Beehive Air Sampling and Sensing Device Operation in Apicultural Applications—Methodological and Technical Aspects
Abstract
:1. Introduction
2. Beehive Air Measurement for Beekeeping
3. Experimental Part
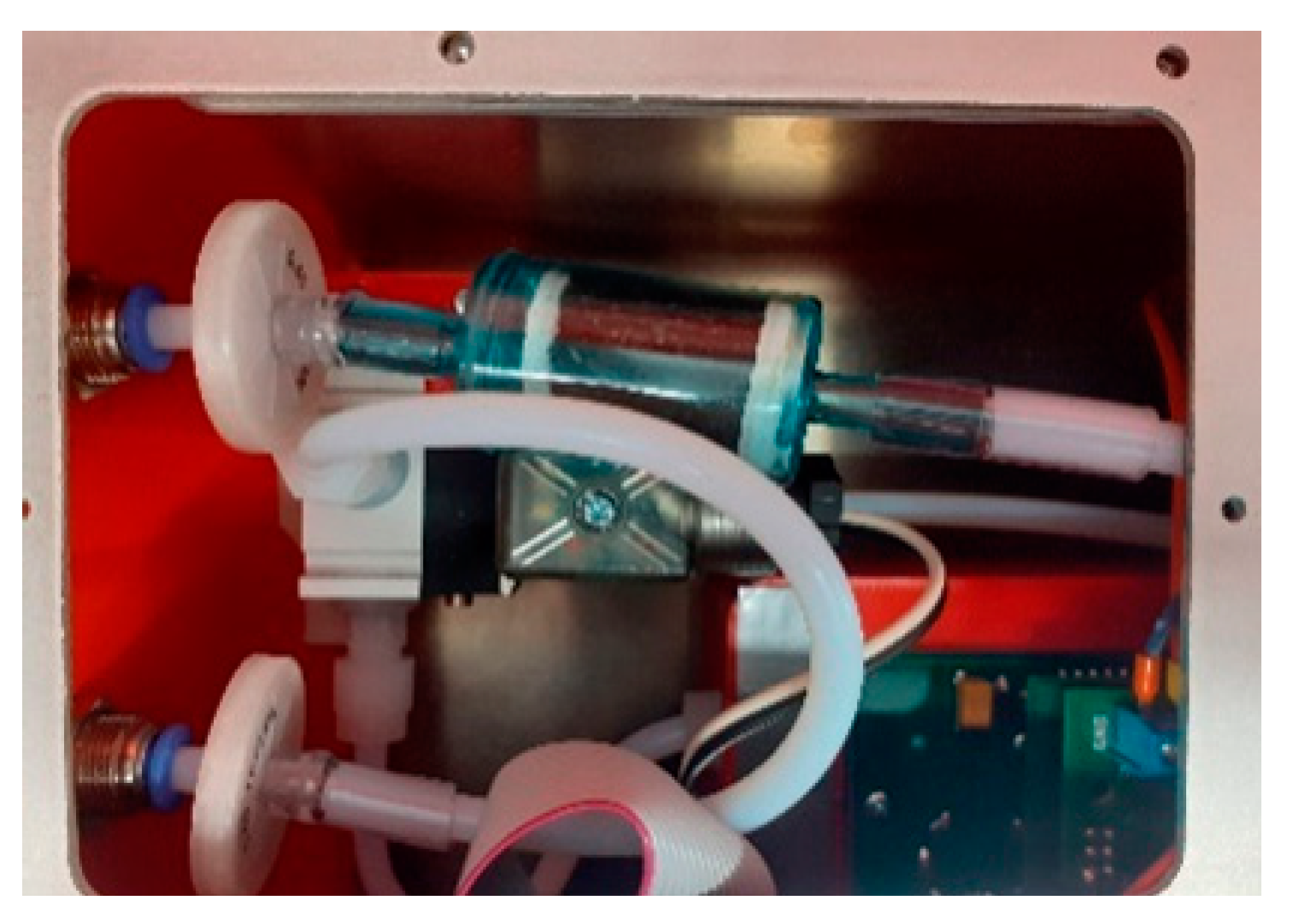
3.1. Gas Sensing Device
- gas sampling unit with sample delivery system;
- array of six low-cost semiconductor gas sensors (TGS822, TGS823, TGS826, TGS2600, TGS2602, TGS2603) with partial or overlapping sensitivities;
- circuit for an analog signal conditioning;
- module for data acquisition, and storage;
- signal preprocessing module;
- pattern recognition machine;
- GSM module for data transmission;
- GPS module for instrument localization.
