B.C.; Kumar, R. Development of WO3-PEDOT: PSS hybrid nanocomposites based devices for liquefied petroleum gas (LPG) sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 13593–13603. [Google Scholar] [CrossRef]
- Yang, P.; Lv, D.; Shen, W.; Wu, T.; Yang, Y.; Zhao, Y.; Tan, R.; Song, W. Porous flexible polyaniline/polyvinylidene fluoride composite film for trace-level NH3 detection at room temperature. Mater. Lett. 2020, 271, 127798. [Google Scholar] [CrossRef]
- Xu, H.; Ju, D.; Li, W.; Gong, H.; Zhang, J.; Wang, J.; Cao, B. Low-working-temperature, fast-response-speed NO2 sensor with nanoporous-SnO2/polyaniline double-layered film. Sens. Actuators B Chem. 2016, 224, 654–660. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Kotresh, S.; Ravikiran, Y.T.; Vijayakumari, S.C.; Thomas, S. Interfacial p-n heterojunction of polyaniline-nickel ferrite nanocomposite as room temperature liquefied petroleum gas sensor. Compos. Interfaces 2017, 24, 549–561. [Google Scholar] [CrossRef]
- Tang, X.; Raskin, J.-P.; Kryvutsa, N.; Hermans, S.; Slobodian, O.; Nazarov, A.N.; Debliquy, M. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens. Actuators B Chem. 2020, 305, 127423. [Google Scholar] [CrossRef]
- Navale, S.; Mane, A.; Chougule, M.; Sakhare, R.; Nalage, S.; Patil, V. Highly selective and sensitive room temperature NO2 gas sensor based on polypyrrole thin films. Synth. Met. 2014, 189, 94–99. [Google Scholar] [CrossRef]
- Adhikari, A.; Tiwary, P.; Rana, D.; Halder, A.; Nath, J.; Basu, A.; Ghoshal, D.; Kar, P.; Chakraborty, A.K.; Chattopadhyay, D. Na-cholate micelle mediated synthesis of polypyrrole nanoribbons for ethanol sensing. J. Environ. Chem. Eng. 2020, 8, 104249. [Google Scholar] [CrossRef]
- Adhikari, A.; Kar, P.; Rana, D.; De, S.; Nath, J.; Dutta, K.; Chattopadhyay, D. Synthesis of sodium cholate mediated rod-like polypyrrole-silver nanocomposite for selective sensing of acetone vapor. Nano Struct. Nano Objects 2020, 21, 100419. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, O.S.; Jang, J. A high-performance hydrogen gas sensor using ultrathin polypyrrole-coated CNT nanohybrids. Chem. Commun. 2013, 49, 4673. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, J.; Jin, H.; Khatib, M.; Li, X.; Wei, Z.; Wang, F.; Horev, Y.D.; Wu, W.; Haick, H. Chemically modified polyaniline for the detection of volatile biomarkers of minimal sensitivity to humidity and bending. Adv. Heal. Mater. 2018, 7, e1800232. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Vishnu, G.K.A.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of electronic-nose technologies and voc-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Huang, Y.; Kam, K.W.; Cheung, W.-F.; Zhao, N.; Zheng, B. Functionalized graphene-based chemiresistive electronic nose for discrimination of disease-related volatile organic compounds. Biosens. Bioelectron. X 2019, 1, 100016. [Google Scholar] [CrossRef]
