Non-Invasive Optical Coherence Tomography Data-Based Quantitative Algorithm for the Assessment of Residual Adhesive on Bracket-Removed Dental Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. OCT System Configuration
2.3. Description of the Customized Residual Adhesive Thickness and Area Detection Algorithm
3. Results
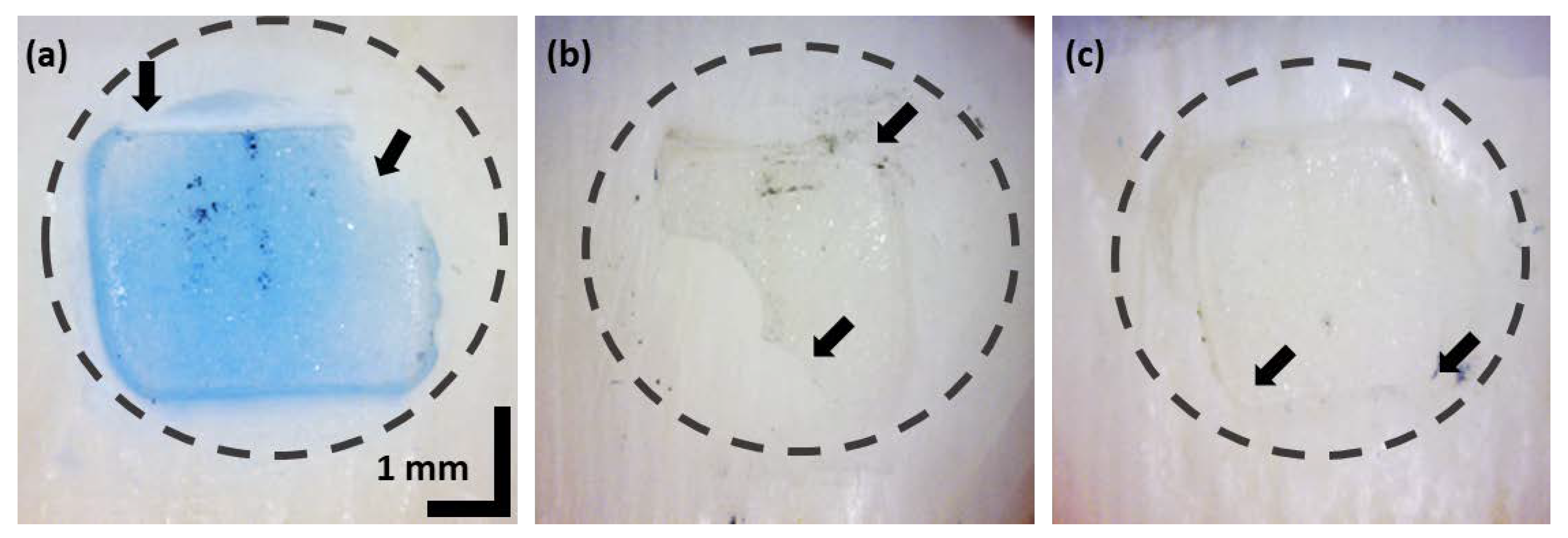
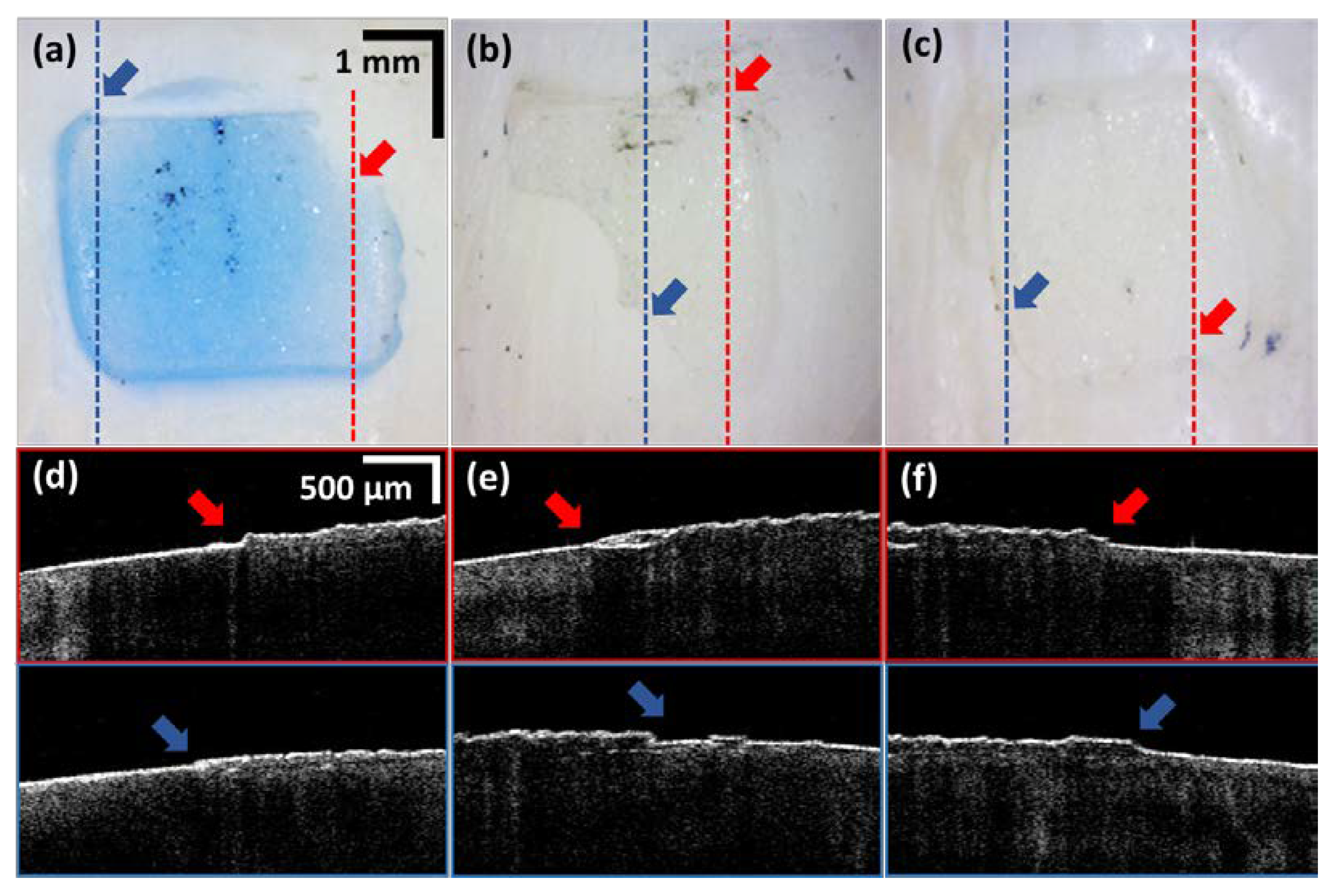
3.1. In-Front Visualizations of Residual Adhesive
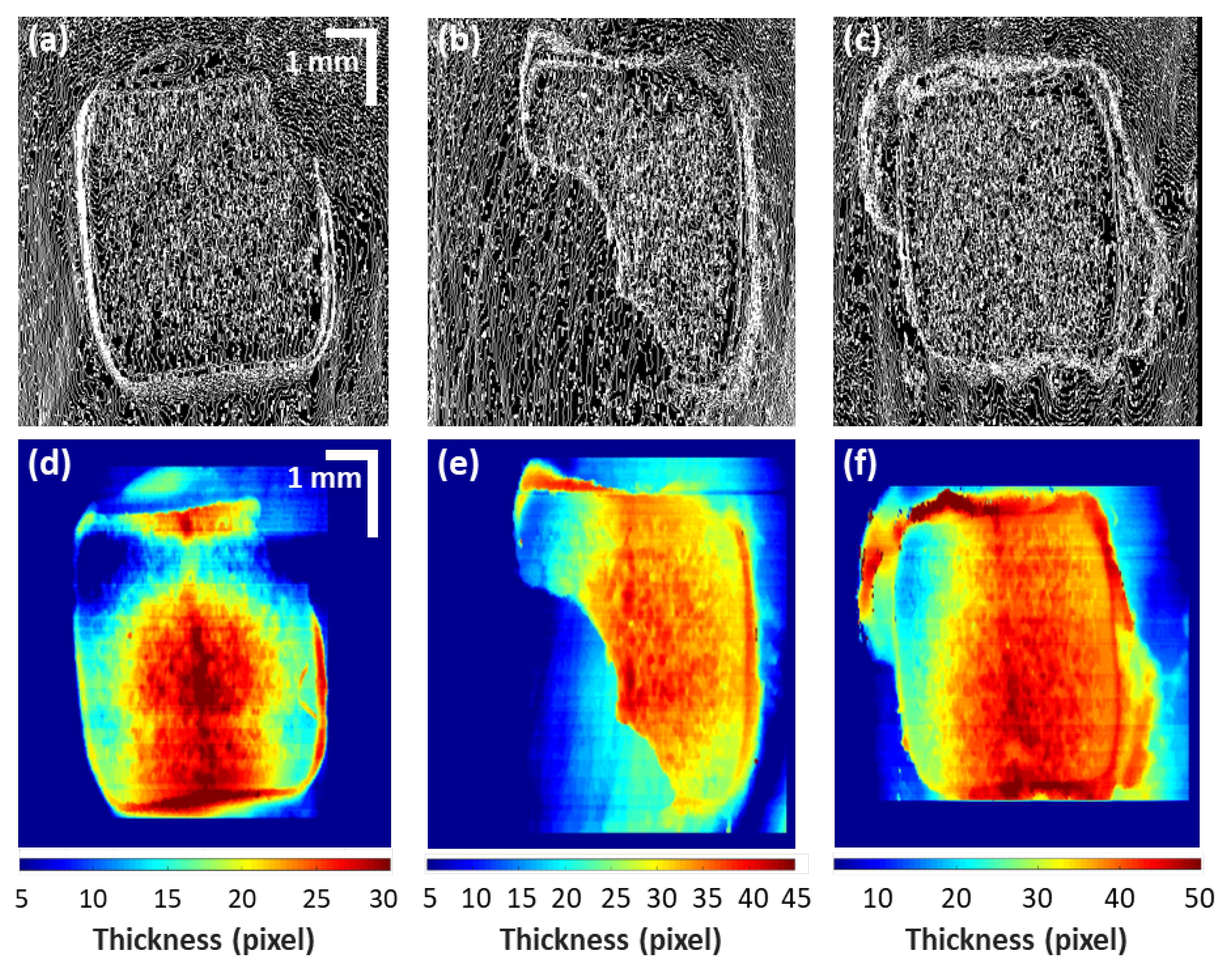
3.2. Residual Adhesive Boundary Detection Algorithm-Based Quatitative Assessments
3.3. A Comparison between Optical Microscope and OCT Visualization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghazanfari, R.; Nokhbatolfoghahaei, H.; Alikhasi, M. Laser-Aided Ceramic Bracket Debonding: A Comprehensive Review. J. Lasers Med. Sci. 2016, 7, 2–11. [Google Scholar] [CrossRef]
- Tehranchi, A.; Fekrazad, R.; Zafar, M.; Eslami, B.; Kalhori, K.A.M.; Gutknecht, N. Evaluation of the effects of CO2 laser on debonding of orthodontics porcelain brackets vs. the conventional method. Lasers Med. Sci. 2010, 26, 563–567. [Google Scholar] [CrossRef]
- Hayakawa, K. Nd: YAG laser for debonding ceramic orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.R.; Von Fraunhofer, J.A. Direct Bonding of Orthodontic Attachments to Teeth: The Relation of Adhesive Bond Strength to Gauze Mesh Size. Br. J. Orthod. 1976, 3, 91–95. [Google Scholar] [CrossRef]
- E Bishara, S.; VonWald, L.; Laffoon, J.F.; Warren, J.J. The effect of repeated bonding on the shear bond strength of a composite resin orthodontic adhesive. Angle Orthod. 2000, 70, 435–443. [Google Scholar] [CrossRef]