3.2. Apiaries
4. Results and Discussion
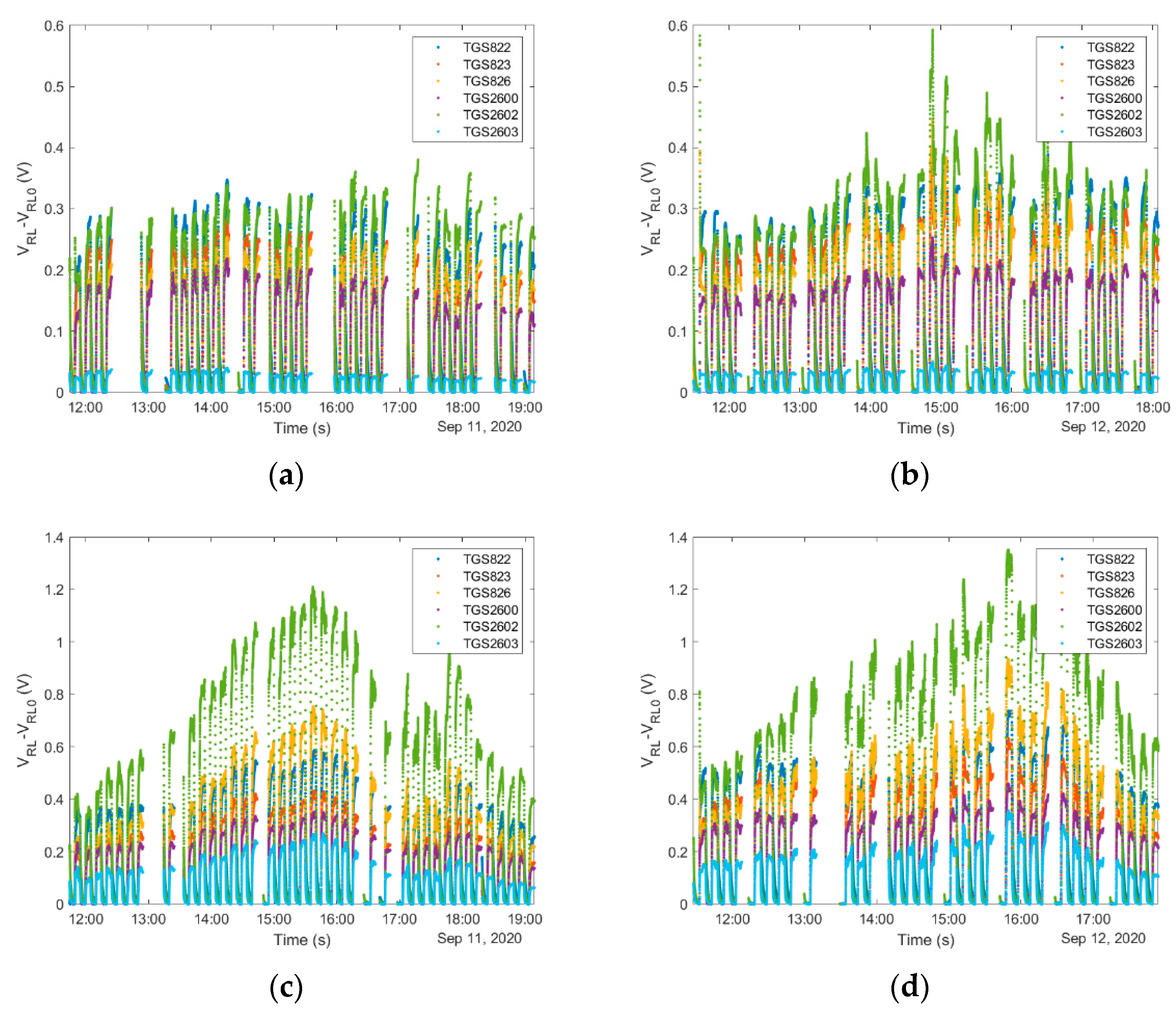
4.1. Sampling Method and Device Operation
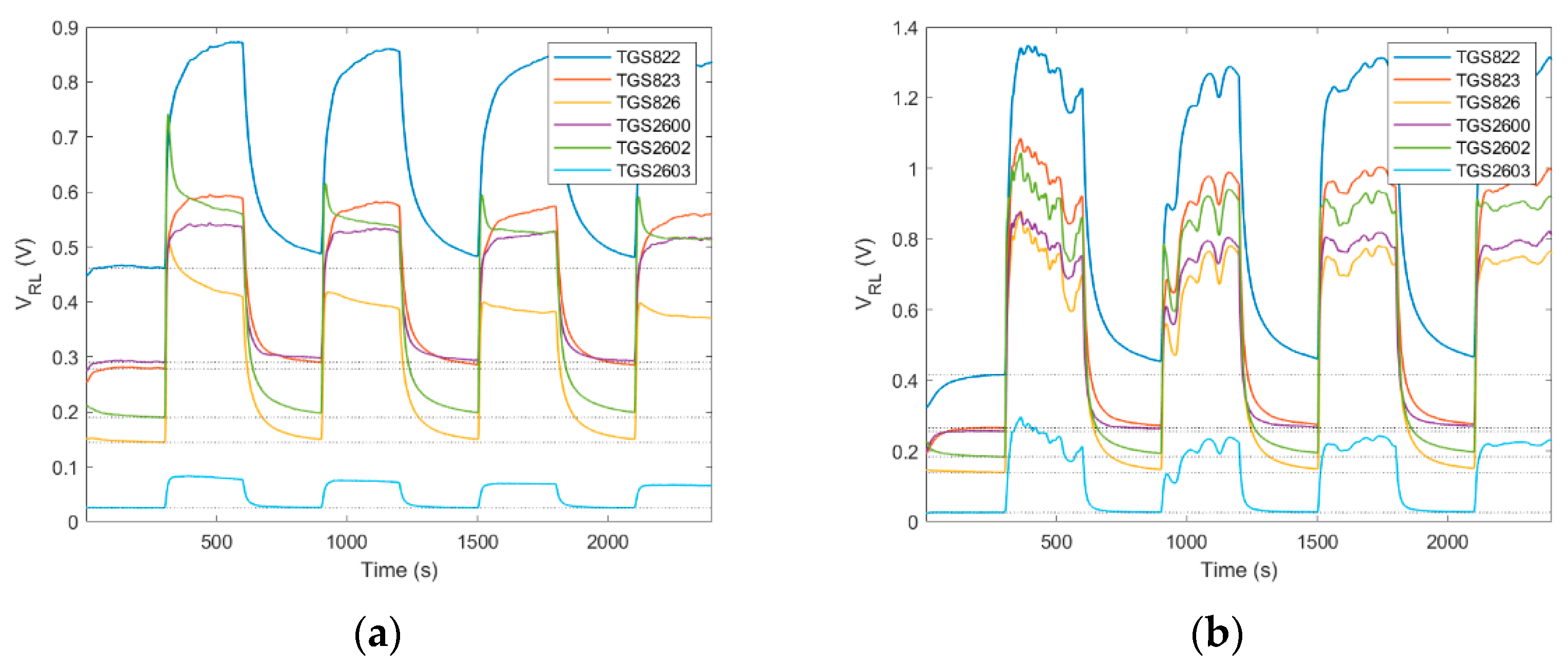
