Which Factors Affect the Stress of Intraoperative Orthopedic Surgeons by Using Electroencephalography Signals and Heart Rate Variability?
Abstract
1. Introduction
- (1)
- Can we recognize intraoperative real-time stress in orthopedic surgeons?
- (2)
- Which factors affecting the stress of intraoperative orthopedic surgeons can be measured using EEG and HRV?
2. Materials and Methods
2.1. Subjects
2.2. Materials
2.3. Methods
2.4. Statistical Analyses
3. Results
3.1. Demographics
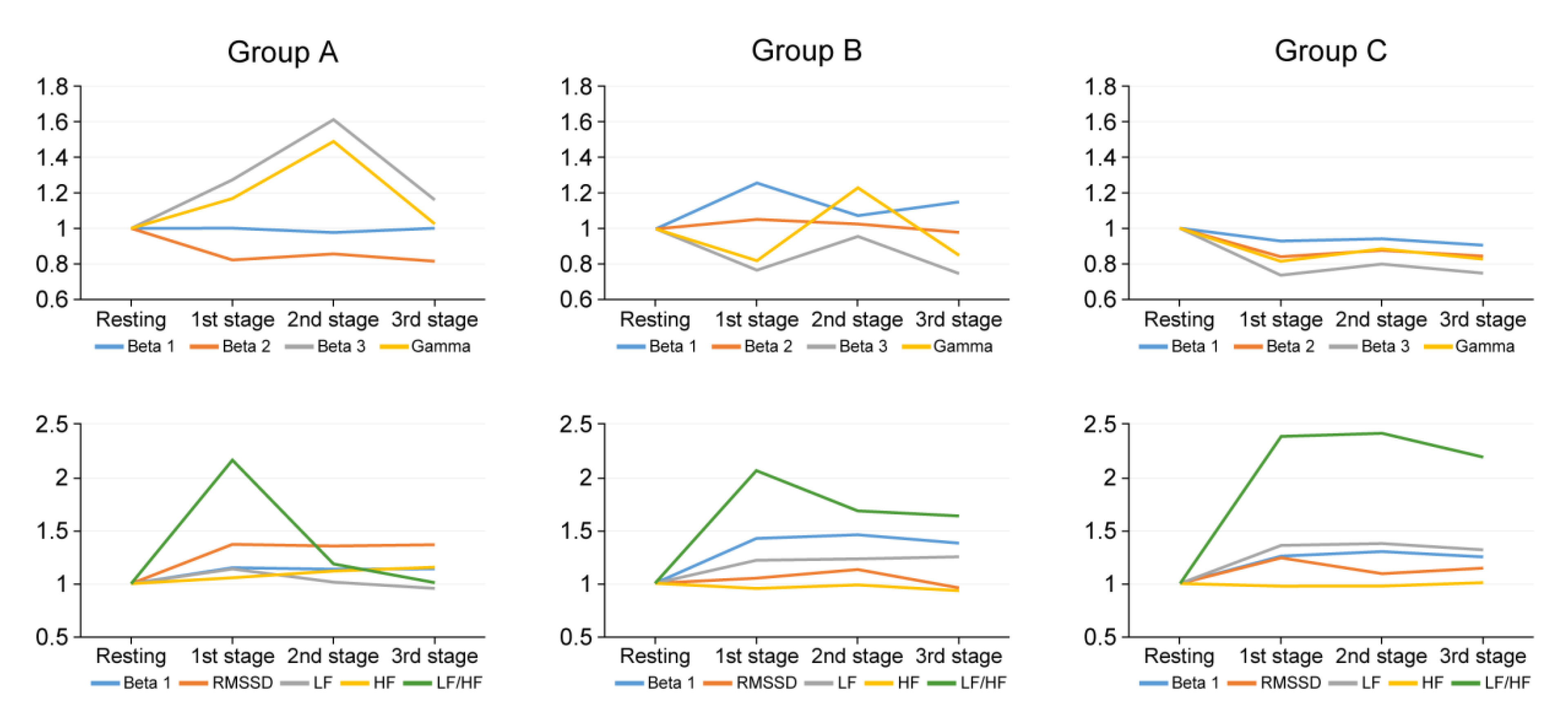
3.2. Stress Analysis According to the Experience of Surgeons
3.3. Subgroup Analysis Whether to Use Tourniquet or Not
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, S.; Sevdalis, N.; Nestel, D.; Tierney, T.; Woloshynowych, M.; Kneebone, R. Managing intraoperative stress: What do surgeons want from a crisis training program? Am. J. Surg. 2009, 197, 537–543. [Google Scholar] [CrossRef]
- Arora, S.; Sevdalis, N.; Nestel, D.; Woloshynowych, M.; Darzi, A.; Kneebone, R. The impact of stress on surgical performance: A systematic review of the literature. Surgery 2010, 147, 318–330.e6. [Google Scholar] [CrossRef]
- Balch, C.M.; Freischlag, J.A.; Shanafelt, T.D. Stress and burnout among surgeons: Understanding and managing the syndrome and avoiding the adverse consequences. Arch. Surg. 2009, 144, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.J.; Samdani, A.F.; Brusalis, C.M.; Blumberg, T.; Asghar, J.; Bastrom, T.P.; Pasha, S.; Refakis, C.A.; Pahys, J.M.; Flynn, J.M.; et al. Youth and Experience: The Effect of Surgeon Experience on Outcomes in Cerebral Palsy Scoliosis Surgery. Spine Deform. 2018, 6, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Carswell, C.M.; Clarke, D.; Seales, W.B. Assessing Mental Workload During Laparoscopic Surgery. Surg. Innov. 2005, 12, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Alloy, L.B. Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clin. Psychol. Rev. 2016, 50, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Järvelin-Pasanen, S.; Sinikallio, S.; Tarvainen, M.P. Heart rate variability and occupational stress—Systematic review. Ind. Health 2018, 56, 500–511. [Google Scholar] [CrossRef]