- Eminkahyagil, N.; Arman, A.; Cetinşahin, A.; Karabulut, E. Effect of resin-removal methods on enamel and shear bond strength of rebonded brackets. Angle Orthod. 2006, 76, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Laffoon, J.F.; VonWald, L.; Warren, J.J. The effect of repeated bonding on the shear bond strength of different orthodontic adhesives. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Ryf, S.; Flury, S.; Palaniappan, S.; Lussi, A.; Van Meerbeek, B.; Zimmerli, B. Enamel loss and adhesive remnants following bracket removal and various clean-up procedures in vitro. Eur. J. Orthod. 2012, 34, 25–32. [Google Scholar] [CrossRef]
- Randklev, R.M. Method and Manufacture for Applying and Removal of Orthodontic Bracket. U.S. Patent 4,435,160, 6 March 1984. [Google Scholar]
- Sandison, R.M. Tooth Surface Appearance after Debonding. Br. J. Orthod. 1981, 8, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Montasser, M.A.; Drummond, J.L. Reliability of the Adhesive Remnant Index Score System with Different Magnifications. Angle Orthod. 2009, 79, 773–776. [Google Scholar] [CrossRef]
- Janiszewska-Olszowska, J.; Tandecka, K.; Szatkiewicz, T.; Sporniak-Tutak, K.; Grocholewicz, K. Three-dimensional quantitative analysis of adhesive remnants and enamel loss resulting from debonding orthodontic molar tubes. Head Face Med. 2014, 10, 37. [Google Scholar] [CrossRef]
- Cehreli, S.B.; Polat-Ozsoy, O.; Sar, C.; Cubukcu, H.E. A comparative study of qualitative and quantitative methods for the assessment of adhesive remnant after bracket debonding. Eur. J. Orthod. 2011, 34, 188–192. [Google Scholar] [CrossRef]