- Nategh, N.A.; Dalvand, M.J.; Anvar, A. Detection of toxic and non-toxic sweet cherries at different degrees of maturity using an electronic nose. J. Food Meas. Charact. 2021, 15, 1213–1224. [Google Scholar] [CrossRef]
- Hatfield, J.; Neaves, P.; Hicks, P.; Persaud, K.; Travers, P. Towards an integrated electronic nose using conducting polymer sensors. Sens. Actuators B Chem. 1994, 18, 221–228. [Google Scholar] [CrossRef]
- Stella, R.; Barisci, J.N.; Serra, G.; Wallace, G.; De Rossi, D. Characterisation of olive oil by an electronic nose based on conducting polymer sensors. Sens. Actuators B Chem. 2000, 63, 1–9. [Google Scholar] [CrossRef]
- Barisci, J.N.; Wallace, G.G.; Andrews, M.K.; Partridge, A.C.; Harris, P.D. Conducting polymer sensors for monitoring aromatic hydrocarbons using an electronic nose. Sens. Actuators B Chem. 2002, 84, 252–257. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wu, Y.; Chen, S.; Liu, S.; Wang, P.; Xue, F.; Liu, L. A flexible and multifunctional electronic nose using polyaniline/cotton fibrous membrane with a hierarchical structure. Mater. Lett. 2018, 233, 324–327. [Google Scholar] [CrossRef]
- Tiggemann, L.; Ballen, S.C.; Bocalon, C.M.; Graboski, A.M.; Manzoli, A.; Steffens, J.; Valduga, E.; Steffens, C. Electronic nose system based on polyaniline films sensor array with different dopants for discrimination of artificial aromas. Innov. Food Sci. Emerg. Technol. 2017, 43, 112–116. [Google Scholar] [CrossRef]
- Graboski, A.M.; Ballen, S.C.; Galvagni, E.; Lazzari, T.; Manzoli, A.; Shimizu, F.M.; Steffens, J.; Steffens, C. Aroma detection using a gas sensor array with different polyaniline films. Anal. Methods 2019, 11, 654–660. [Google Scholar] [CrossRef]
- Manzoli, A.; Steffens, C.; Paschoalin, R.T.; Graboski, A.M.; Brandão, H.; de Carvalho, B.C.; Bellini, J.L.; Herrmann, P.S.D.P. Volatile compounds monitoring as indicative of female cattle fertile period using electronic nose. Sens. Actuators B Chem. 2019, 282, 609–616. [Google Scholar] [CrossRef]
- Manzoli, A.; Steffens, C.; Paschoalin, R.T.; Correa, A.A.; Alves, W.F.; Leite, F.L.; Herrmann, P.S.P. Low-Cost gas sensors produced by the graphite line-patterning technique applied to monitoring banana ripeness. Sensors 2011, 11, 6425–6434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballen, S.C.; Graboski, A.M.; Manzoli, A.; Steffens, J.; Steffens, C. Monitoring aroma release in gummy candies during the storage using electronic nose. Food Anal. Methods 2019, 13, 3–12. [Google Scholar] [CrossRef]