- Kuhn, E.W.; Choi, Y.-H.; Schönherr, M.; Liakopoulos, O.J.; Rahmanian, P.B.; Choi, C.Y.-U.; Wittwer, T.; Wahlers, T. Intraoperative stress in cardiac surgery: Attendings versus residents. J. Surg. Res. 2013, 182, e43–e49. [Google Scholar] [CrossRef]
- Patti, M.G.; Schlottmann, F.; Sarr, M.G. The Problem of Burnout Among Surgeons. JAMA Surg. 2018, 153, 403–404. [Google Scholar] [CrossRef]
- Weenk, M.; Alken, A.P.; Engelen, L.J.; Bredie, S.J.; Van De Belt, T.H.; Van Goor, H. Stress measurement in surgeons and residents using a smart patch. Am. J. Surg. 2018, 216, 361–368. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Sung, S.; Lee, S.-B.; Lee, H.-M.; Moon, S.-H.; Lee, B.H. Intraoperative real-time stress in degenerative lumbar spine surgery: Simultaneous analysis of electroencephalography signals and heart rate variability: A pilot study. Spine J. 2020, 20, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-I.; Shim, M.; Lee, S.M.; Huh, H.J.; Huh, S.; Joo, J.-Y.; Lee, S.-H.; Chae, J.-H. Increased beta power in the bereaved families of the Sewol ferry disaster: A paradoxical compensatory phenomenon? A two-channel electroencephalography study. Psychiatry Clin. Neurosci. 2017, 71, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Technical Aspects of Brain Rhythms and Speech Parameters. In Introduction to EEG and Speech-Based Emotion Recognition; Abhang, P.A., Gawali, B.W., Mehrotra, S.C., Eds.; Academic Press: Aurangabad, India, 2016; Chapter 3; pp. 51–79. [Google Scholar]
- Vescio, B.; Salsone, M.; Gambardella, A.; Quattrone, A. Comparison between Electrocardiographic and Earlobe Pulse Photoplethysmographic Detection for Evaluating Heart Rate Variability in Healthy Subjects in Short and Long-Term Recordings. Sensors 2018, 18, 844. [Google Scholar] [CrossRef] [PubMed]
- Malik, B. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Saeed, S.M.U.; Anwar, S.M.; Khalid, H.; Majid, M.; Bagci, A.U. EEG Based Classification of Long-Term Stress Using Psychological Labeling. Sensors 2020, 20, 1886. [Google Scholar] [CrossRef]