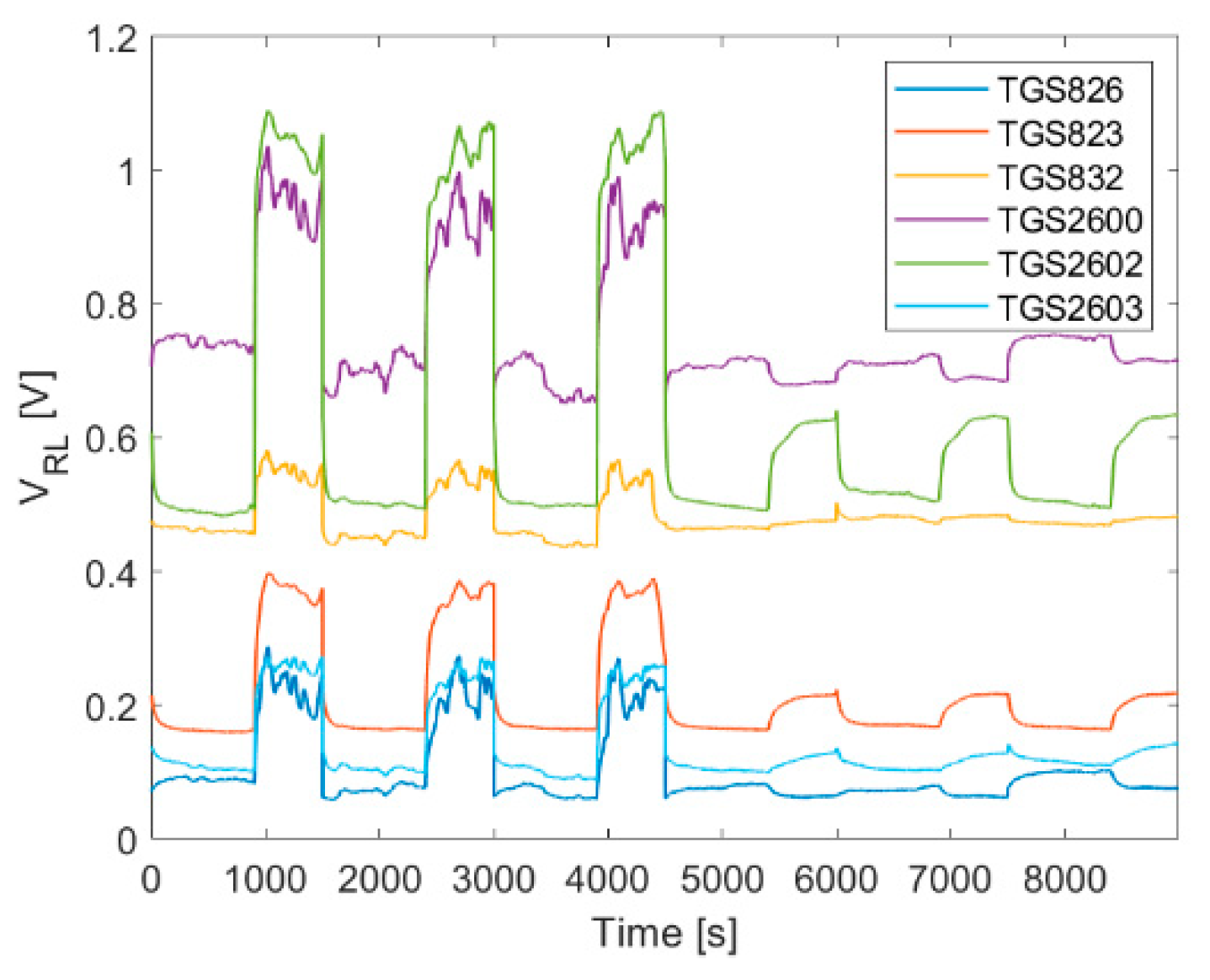
4.1.1. Diffusive Sampling
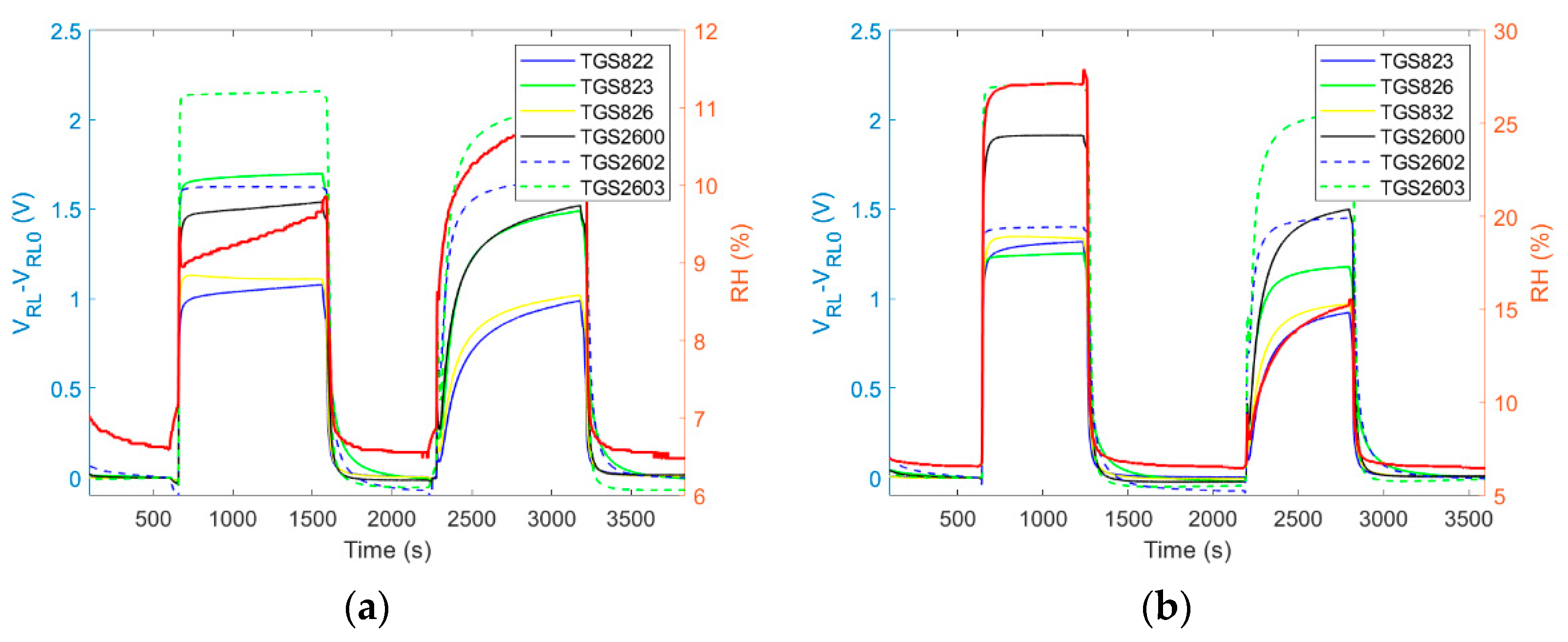
4.1.2. Dynamic Sampling
4.1.3. Continuous Sampling
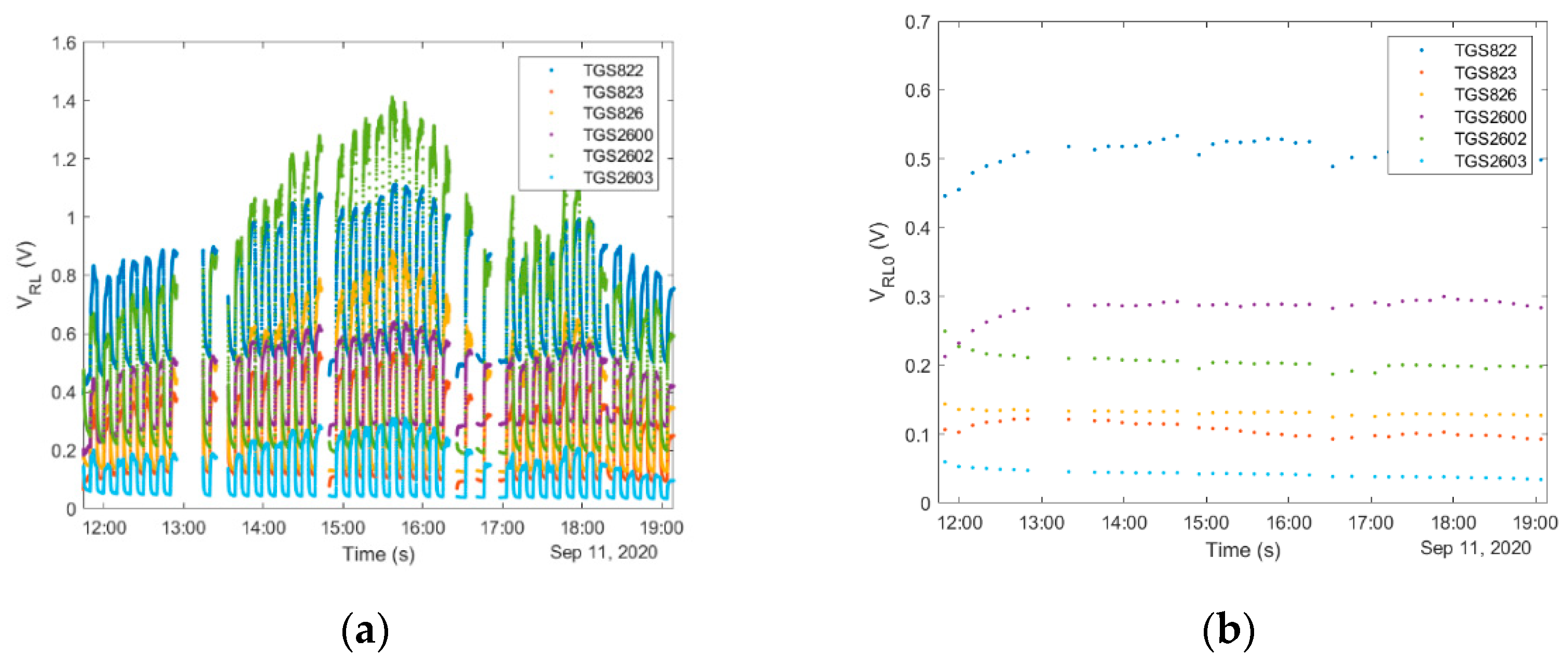