- Le Maout, P.; Wojkiewicz, J.-L.; Redon, N.; Lahuec, C.; Seguin, F.; Dupont, L.; Mikhaylov, S.; Noskov, Y.; Ogurtsov, N.; Pud, A. Polyaniline nanocomposites based sensor array for breath ammonia analysis. Portable e-nose approach to non-invasive diagnosis of chronic kidney disease. Sens. Actuators B Chem. 2018, 274, 616–626. [Google Scholar] [CrossRef]
- Le Maout, P.; Laquintinie, P.; Lahuec, C.; Seguin, F.; Wojkiewicz, J.-L.; Redon, N.; Dupont, L. A Low Cost, Handheld E-Nose for Renal Diseases Early Diagnosis. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 2817–2820. [Google Scholar]
- Graboski, A.M.; Zakrzevski, C.A.; Shimizu, F.M.; Paschoalin, R.T.; Soares, A.C.; Steffens, J.; Paroul, N.; Steffens, C. Electronic nose based on carbon nanocomposite sensors for clove essential oil detection. ACS Sens. 2020, 5, 1814–1821. [Google Scholar] [CrossRef]
- Tung, T.T.; Castro, M.; Feller, J.-F.; Kim, T.Y.; Suh, K.S. Hybrid film of chemically modified graphene and vapor-phase-polymerized PEDOT for electronic nose applications. Org. Electron. 2013, 14, 2789–2794. [Google Scholar] [CrossRef]
- Hamilton, S.; Hepher, M.; Sommerville, J. Polypyrrole materials for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2005, 107, 424–432. [Google Scholar] [CrossRef]
- Alizadeh, N.; Babaei, M.; Alizadeh, M.S.; Mani-Varnosfaderani, A. Simultaneous Analysis of Aliphatic Alcohols Mixtures Using an Electronic Nose Based on Nano/Microstructured Conducting Polypyrrole Film Prepared by Catalytic Electropolymerization on Cu/Au Interdigital Electrodes Using Multivariate Calibration. IEEE Sens. J. 2015, 16, 418–425. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Izquierdo, J.E.E.; Braga, G.S.; Dirani, E.A.T.; Pereira-Da-Silva, M.A.; Rodríguez, E.F.G.; Fonseca, F.J. Enhanced sensitivity of gas sensor based on poly(3-hexylthiophene) thin-film transistors for disease diagnosis and environment monitoring. Sensors 2015, 15, 9592–9609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, C.H.A.; Iglesias, B.A.; Ogawa, T.; Araki, K.; Hoehne, L.; Gruber, J. Identification of tobacco types and cigarette brands using an electronic nose based on conductive polymer/porphyrin composite sensors. ACS Omega 2018, 3, 6476–6482. [Google Scholar] [CrossRef]
- Abbas, N.K.; Ibrahim, I.M.; Saleh, M.A. Characteristics of MEH-PPV/Si and MEH-PPV/PS heterojunctions as NO2 Gas sensors. Silicon 2018, 10, 1345–1350. [Google Scholar] [CrossRef]
- Jafari, A.; Amini, A. Lactic acid gas sensor based on polypyrrole thin film. Mater. Lett. 2019, 236, 175–178. [Google Scholar] [CrossRef]