- Gwinnett, A.; Ceen, R.F. Plaque distribution on bonded brackets: A scanning microscope study. Am. J. Orthod. 1979, 75, 667–677. [Google Scholar] [CrossRef]
- Bishara, S.E.; Olsen, M.E.; VonWald, L.; Jakobsen, J.R. Comparison of the debonding characteristics of two innovative ceramic bracket designs. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 86–92. [Google Scholar] [CrossRef]
- Baan, F.; Bruggink, R.; Nijsink, J.; Maal, T.J.J.; Ongkosuwito, E.M. Fusion of intra-oral scans in cone-beam computed tomography scans. Clin. Oral Investig. 2021, 25, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Read-Fuller, A. Cone Beam Computed Tomography in Oral and Maxillofacial Surgery: An Evidence-Based Review. Dent. J. 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Goymen, M.; Gulec, A. Effect of photobiomodulation therapies on the root resorption associated with orthodontic forces: A pilot study using micro computed tomography. Clin. Oral Investig. 2020, 24, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Erpaçal, B.; Adıgüzel, Ö.; Cangül, S. The use of micro-computed tomography in dental applications. Int. Dent. Res. 2019, 9, 78–91. [Google Scholar] [CrossRef]
- Akli, E.; Araujo, E.A.; Kim, K.B.; McCray, J.F.; Hudson, M.J. Enamel thickness of maxillary canines evaluated with microcomputed tomography scans. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 391–399. [Google Scholar] [CrossRef]
- Abdelkarim, A. Cone-Beam Computed Tomography in Orthodontics. Dent. J. 2019, 7, 89. [Google Scholar] [CrossRef]