- Tatum, W.O., IV; Husain, A.M.; Benbadis, S.R.; Kaplan, P.W. Normal adult EEG and patterns of uncertain significance. J. Clin. Neurophysiol. 2006, 23, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.P.; Brodie, D.A. Effects of short-term psychological stress on the time and frequency domains of heart-rate variability. Percept. Mot. Skills 2000, 91, 515–524. [Google Scholar] [CrossRef]
- Hoogerwerf, M.D.; Veldhuizen, I.J.T.; Tarvainen, M.P.; Merz, E.M.; Huis in ‘t Veld, E.M.J.; de Kort, W.L.A.M.; Sluiter, J.K.; Frings-Dresen, M.H.W. Physiological stress response patterns during a blood donation. Vox Sang. 2018, 113, 357–367. [Google Scholar] [CrossRef]
- Jarczewski, J.; Furgała, A.; Winiarska, A.; Kaczmarczyk, M.; Poniatowski, A. Cardiovascular response to different types of acute stress stimulations. Folia Med. Cracov. 2019, 59, 95–110. [Google Scholar]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef]
- Cai, D.F.; Fan, Q.H.; Zhong, H.H.; Peng, S.; Song, H. The effects of tourniquet use on blood loss in primary total knee arthroplasty for patients with osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Noh, J.H.; Lee, J.W.; Nam, Y.J.; Choi, K.Y. Is Intraoperative Use of QuikClot Combat Gauze Effective for Hemostasis after Total Knee Arthroplasty? Clin. Orthop. Surg. 2017, 9, 43–49. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Sukucu, S.; Erdogan, S.; Kabukcuoglu, Y.S. Tourniquet use in orthopedic surgery: A descriptive survey study among Turkish orthopedic surgeons and residents in Istanbul. Acta Orthop. Traumatol. Turc. 2014, 48, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Broeders, J.A.J.L.; Draaisma, W.A.; Van Lanschot, J.J.B.; Gooszen, H.G. Impact of Surgeon Experience on 5-Year Outcome of Laparoscopic Nissen Fundoplication. Arch. Surg. 2011, 146, 340. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.L. A cumulative analysis of an individual surgeon’s early experience with elective open abdominal aortic aneurysm repair. Am. J. Surg. 2005, 189, 469–473. [Google Scholar] [CrossRef]