- Rañola, R.A.G.; Santiago, K.S.; Sevilla, F.B. Chemiresistor gas sensor array based on conducting polymers for the discrimination of virgin coconut oil. Appl. Mech. Mater. 2015, 789–790, 554–559. [Google Scholar] [CrossRef]
- Gruber, J.; Nascimento, H.M.; Yamauchi, E.Y.; Li, R.W.; Esteves, C.H.; Rehder, G.; Gaylarde, C.C.; Shirakawa, M.A. A conductive polymer based electronic nose for early detection of Penicillium digitatum in post-harvest oranges. Mater. Sci. Eng. C 2013, 33, 2766–2769. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Kumar, B.; Feller, J.; Haddi, Z.; Amari, A.; Bouchikhi, B. Novel e-nose for the discrimination of volatile organic biomarkers with an array of carbon nanotubes (CNT) conductive polymer nanocomposites (CPC) sensors. Sens. Actuators B Chem. 2011, 159, 213–219. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Shabanian, M. A bulky aromatic functional polyimide composite as a sensitive layer for the detection of organic compound biomarkers. Iran. Polym. J. 2019, 28, 203–211. [Google Scholar] [CrossRef]
- Jiménez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In Situ polymerization deposition of porous conducting polymer on reduced graphene oxide for gas sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef]
- Kwon, O.S.; Hong, J.-Y.; Park, S.J.; Jang, Y.; Jang, J. Resistive gas sensors based on precisely size-controlled polypyrrole nanoparticles: Effects of particle size and deposition method. J. Phys. Chem. C 2010, 114, 18874–18879. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly sensitive, printable nanostructured conductive polymer wireless sensor for food spoilage detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef]
- Bertoni, C.; Naclerio, P.; Viviani, E.; Zilio, S.D.; Carrato, S.; Fraleoni-Morgera, A. Nanostructured p3ht as a promising sensing element for real-time, dynamic detection of gaseous acetone. Sensors 2019, 19, 1296. [Google Scholar] [CrossRef] [Green Version]
- Di Rosa, A.R.; Marino, A.M.F.; Leone, F.; Corpina, G.G.; Giunta, R.P.; Chiofalo, V. Characterization of sicilian honeys pollen profiles using a commercial e-tongue and melissopalynological analysis for rapid screening: A pilot study. Sensors 2018, 18, 4065. [Google Scholar] [CrossRef] [Green Version]
- Major, N.; Marković, K.; Krpan, M.; Šarić, G.; Hruškar, M.; Vahčić, N. Rapid honey characterization and botanical classification by an electronic tongue. Talanta 2011, 85, 569–574. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Meng, Q.; Li, N.; Ren, L. Evaluation of beef by electronic tongue system TS-5000Z: Flavor assessment, recognition and chemical compositions according to its correlation with flavor. PLoS ONE 2015, 10, e0137807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Li, D.; Wang, M. Electronic tongue coupled with physicochemical analysis for the recognition of orange beverages. J. Food Qual. 2012, 35, 429–441. [Google Scholar] [CrossRef]