- Schmitt, J. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.; Brezinski, M.E. Optical Coherence Tomography: An Emerging Technology for Biomedical Imaging and Optical Biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Brezinski, M.E.; Tearney, G.J.; Boppart, S.; Bouma, B.; Hee, M.R.; Southern, J.F.; Swanson, E.A. Optical biopsy and imaging using optical coherence tomography. Nat. Med. 1995, 1, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Seong, D.; Han, S.; Jeon, D.; Kim, Y.; Wijesinghe, R.E.; Ravichandran, N.K.; Lee, J.; Lee, J.; Kim, P.; Lee, D.-E.; et al. Dynamic Compensation of Path Length Difference in Optical Coherence Tomography by an Automatic Temperature Control System of Optical Fiber. IEEE Access 2020, 8, 77501–77510. [Google Scholar] [CrossRef]
- Chong, S.L.; Darling, C.L.; Fried, D. Nondestructive measurement of the inhibition of demineralization on smooth surfaces using polarization-sensitive optical coherence tomography. Lasers Surg. Med. 2007, 39, 422–427. [Google Scholar] [CrossRef]
- Le Bs, M.H.; Darling, C.L.; Fried, D. Automated analysis of lesion depth and integrated reflectivity in PS-OCT scans of tooth demineralization. Lasers Surg. Med. 2010, 42, 62–68. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.; Shirazi, M.F.; Jo, H.; Kim, P.; Wijesinghe, R.E.; Jeon, M.; Kim, J. Classification of human gingival sulcus using swept-source optical coherence tomography: In vivo imaging. Infrared Phys. Technol. 2019, 98, 155–160. [Google Scholar] [CrossRef]
- Baumgartner, A.; Dichtl, S.; Hitzenberger, C.; Sattmann, H.; Robl, B.; Moritz, A.; Fercher, A.; Sperr, W. Polarization–Sensitive Optical Coherence Tomography of Dental Structures. Caries Res. 1999, 34, 59–69. [Google Scholar] [CrossRef]