- Nüssler, E.; Eskildsen, J.K.; Bixo, M.; Löfgren, M. Impact of surgeon experience on routine prolapse operations. Int. Urogynecol. J. 2018, 29, 297–306. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kanemitsu, S. Surgeons’ stress from surgery and night duty: A multi-institutional study. Arch. Surg. 2011, 146, 271–278. [Google Scholar] [CrossRef]
- Kragh, J.F.; Swan, K.G.; Smith, D.C.; Mabry, R.L.; Blackbourne, L.H. Historical review of emergency tourniquet use to stop bleeding. Am. J. Surg. 2012, 203, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Reisener, M.-J.; Hughes, A.P.; Schadler, P.; Forman, A.; Sax, O.C.; Shue, J.; Cammisa, F.P.; Sama, A.A.; Girardi, F.P.; Mancuso, C.A. Expectations of Lumbar Surgery Outcomes among Opioid Users Compared with Non-Users. Asian Spine J. 2020, 14, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.J.; Yeo, W.; Ling, M.Z.; Fong, P.L.; Guo, C.M.; Chen, J.L.T.; Soh, R.C.C. The Effect of Body Mass Index on Long-Term Patient-Reported Outcome Scores after Anterior Cervical Discectomy and Fusion in an Asian Population: A 2-Year Study. Asian Spine J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.M.; Lee, H.-J.; Kang, T.; Chang, S.Y.; Kim, S.-M.; Chang, B.-S.; Lee, C.-K.; Kim, H. Clinical Significance of Improved Intraoperative Neurophysiological Monitoring Signal during Spine Surgery: A Retrospective Study of a Single-Institution Prospective Cohort. Asian Spine J. 2020, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
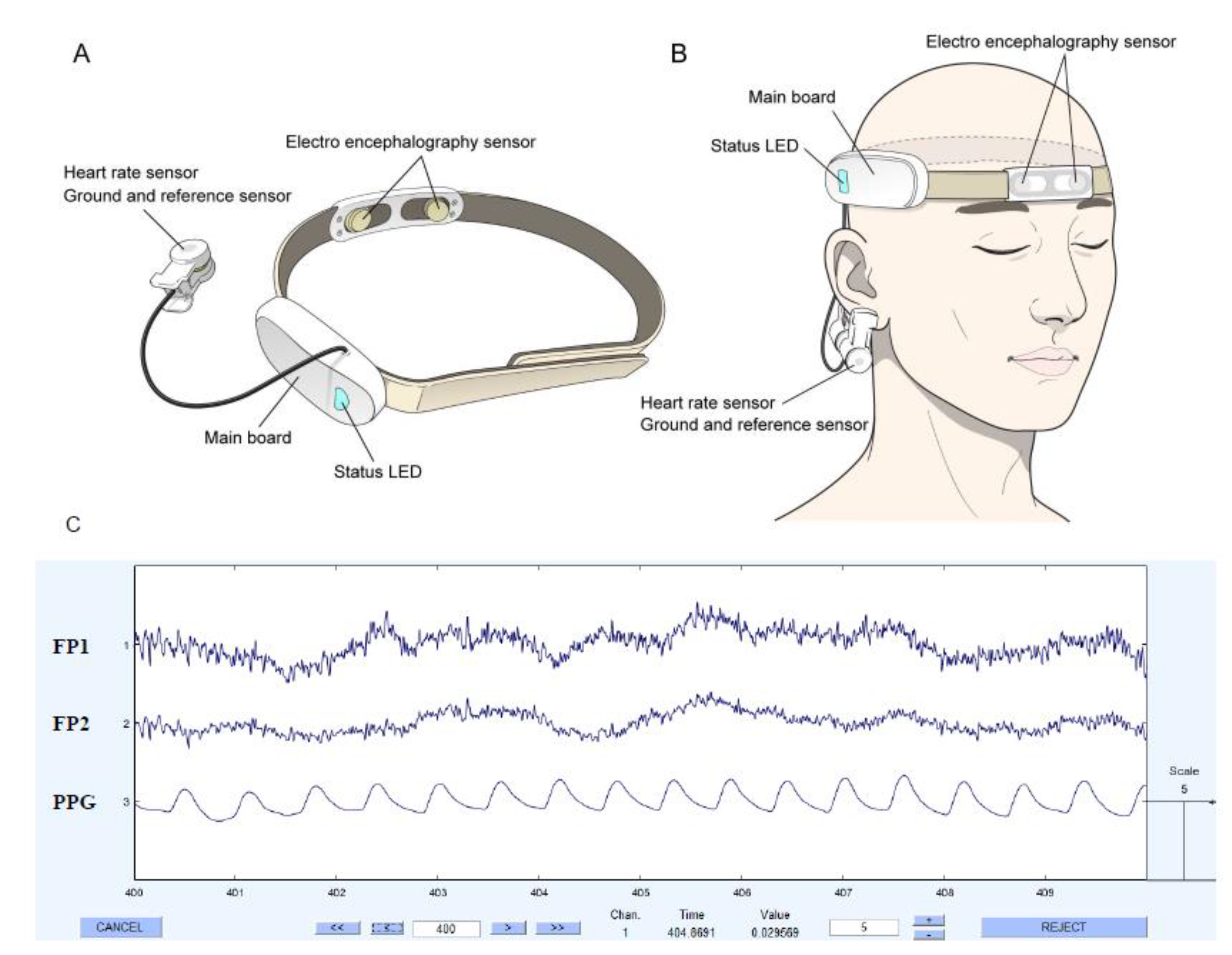
| Surgeon | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 43 | 35 | 35 | 36 | 43 | 56 | 50 | 38 |
| Experience in orthopedic subspecialty (years) | 10 | 5 | 2 | 3 | 9 | 23 | 16 | 4 |
| Orthopedic subspecialty | Spine | Hip | Spine | Hand and wrist | Knee | Knee | Elbow | Foot and ankle |
| Enrolled cases (cases) | 35 | 35 | 32 | 33 | 30 | 32 | 33 | 35 |
| Whether to use tourniquet | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Baseline of the EEG (resting status) | ||||||||
| Beta 1 wave | 0.05 ± 0.004 | 0.05 ± 0.004 | 0.03 ± 0.005 | 0.05 ± 0.001 | 0.06 ± 0.006 | 0.04 ± 0.004 | 0.03 ± 0.045 | 0.04 ± 0.005 |