- Pein, M.; Kirsanov, D.; Ciosek, P.; Del Valle, M.; Yaroshenko, I.; Wesoły, M.; Zabadaj, M.; González-Calabuig, A.; Wróblewski, W.; Legin, A. Independent comparison study of six different electronic tongues applied for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2015, 114, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Tirzīte, M.; Bukovskis, M.; Strazda, G.; Jurka, N.; Taivans, I. Detection of lung cancer with electronic nose and logistic regression analysis. J. Breath Res. 2018, 13, 016006. [Google Scholar] [CrossRef] [Green Version]
- Herman-Saffar, O.; Boger, Z.; Libson, S.; Lieberman, D.; Gonen, R.; Zeiri, Y. Early non-invasive detection of breast cancer using exhaled breath and urine analysis. Comput. Biol. Med. 2018, 96, 227–232. [Google Scholar] [CrossRef]
- De León-Martíne, L.D.; Rodríguez-Aguilar, M.; Gorocica-Rosete, P.; Domínguez-Reyes, C.A.; Martínez-Bustos, V.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Cruz-Ramos, J.A.; Balderas-Segura, B.; Flores-Ramírez, R. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: A case-control study. J. Breath Res. 2020, 14, 046009. [Google Scholar] [CrossRef]
- Farraia, M.; Rufo, J.C.; Paciência, I.; Mendes, F.C.; Rodolfo, A.; Rama, T.A.; Rocha, S.; Delgado, L.; Brinkman, P.; Moreira, A. Human volatilome analysis using eNose to assess uncontrolled asthma in a clinical setting. Allergy 2020, 75, 1630–1639. [Google Scholar] [CrossRef]
- Tenero, L.; Sandri, M.; Piazza, M.; Paiola, G.; Zaffanello, M.; Piacentini, G.L. Electronic nose in discrimination of children with uncontrolled asthma. J. Breath Res. 2020, 14, 046003. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.N.; Carratu, P.; Ranieri, T.; Marra, L.; D’Alba, G.; Resta, O. An electronic nose may sniff out amyotrophic lateral sclerosis. Respir. Physiol. Neurobiol. 2016, 232, 22–25. [Google Scholar] [CrossRef]
- Zheng, X.-Z.; Lan, Y.-B.; Zhu, J.-M.; Westbrook, J.; Hoffmann, W.C.; Lacey, R.E. Rapid identification of rice samples using an electronic nose. J. Bionic Eng. 2009, 6, 290–297. [Google Scholar] [CrossRef]
- Li, C.; Krewer, G.W.; Ji, P.; Scherm, H.; Kays, S.J. Gas sensor array for blueberry fruit disease detection and classification. Postharvest Biol. Technol. 2010, 55, 144–149. [Google Scholar] [CrossRef]
- Autelitano, F.; Giuliani, F. Analytical assessment of asphalt odor patterns in hot mix asphalt production. J. Clean. Prod. 2018, 172, 1212–1223. [Google Scholar] [CrossRef]