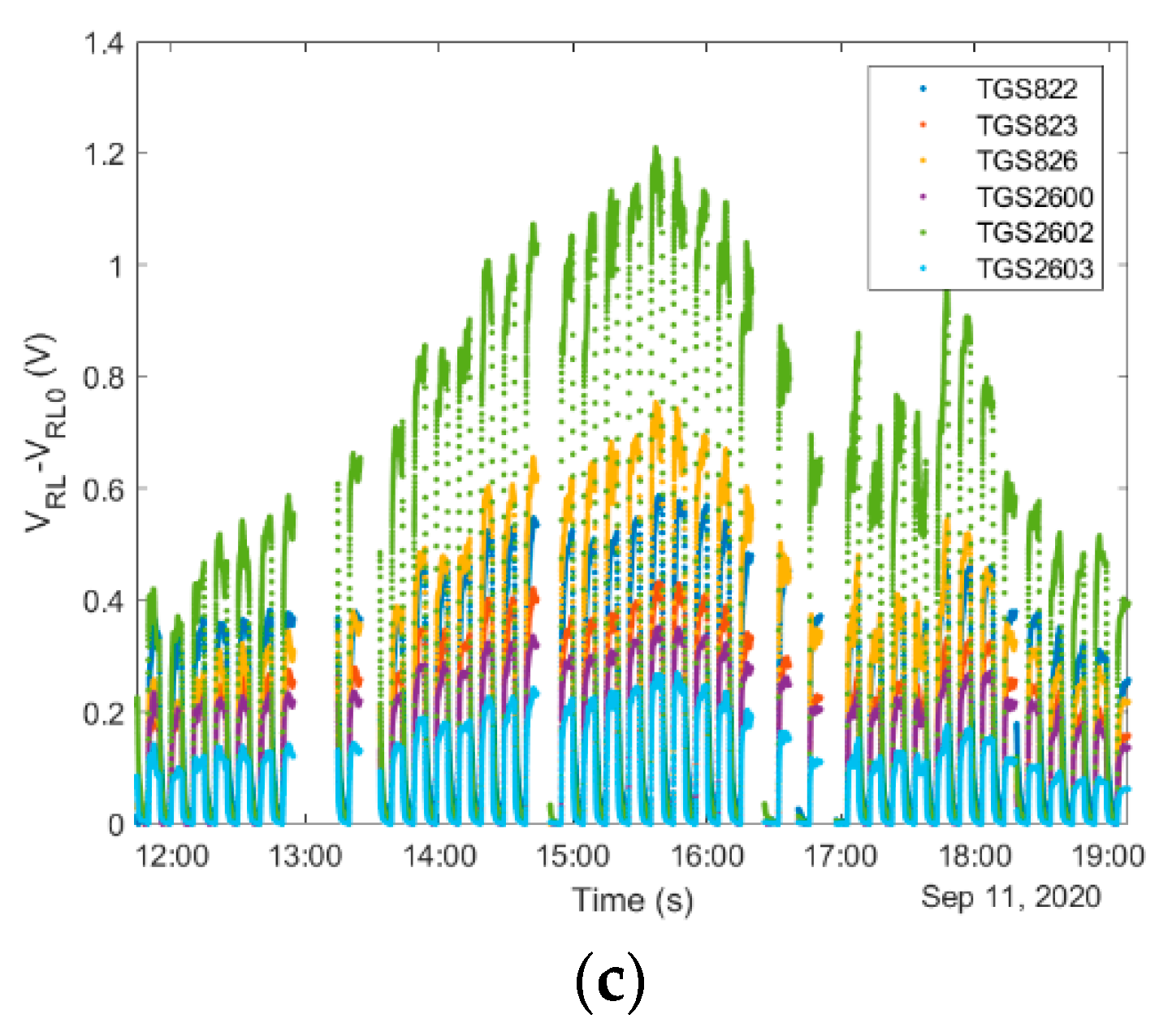
4.1.4. ‘Exposure-Cleaning’ Operation
4.1.5. Consideration of Temporal Factors Influencing Operation of Gas Sensing Devices in Beekeeping
4.2. Sampling System
4.2.1. Sample Probe
4.2.2. Sampling Point Selection
4.2.3. Sample Conditioning Unit
4.2.4. System of Gas Delivery
5. Conclusions
- Dynamic sampling allows for the collection of representative samples of beehive air, and its unobstructed delivery to sensors chamber, which results in high accuracy of measurement. Proper gas flow rate selection is crucial.