| Beta 2 wave | 0.07 ± 0.009 | 0.08 ± 0.006 | 0.07 ± 0.008 | 0.09 ± 0.003 | 0.10 ± 0.002 | 0.08 ± 0.011 | 0.06 ± 0.010 | 0.09 ± 0.010 |
| Beta 3 wave | 0.13 ± 0.017 | 0.16 ± 0.009 | 0.15 ± 0.015 | 0.05 ± 0.011 | 0.15 ± 0.009 | 0.17 ± 0.022 | 0.14 ± 0.178 | 0.14 ± 0.020 |
| Gamma wave | 0.15 ± 0.021 | 0.19 ± 0.135 | 0.21 ± 0.026 | 0.10 ± 0.017 | 0.15 ± 0.004 | 0.18 ± 0.025 | 0.16 ± 0.217 | 0.13 ± 0.028 |
| Baseline of the HRVs (resting status) | ||||||||
| BPM | 58.0 ± 4.60 | 76.9 ± 8.48 | 76.4 ± 8.30 | 83.0 ± 4.42 | 59.4 ± 4.94 | 86.0 ± 4.80 | 61.4 ± 4.52 | 83.7 ± 6.35 |
| RMSSD | 41.9 ± 3.74 | 37.9 ± 5.89 | 39.5 ± 5.87 | 26.1 ± 4.63 | 27.3 ± 0.70 | 34.7 ± 7.46 | 23.5 ± 2.07 | 21.2 ± 3.91 |
| LF/HF | 1.2 ± 0.55 | 0.4 ± 0.84 | 0.7 ± 0.84 | 0.4 ± 0.32 | 0.6 ± 0.31 | 0.5 ± 0.46 | 1.3 ± 0.81 | 1.4 ± 0.34 |
| Group A | Group B | Group C | |
|---|---|---|---|
| Surgeons | No 3, 4, 8 | No 1, 2, 5 | No 6, 7 |
| Mean Age (years) | 36.3 | 40.3 | 53.0 |
| Enrolled cases (cases) | 100 | 100 | 65 |
| Orthopedic subspecialty | spine, hand and wrist, and foot and ankle | spine, hip, and knee | knee and elbow |
| Operation time (minutes) | 166.1 ± 90.3 | 143.3 ± 64.6 | 103.7 ± 35.7 |
| Intraoperative blood loss (cc) | 289.1 ± 526.4 | 209.7 ± 308.7 | 54.9 ± 44.0 |
| Beta 1 | Beta 2 | Beta 3 | Gamma | BPM | RMSSD | LF/HF | |
|---|---|---|---|---|---|---|---|
| 1st stage | |||||||
| Operation time | −0.004 | −0.086 | −0.082 | −0.178 | −0.097 | 0.233 * | −0.053 |
| Accumulated intraoperative blood loss | 0.325 ** | 0.251 * | 0.385 ** | −0.452 ** | 0.148 | −0.227 * | −0.042 |
| 2nd stage | |||||||
| Operation time | −0.053 | −0.167 | −0.197 | −0.233 * | −0.101 | 0.225 * | −0.041 |
| Accumulated intraoperative blood loss | 0.357 ** | 0.288 ** | 0.373 ** | −0.393 ** | −0.142 | −0.148 | −0.147 |
| 3rd stage | |||||||
| Operation time | −0.043 | −0.071 | −0.013 | −0.032 | −0.089 | 0.158 | −0.001 |
| Accumulated intraoperative blood loss | 0.411 ** | 0.366 ** | 0361 ** | −0.431 ** | −0.061 | −0.201 * | −0.308 ** |