- Autelitano, F.; Giuliani, F. Influence of chemical additives and wax modifiers on odor emissions of road asphalt. Constr. Build. Mater. 2018, 183, 485–492. [Google Scholar] [CrossRef]
- Hosfield, B.D.; Pecoraro, A.R.; Baxter, N.T.; Hawkins, T.B.; Markel, T.A. The assessment of fecal volatile organic compounds in healthy infants: Electronic nose device predicts patient demographics and microbial enterotype. J. Surg. Res. 2020, 254, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A.D.; Bassi, D.; Ferrini, F. Evaluation of three electronic noses for detecting incipient wood decay. Sensors 2010, 10, 1062–1092. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Oberle, C.S.; Oberle, D.F. Detection of off-flavor in catfish using a conducting polymer electronic-nose technology. Sensors 2013, 13, 15968–15984. [Google Scholar] [CrossRef]
- Voss, H.G.J.; Júnior, J.J.A.M.; Farinelli, M.E.; Stevan, J.S.L. A prototype to detect the alcohol content of beers based on an electronic nose. Sensors 2019, 19, 2646. [Google Scholar] [CrossRef] [Green Version]



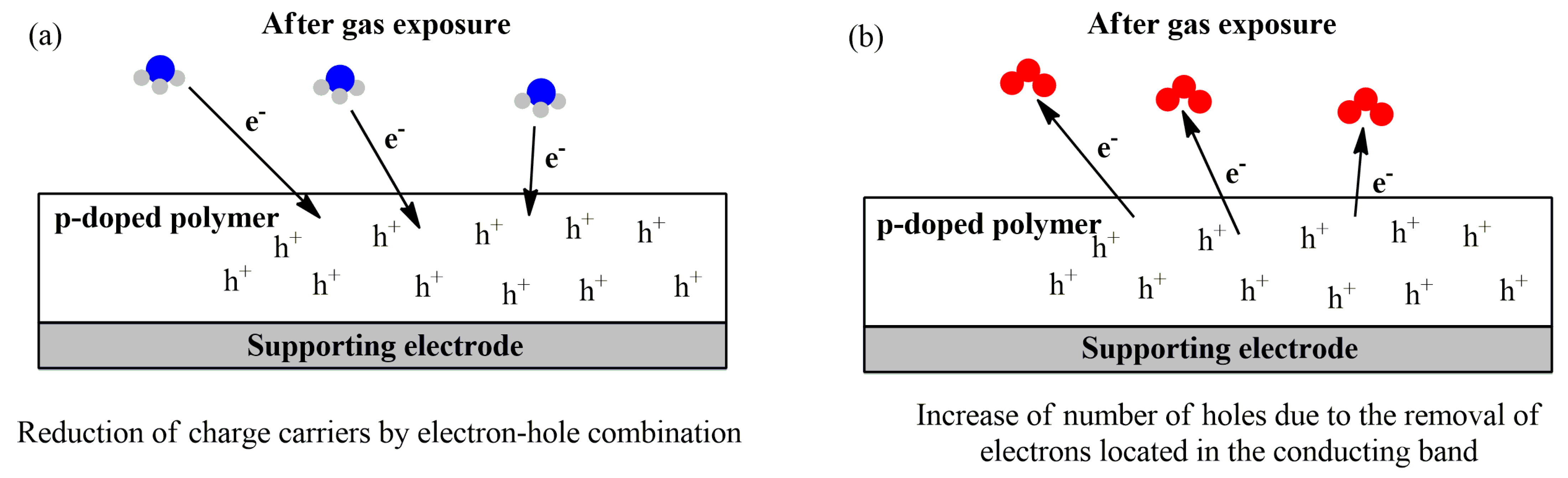
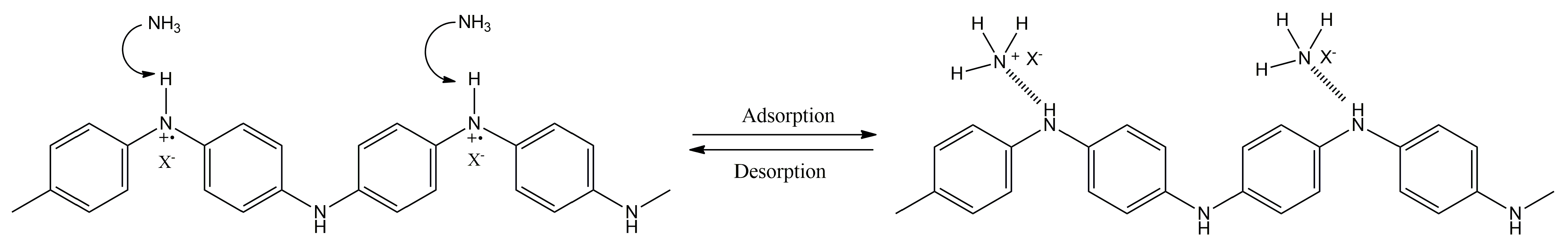
| Electrochemical Device | Analyte | Working Media | Sample | Analytical Parameters | Ref. | |
|---|---|---|---|---|---|---|
| LD (µM) | LR (µM) | |||||
| PTh | ||||||
| MWCNT/PTh/Pt | BPA | PBS pH 7.5 | Water | 0.009 | 0.05–0.4 | [62] |
| MnO2/PTh/rGO/GCE | MP | PBS pH 7 | Human urine and blood | 0.0057 | 0.5–10 | [63] |
| GO-4-ATP-Au-PTh/Au GCE | Nicotine | PBS pH 7 | Serum, urine, cigarette | 0.17 | 1.0–30 | [64] |
| PTh-AgBr | Glucose | NaOH | Human blood plasma | 0.31 | 4–5000 | [65] |
| PTh-Ag/GCE | L-Tryp | PBS pH 7 | Soybeans extract | 0.020 | 0.2–400 | [66] |
| PEDOT | ||||||
| PEDOT/IL/GCE | DA | PBS pH 7.4 | Human urine | 0.033 | 0.2–328 | [67] |
| UiO-66-NH2@PEDOT/GA/GCE | PCMC | ABS pH 6 | Tap water | 0.2 | 0.6–18 | [68] |
| PEDOT/AG/GCE | AC | PBS pH 7 | Local tablets | 0.041 | 0.15–5881 | [69] |
| Cu2O/PEDOT/MWCNT | Glucose | NaOH | Human blood serum | 0.04 | 0.495–374 | [70] |
| GC/PEDOT-AuNPs-SV | CA | PBS pH 7 | Juice | 4.24 | 10–1000 | [71] |
| PEDOT-Tyr/SNG-C | CA | PBS pH 7 | Wine, beer | 4.33 | 10–300 | [72] |
| PEDOT/PEDOT-SH/Au | Nitrite | PBS pH 6.9 | Tap water, milk | 0.051 | 0.15–1000 | [73] |
| PEDOT/Au | UA | PBS pH 6.6 | Milk | 7.0 | 6–200 | [74] |
| GCE/PEDOT-MC/AgNPs | Rutin | PBS pH 3 | Tablets | 0.0035 | 0.005–0.5 | [75] |
| Pt/PEDOT-PBNPS | H2O2 | ABS pH 5.5 | Human blood | 1.4 | 5–1000 | [76] |
| PANI | ||||||
| Co3O4@PANINFs/GCE | Glucose | PBS pH 7.4 | Human serum | 60 | 100–8000 | [77] |
| TiO2@PANI@Au/GCE | Hydrazine | NH3/NH4+ pH 9 | Power plant sewage | 0.15 | 0.9–1200 | [78] |
| PANI/SnO2/GCE | Nitrite | PBS pH 6 | - | 0.04 | 0.12–7777 | [79] |