- Otis, L.L.; Everett, M.J.; Sathyam, U.S.; Colston, B.W., Jr. Optical coherence tomography: A new imaging: Technology for dentistry. J. Am. Dent. Assoc. 2000, 131, 511–514. [Google Scholar] [CrossRef]
- Fried, D.; Xie, J.; Shafi, S.; Featherstone, J.D.B.; Breunig, T.M.; Le, C.Q. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J. Biomed. Opt. 2002, 7, 618–628. [Google Scholar] [CrossRef]
- Ravichandran, N.K.; Lakshmikantha, H.T.; Park, H.-S.; Jeon, M.; Kim, J. Analysis of Enamel Loss by Prophylaxis and Etching Treatment in Human Tooth Using Optical Coherence Tomography: An In Vitro Study. J. Healthc. Eng. 2019, 2019, 8973825. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Son, K.; Park, J.; Lee, J.; Kang, S.H.; Wijesinghe, R.E.; Kim, P.; Hwang, J.H.; Park, S.; Yun, B.-J.; et al. Non-Ionized, High-Resolution Measurement of Internal and Marginal Discrepancies of Dental Prosthesis Using Optical Coherence Tomography. IEEE Access 2019, 7, 6209–6218. [Google Scholar] [CrossRef]
- Zhou, Y.; Matin, K.; Shimada, Y.; Wang, G.; Sadr, A.; Tagami, J. Detection and analysis of early degradation at resin-dentin interface by optical coherence tomography (OCT) and confocal laser scanning microscope (CLSM). J. Dent. 2021, 106, 103583. [Google Scholar] [CrossRef] [PubMed]
- Sinescu, C.; Manescu, A.; Negrutiu, M.L.; Rominu, M.; Marsavina, L.; Giuliani, A.; Podoleanu, A.G. Imagistic Evaluation of the Orthodontics Interfaces. Adv. Eng. Forum 2013, 8–9, 317–326. [Google Scholar] [CrossRef]


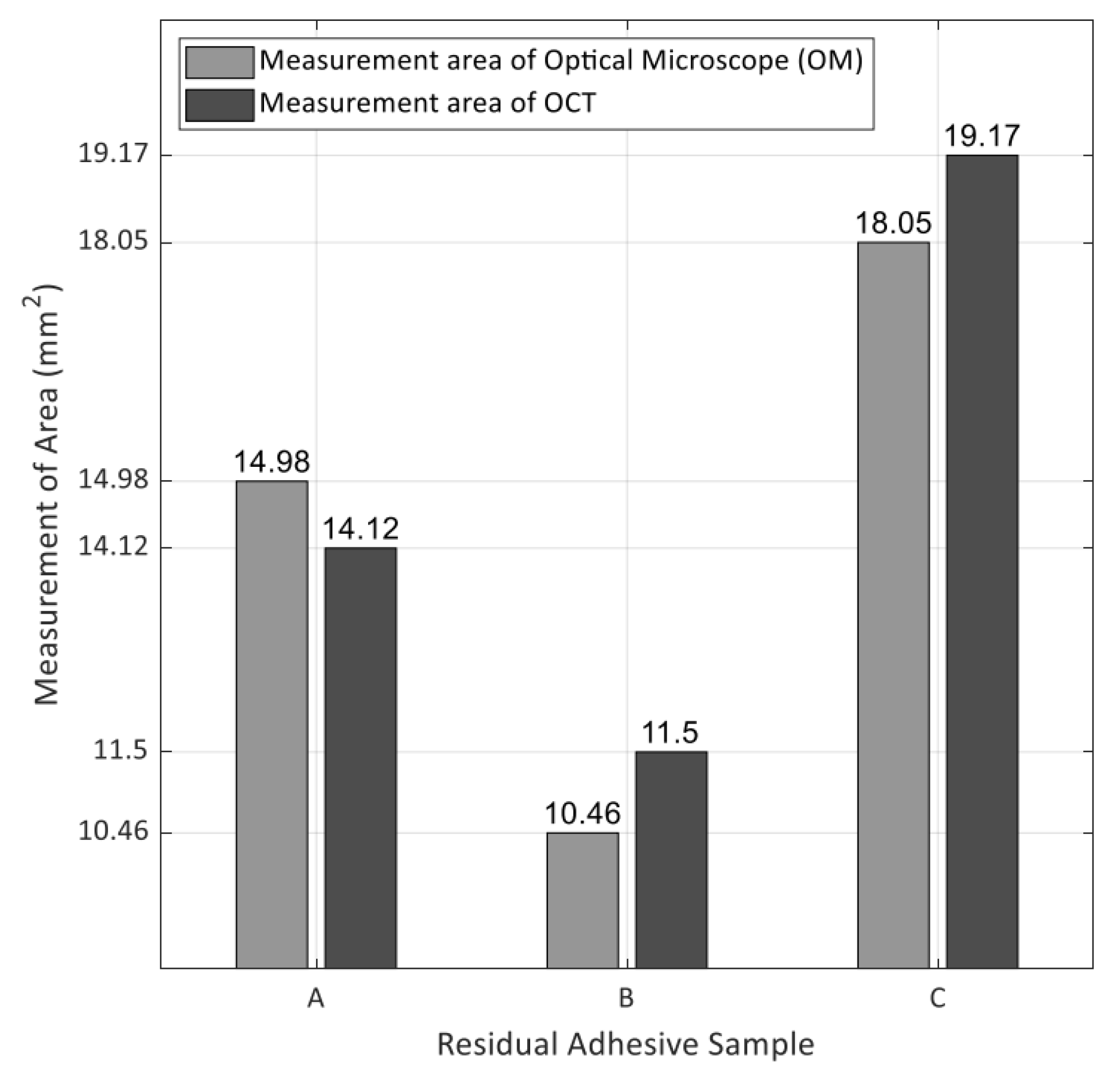
| Sample | Measurement Area of Optical Microscope (mm2) | Measurement Area of OCT (mm2) | Difference/Ratio |