| Beta 1 | Beta 2 | Beta 3 | Gamma | BPM | RMSSD | LF/HF | |
|---|---|---|---|---|---|---|---|
| 1st stage | |||||||
| Operation time | 0.308 ** | 0.501 ** | 0.303 ** | 0.042 | 0.303 ** | 0.035 | −0.046 |
| Accumulated intraoperative blood loss | −0.032 | 0.058 | 0.047 | 0.001 | 0.109 | −0.385 ** | 0.348 ** |
| 2nd stage | |||||||
| Operation time | 0.289 ** | 0.372 ** | 0.094 * | −0.305 ** | 0.259 ** | 0.048 | −0.008 |
| Accumulated intraoperative blood loss | −0.049 | 0.202 ** | 0.081 | 0.072 | 0.339 ** | −0.425 ** | 0.432 ** |
| 3rd stage | |||||||
| Operation time | 0.115 | 0.363 ** | 0.171 * | −0.013 | 0.205 ** | 0.002 | −0.142 |
| Accumulated intraoperative blood loss | −0.254 ** | −0.024 | 0.066 | 0.068 | 0.202 ** | −0.384 ** | 0.333 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.-W.; Lee, S.-B.; Sung, S.; Park, Y.; Ha, J.-W.; Kim, G.; Suk, K.-S.; Kim, H.-S.; Lee, H.-M.; Moon, S.-H.; et al. Which Factors Affect the Stress of Intraoperative Orthopedic Surgeons by Using Electroencephalography Signals and Heart Rate Variability? Sensors 2021, 21, 4016. https://doi.org/10.3390/s21124016
Kwon J-W, Lee S-B, Sung S, Park Y, Ha J-W, Kim G, Suk K-S, Kim H-S, Lee H-M, Moon S-H, et al. Which Factors Affect the Stress of Intraoperative Orthopedic Surgeons by Using Electroencephalography Signals and Heart Rate Variability? Sensors. 2021; 21(12):4016. https://doi.org/10.3390/s21124016
Chicago/Turabian StyleKwon, Ji-Won, Soo-Bin Lee, Sahyun Sung, Yung Park, Joong-Won Ha, Gihun Kim, Kyung-Soo Suk, Hak-Sun Kim, Hwan-Mo Lee, Seong-Hwan Moon, and et al. 2021. "Which Factors Affect the Stress of Intraoperative Orthopedic Surgeons by Using Electroencephalography Signals and Heart Rate Variability?" Sensors 21, no. 12: 4016. https://doi.org/10.3390/s21124016
APA StyleKwon, J.-W., Lee, S.-B., Sung, S., Park, Y., Ha, J.-W., Kim, G., Suk, K.-S., Kim, H.-S., Lee, H.-M., Moon, S.-H., & Lee, B. H. (2021). Which Factors Affect the Stress of Intraoperative Orthopedic Surgeons by Using Electroencephalography Signals and Heart Rate Variability? Sensors, 21(12), 4016. https://doi.org/10.3390/s21124016