| GCE/PANI-Fe3O4 | DA | PBS pH 7 | Water | 0.176 | 0.2–2.4 | [80] |
| GCE/PANI-NiO | DA | PBS pH 7 | Water | 0.166 | 0.2–2.4 | [80] |
| α-Fe2O3/PANI/GCE | UA | PBS pH 7 | Human urine | 0.038 | 0.01–5 | [81] |
| NiO-NPs@PANINS/SPE | Glucose | NaOH | Human blood serum | 0.06 | 1–3000 | [82] |
| MeGO/PANI | AA | PBS pH 7.4 | - | 2.0 | 8–5000 | [83] |
| PPy | ||||||
| Fe3O4@PPy/MWCNTs/GE | AT | BR pH 4 | Serum, tablets | 0.0230 | 0.0314–201 | [84] |
| AuNP/PPy/GCE | L-dopa | PBS pH 7 | Urine | 0.075 | 0.1–6.0 | [85] |
| PDA/PPy/GCE | UA | PBS pH 8 | Human serum, urine | 0.11 | 0.5–40 | [86] |
| PGE/CuO-NPs/PPy | TR | PBS pH 8.5 | Tablets | 0.001 | 0.005–380 | [87] |
| PPy:LAC | Lactate | KNO3 | Human tear, rat blood | 81.0 | 100–10,000 | [88] |
| AuCu/PPy/Cu-TCCP | H2O2 | PBS pH 8 | Medical H2O2 solution | 0.0067 | 0.71–24,100 | [89] |
| Sensor Array | Sample | Use | Multivariate Calibration | Ref. | |
|---|---|---|---|---|---|
| No CP Sensor | CP Sensor | ||||
| SNG-C | PEDOT/Pt | Musts | Discrimination of samples collected at different ripening times | PCA iPLS PLS | [114] |
| - | PEDOT/Pt | Red wines | Classification of different samples and origin | PCA PLS | [115] |
| Pt Au | PEDOT/Pt | Fruit juice | Discrimination between samples from different fruits | PCA PLS-LDA | [116] |
| IDE PA6/IDE | PA6/PANI/IDE (0.25–5.0% PANI) | Bovine milk | Discrimination of samples according to tetracycline residue content | PCA | [117] |
| CE AuCE rGO-CE rGO-AuCE | PANI-CE PANI-AuCE | Vinegar, sugar | Multiflavor detection | PCA | [118] |
| C/SPE NiO/C/SPE MWCNT/C/SPE SWCNT/C/SPE Pt | PANI/C/SPE | Red wine | Phenolic content | PCA | [119] |
| SWCNT/SPCE MWCNT/SPCE | PPy-DSA/SPCE | White wine | Discrimination according to varietal origin | PCA LDA | [120] |
| CPE-CoPc CPE-LuPc2 CPE-LuPc2 | PPy-dopant/Au Dopant: SO4, DSA, FCN, AQDS, PWA, TSA | Red wine | Evaluation of chemical adulteration | PCA PLS | [121] |
| GdPc2/CSPE DyPc2/CSPE CSPE | PPy-dopant/CSPE Dopant: FeCN, NP, Mo | Beef | Determination of ammonia and putresceine | PCA PLS-LDA | [122] |
| - | PPy- dopant/Pt Dopant: DSA, H2SO4, FCN, AQDS, PWA, TSA | Beer | Evaluation of bitterness and alcoholic strength | PCA PLS | [123] |
| - | PPy-dopant/Pt Dopant: FCN, NP, PWA, H2SO4, MO, AQS | Olive oil | Evaluation of bitterness | PCA PLS | [124] |
| - | PPy-dopant/SPCE Dopant: DSA, SO4, FCN | Wine | Classification of wines according to vintage year | PCA LDA | [125] |
| Graphite-epoxy PtNPs CuNPs | PANI PPy | Wine | Classification of wines and recognition of the oxygenation effect | PCA | [126] |
| Gas Sensor Device | Target Gas | Range (ppm) | Sensing Performance | Ref. | ||
|---|---|---|---|---|---|---|