- The ‘exposure-cleaning’ operation allows for performing short-term measurements as well as long-term monitoring of beehive air. It prevents measurement bias and assures good temporal resolution of the acquired information. An adequate duration of exposure and cleaning operations is important.
- The sampling system cannot change the composition of the gas sample. Proper construction materials are needed for this purpose; we chose PTFE and PE.
- The sample probe geometry has to be defined on the basis of the intended localization of the sampling point in the beehive. Assess constraints apply.
- The location of the sampling point in the beehive should be strictly defined, as it influences gas sensor responses.
- The sampling system has to be fitted with elements that protect the sensing device from particulate matter and water condensate. This role is fulfilled by adequate filters. A Nafion drier was excluded as it changed the chemical composition of the gas sample.
- Elements of the sampling system have to be checked, cleaned, dried, or replaced as a part of regular daily maintenance of the gas sensing device. It is necessary for attaining proper measurement results and to prevent the damage of the instrument.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Watson, K.; Stallins, J.A. Honey Bees and Colony Collapse Disorder: A Pluralistic Reframing. Geogr. Compass 2016, 10, 222–236. [Google Scholar] [CrossRef]
- Sperandio, G.; Simonetto, A.; Carnesecchi, E.; Costa, C.; Hatjina, F.; Tosig, S.; Gilioli, G. Beekeeping and honey bee colony health: A review and conceptualization of beekeeping management practices implemented in Europe. Sci. Total Environ. 2019, 696, 133795. [Google Scholar] [CrossRef]
- Zacepins, A.; Stalidzans, E.; Meitalovs, J. Application of information technologies in precision apiculture. In Proceedings of the 13th International Conference on Precision Agriculture (ICPA 2012), Indianapolis, IN, USA, 20–23 July 2012. [Google Scholar]
- Bozek, K.; Hebert, L.; Portugal, Y.; Stephens, G.J. Markerless tracking of an entire honey bee colony. Nat. Commun. 2021, 12, 1733. [Google Scholar] [CrossRef]
- Ferrari, S.; Silva, M.; Guarino, M.; Berckmans, D. Monitoring of swarming sounds in bee hives for early detection of the swarming period. Comput. Electron. Agric. 2008, 64, 72–77. [Google Scholar] [CrossRef]
- Sánchez, V.; Gil, S.; Flores, J.M.; Quiles, F.J.; Ortiz, M.A.; Luna, J.J. Implementation of an electronic system to monitor the thermoregulatory capacity of honeybee colonies in hives with open-screened bottom boards. Comput. Electron. Agric. 2015, 119, 209–216. [Google Scholar] [CrossRef]
- Meikle, W.G.; Weiss, M.; Maes, P.W.; Fitz, W.; Snyder, L.A.; Sheehan, T.; Mott, B.M.; Anderson, K.E. Internal hive temperature as a means of monitoring honey bee colony health in a migratory beekeeping operation before and during winter. Apidologie 2017, 48, 666–680. [Google Scholar] [CrossRef] [Green Version]
- Bilik, S.; Kratochvila, L.; Ligocki, A.; Bostik, O.; Zemcik, T.; Hybl, M.; Horak, K.; Zalud, L. Visual Diagnosis of the Varroa Destructor Parasitic Mite in Honeybees Using Object Detector Techniques. Sensors 2021, 21, 2764. [Google Scholar] [CrossRef]
- Arenas, A.; Fernandez, V.M.; Farina, W.M. Floral scents experienced within the colony affect long-term foraging preferences in honeybees. Apidologie 2008, 39, 714–772. [Google Scholar] [CrossRef]
- Mas, F.; Horner, R.; Brierley, S.; Harper, A.; Suckling, D.M. The Scent of Individual Foraging Bees. J. Chem. Ecol. 2020, 46, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Murphy, F.; Magno, M.; Whelan, P.M.; O’Halloran, J.; Popovici, E.M. b+WSN: Smart beehive with preliminary decision tree analysis for agriculture and honey bee health monitoring. Comput. Electron. Agric. 2016, 124, 211–219. [Google Scholar] [CrossRef]
- El-Wahed, A.A.A.; Farag, M.A.; Eraqi, W.A.; Mersal, G.A.M.; Zhao, C.; Khalifa, S.A.M.; El-Seedi, H.R. Unravelling the beehive air volatiles profile as analysed via solid-phase microextraction (SPME) and chemometrics. J. King Saud Univ. Sci. 2021, 33, 101449. [Google Scholar] [CrossRef]