|---|---|---|---|
| A | 14.978 | 14.116 | 0.862/−6% |
| B | 10.459 | 11.499 | −1.04/+10% |
| C | 18.051 | 19.174 | −1.123/+6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Jung, G.-I.; Jeon, D.; Wijesinghe, R.E.; Seong, D.; Lee, J.; Do, W.J.; Kwon, S.-M.; Lee, J.H.; Hwang, J.H.; et al. Non-Invasive Optical Coherence Tomography Data-Based Quantitative Algorithm for the Assessment of Residual Adhesive on Bracket-Removed Dental Surface. Sensors 2021, 21, 4670. https://doi.org/10.3390/s21144670
Kim Y, Jung G-I, Jeon D, Wijesinghe RE, Seong D, Lee J, Do WJ, Kwon S-M, Lee JH, Hwang JH, et al. Non-Invasive Optical Coherence Tomography Data-Based Quantitative Algorithm for the Assessment of Residual Adhesive on Bracket-Removed Dental Surface. Sensors. 2021; 21(14):4670. https://doi.org/10.3390/s21144670
Chicago/Turabian StyleKim, Yoonseok, Gu-In Jung, Deokmin Jeon, Ruchire Eranga Wijesinghe, Daewoon Seong, Jaeyul Lee, Woo Jong Do, Sung-Min Kwon, Jong Hoon Lee, Jun Ho Hwang, and et al. 2021. "Non-Invasive Optical Coherence Tomography Data-Based Quantitative Algorithm for the Assessment of Residual Adhesive on Bracket-Removed Dental Surface" Sensors 21, no. 14: 4670. https://doi.org/10.3390/s21144670
APA StyleKim, Y., Jung, G.-I., Jeon, D., Wijesinghe, R. E., Seong, D., Lee, J., Do, W. J., Kwon, S.-M., Lee, J. H., Hwang, J. H., Kim, H. D., Lee, K.-B., Jeon, M., & Kim, J. (2021). Non-Invasive Optical Coherence Tomography Data-Based Quantitative Algorithm for the Assessment of Residual Adhesive on Bracket-Removed Dental Surface. Sensors, 21(14), 4670. https://doi.org/10.3390/s21144670