| Gas Conc. (ppm) | Recovery Time (s) | Response Time (s) | ||||
| SnO2/PTh | NO2 | 10–200 | 10 | - | 2.07 | [153] |
| P3CT/CNT | NMPEA | 0.004–0.032 | 0.004 | 40 | 20 | [154] |
| PEDOT:PSS/FeCl3 | NH3 | 0.2–200 | 0.5 | - | 20 | [155] |
| WO3-PEDOT:PSS | LPG | 500–3000 | 500 | 54 | 29.4 | [156] |
| PANI/PVDF | NH3 | 0.2–5 | 0.2 | 235 | 174 | [157] |
| PANI/SnO2 | NO2 | 5–55 | 37 | 25 | 17 | [158] |
| SnO2/rGO/PANI | H2S | 0.05–10 | 2 | 78 | 82 | [159] |
| PANI-NF | LPG | 100–1000 | 700 | 200 | 50 | [160] |
| PPy/rGO | NH3 | 1.0–4.0 | 1.0 | 300 | 60 | [161] |
| PPy thin film | NO2 | 10–100 | 10 | 374 | 218 | [162] |
| PPy nanoribbons | CH3CH2OH | - | 100 | 31 | 2 | [163] |
| PPy-Ag | CH3COCH3 | 25–600 | 580 | 150 | 175 | [164] |
| PPy-CNT | H2 | 1–100 | 10 | - | >1.0 | [165] |
| PANI Sensor Array | Sample | Use | Multivariate Calibration | Ref. |
|---|---|---|---|---|
| PANI-dopant/IDGEs Dopant: CSA, DBSA, HCl | Strawberry Grape Apple | Discrimination of samples according to aromatic substances | PCA | [177] |
| PANI-HCl/PGIEs PANI-HCl/IDEs | Strawberry Grape Apple | Detection of different aromas | PCA | [178] |
| PANI-dopant/IDGEs Dopant: HCl, TSA, CSA, MSA | Cow’s estrus | Determination of estrus times of cows | PCA | [179] |
| PANI-dopant/IDEs Dopant: HCl, TSA, CSA, MSA | Bananas | Monitoring of bananas ripeness | PCA | [180] |
| PANI-dopant/PGIEs Dopant: CSA, HCl, DBSA | Gummy candies | Monitoring of aromas during candy storage | PCA | [181] |
| PANI-CSA/Chitosan PANI-DBSA/TiO2 PANI-DBSA/CNT | Simulated human breath | Preliminary diagnoses of kidney disease | PCA LDA | [182] |
| PANI/AuNPs | Human breath | Early diagnoses of renal diseases | PCA LDA | [183] |
| PANI-dopant/MWCNT PANI-dopant/GO Dopant: CSA, DBSA, HCl | Essential oils | Determination of quality of essential oils | PCA | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing. Sensors 2021, 21, 4976. https://doi.org/10.3390/s21154976
Sierra-Padilla A, García-Guzmán JJ, López-Iglesias D, Palacios-Santander JM, Cubillana-Aguilera L. E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing. Sensors. 2021; 21(15):4976. https://doi.org/10.3390/s21154976
Chicago/Turabian StyleSierra-Padilla, Alfonso, Juan José García-Guzmán, David López-Iglesias, José María Palacios-Santander, and Laura Cubillana-Aguilera. 2021. "E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing" Sensors 21, no. 15: 4976. https://doi.org/10.3390/s21154976
APA StyleSierra-Padilla, A., García-Guzmán, J. J., López-Iglesias, D., Palacios-Santander, J. M., & Cubillana-Aguilera, L. (2021). E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing. Sensors, 21(15), 4976. https://doi.org/10.3390/s21154976