- Cecchi, S.; Terenzi, A.; Orcioni, S.; Spinsante, S.; MarianiPrimiani, V.; Moglie, F.; Ruschioni, S.; Mattei, C.; Riolo, P.; Isidoro, N. Multi-sensor platform for real time measurements of honey bee hive parameters. IOP Conf. Series Earth Environ. Sci. 2019, 275, 012016. [Google Scholar] [CrossRef]
- Meikle, W.G.; Holst, N. Application of continuous monitoring of honeybee colonies. Apidologie 2015, 46, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Meng, Q.-H.; Lilienthal, A.J.; Qi, P.F. Development of compact electronic noses: A review. Meas. Sci. Technol. 2021, 32, 062002. [Google Scholar] [CrossRef]
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Gas sensor array and classifiers as a means of varroosis detection. Sensors 2020, 20, 117. [Google Scholar] [CrossRef] [Green Version]
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Detecting varroosis using a gas sensor system as a way to face the environmental threat. Sci. Total Environ. 2020, 722, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borecki, M.; Duk, M.; Kociubiński, A.; Korwin-Pawlowski, M.L. Multiparametric Methane Sensor for Environmental Monitoring. In Proceedings of the SPIE 10175, Electron Technology Conference 2016, Wisla, Poland, 22 December 2016; Volume 10715, p. 101750M. [Google Scholar]
- Miskell, G.; Salmond, J.; Grange, S.; Weissert, L.; Henshaw, G.; Williams, D. Reliable Long-Term Data from Low-Cost Gas Sensor Networks in the Environment. Proceedings 2017, 1, 400. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Szabo, C.; Sheng, Q.Z. Cleaning Environmental Sensing Data Streams Based on Individual Sensor Reliability. In Lecture Notes in Computer Science, Proceedings of the Web Information Systems Engineering—WISE 2014, Thessaloniki, Greece, 12–14 October 2014; Benatallah, B., Bestavros, A., Manolopoulos, Y., Vakali, A., Zhang, Y., Eds.; Springer: Cham, Switzerland, 2014; Volume 8787. [Google Scholar]
- Betty, C.A.; Choudhury, S.; Girija, K.G. Reliability studies of highly sensitive and specific multi-gas sensor based on nanocrystalline SnO2 film. Sens. Actuators B Chem. 2014, 193, 484–491. [Google Scholar] [CrossRef]
- Szczurek, A.; Maciejewska, M. Gas sensing method applicable to real conditions. Meas. Sci. Technol. 2013, 24, 045103. [Google Scholar] [CrossRef]
- Szczurek, A.; Maciejewska, M.; Zelek, M. Influence of gas sampling on MOS response in real measurement conditions. Procedia Eng. 2014, 87, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Figaro Sensor. Available online: https://www.figaro.co.jp/en/product/sensor/ (accessed on 6 June 2021).
- Szczurek, A.; Maciejewska, M.; Zajiczek, Ż.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. The effectiveness of Varroa destructor infestation classification using an E-nose depending on the time of day. Sensors 2020, 20, 2532. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Beehive indoor air measurement for bee colony diagnostics. In Indoor Air 2020: Creative & Smart Solutions for Better Built Environments, Proceedings of the 16th Conference of the International Society of Indoor Air Quality & Climate, Seoul, Korea, 1 November 2020; The International Society of Indoor Air Quality & Climate: Philadelphia, PA, USA, 2020; pp. 2439–2444. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurek, A.; Maciejewska, M. Beehive Air Sampling and Sensing Device Operation in Apicultural Applications—Methodological and Technical Aspects. Sensors 2021, 21, 4019. https://doi.org/10.3390/s21124019
Szczurek A, Maciejewska M. Beehive Air Sampling and Sensing Device Operation in Apicultural Applications—Methodological and Technical Aspects. Sensors. 2021; 21(12):4019. https://doi.org/10.3390/s21124019
Chicago/Turabian StyleSzczurek, Andrzej, and Monika Maciejewska. 2021. "Beehive Air Sampling and Sensing Device Operation in Apicultural Applications—Methodological and Technical Aspects" Sensors 21, no. 12: 4019. https://doi.org/10.3390/s21124019
APA StyleSzczurek, A., & Maciejewska, M. (2021). Beehive Air Sampling and Sensing Device Operation in Apicultural Applications—Methodological and Technical Aspects. Sensors, 21(12), 4019. https://doi.org/10.3390/s21124019




