Construction and Application of Graphene Oxide-Bovine Serum Albumin Modified Extended Gate Field Effect Transistor Chiral Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. FTIR and UV-Vis Characterization
2.3. X-ray Photoelectron Spectroscopy
2.4. Electrochemical Measurements
2.5. Preparation of GO@BSA
2.6. Immobilization of BSA, GO or GO@BSA on Au Electrode
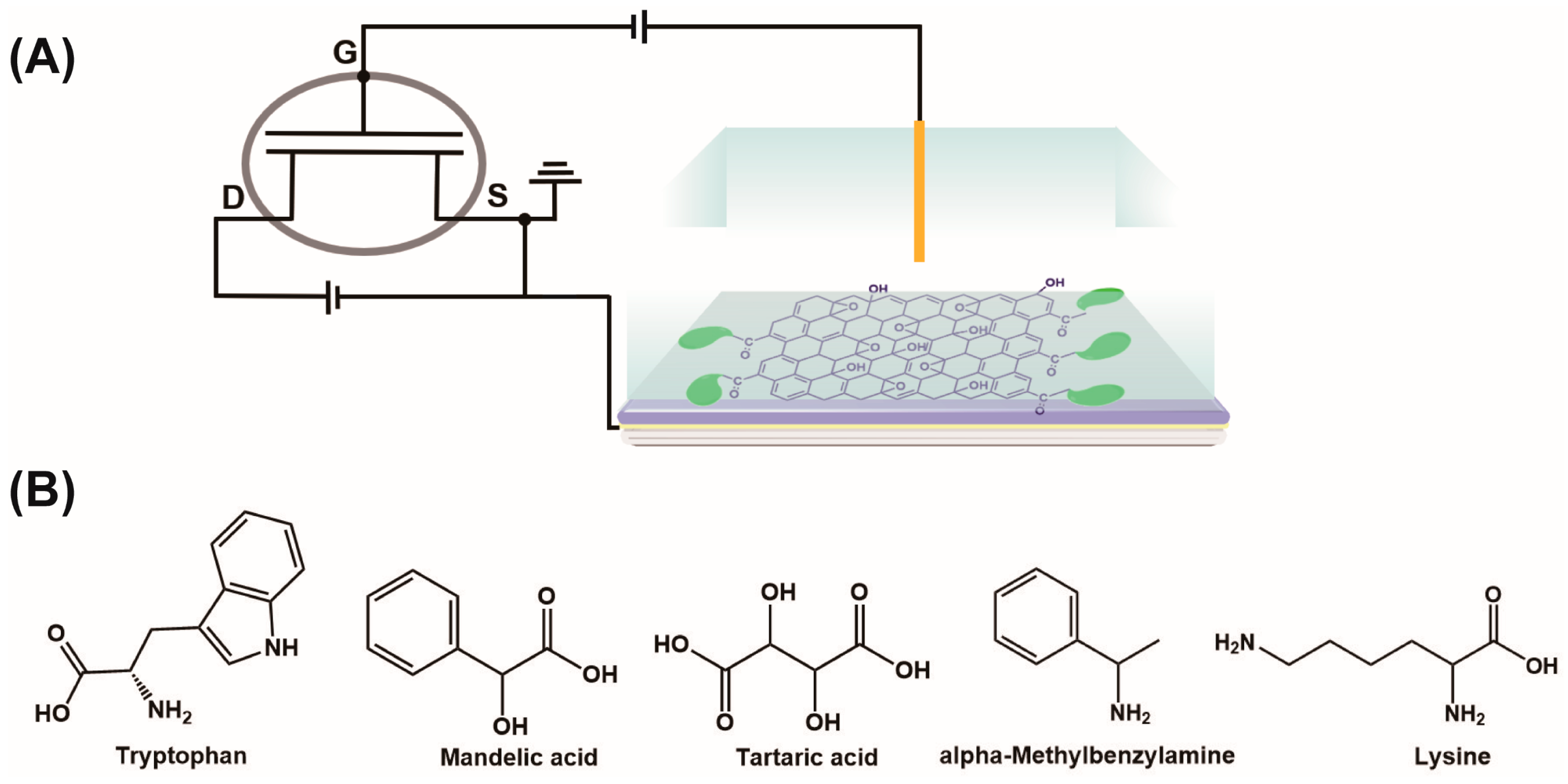
2.7. FET Sensing Procedure
3. Results and Discussion
3.1. Characterization of GO@BSA
3.2. Characterization of Sensitized Au Electrode
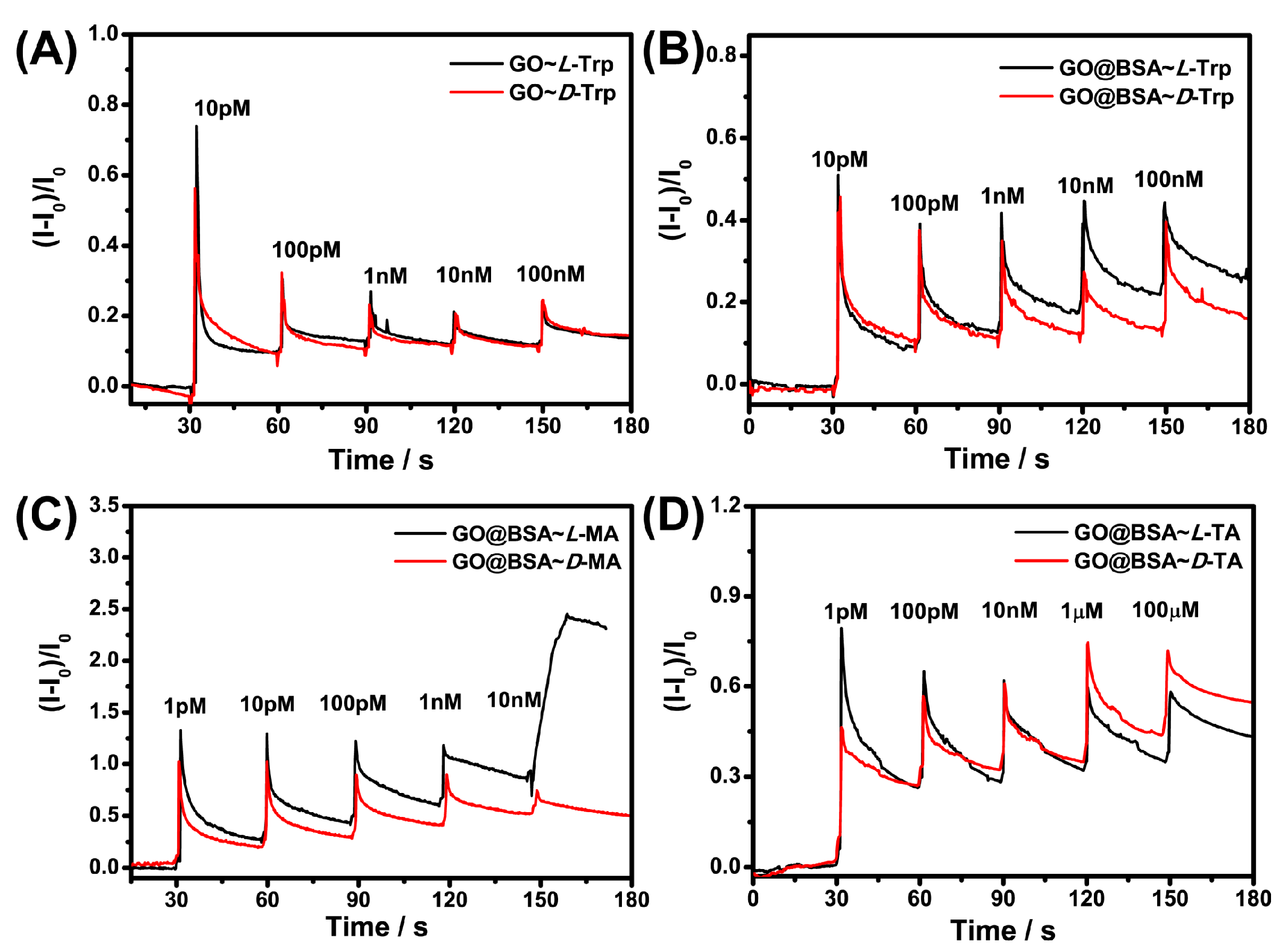
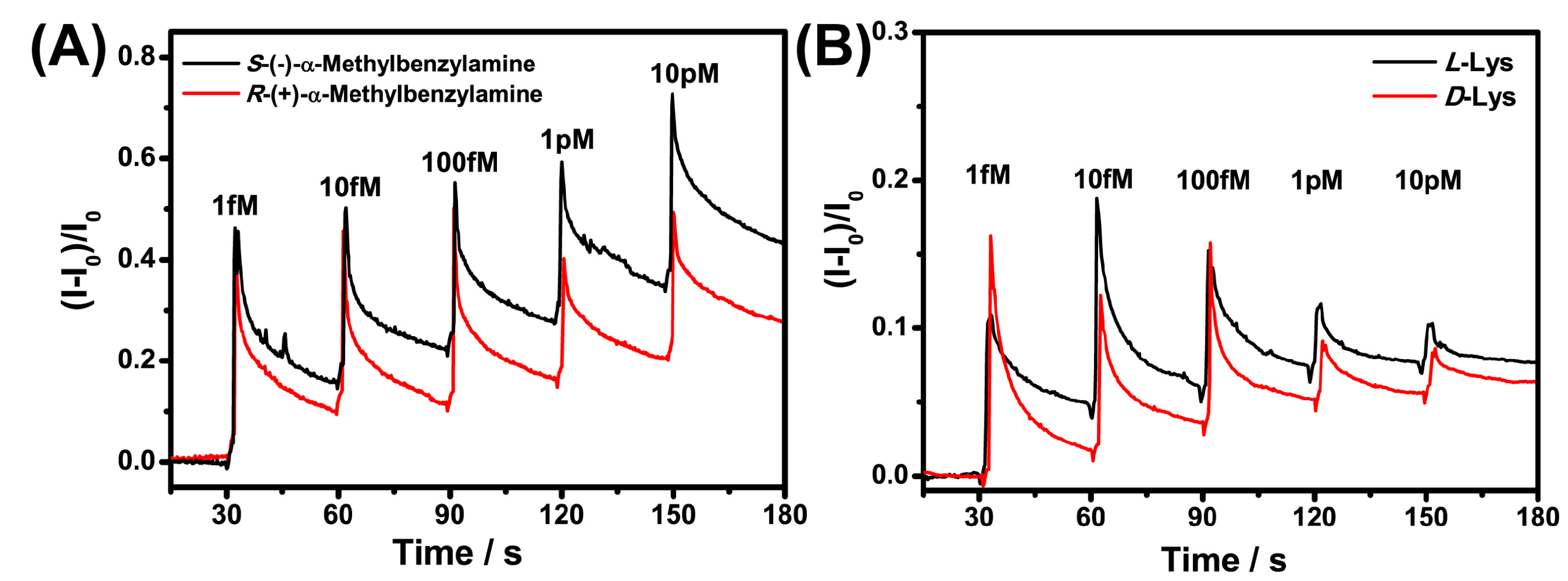
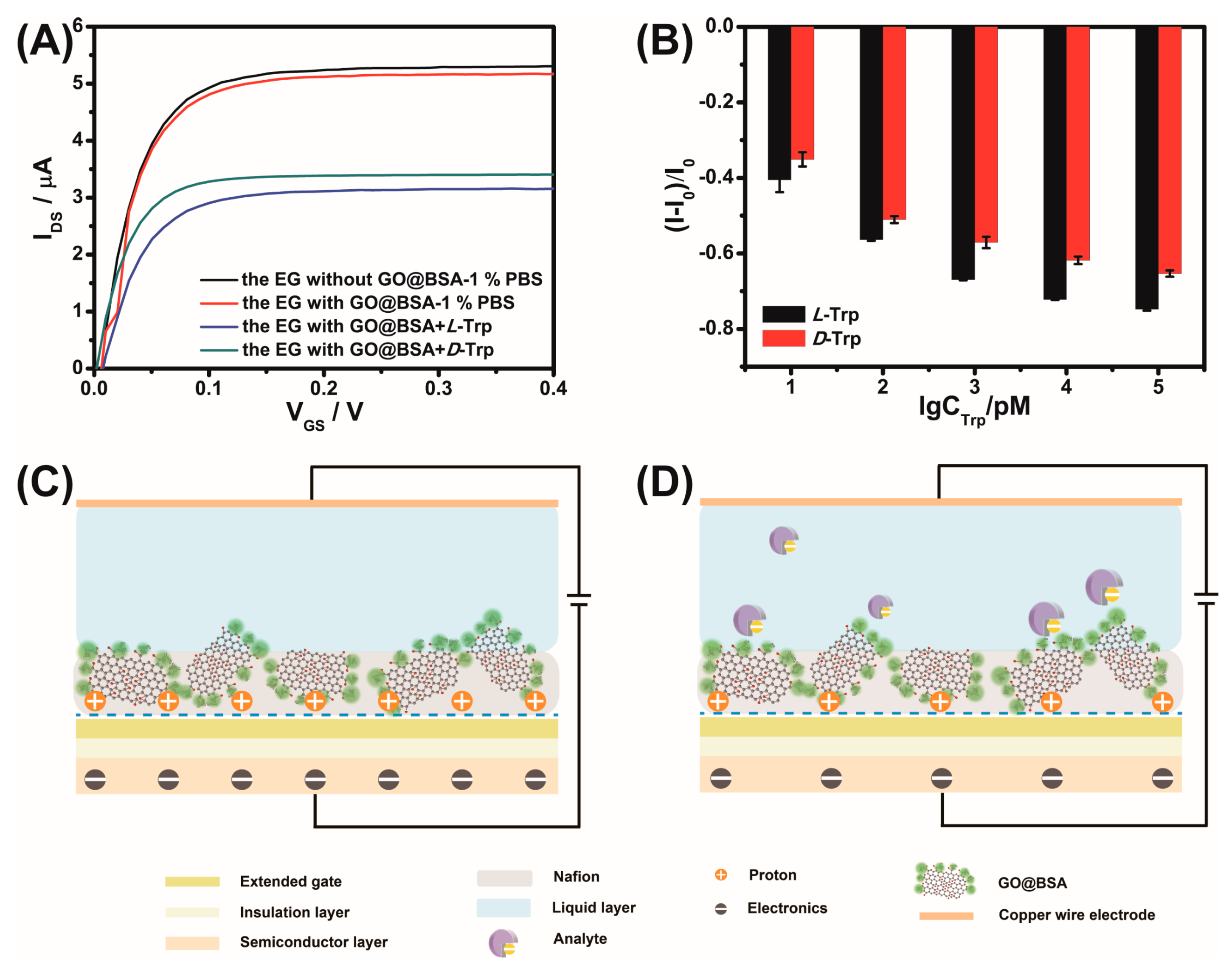
3.3. Response of Sensors to Different Chiral Molecules
3.4. Mechanism Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Zang, S.-Q.; Chen, X. Stereospecific Interactions between Chiral Inorganic Nanomaterials and Biological Systems. Chem. Soc. Rev. 2020, 49, 2481–2503. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Bounoua, N.; Rebizi, M.; Wagdy, H. Application of Nanoparticles in Chiral Analysis and Chiral Separation. Chirality 2021, 33, 196–208. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Tang, X.; Shi, Y.; Li, C.; Wang, Y. One-Pot Click Access to a Cyclodextrin Dimer-Based Novel Aggregation Induced Emission Sensor and Monomer-Based Chiral Stationary Phase. Sensors 2016, 16, 1985. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Budau, M.; Muntean, D.L.; Gagyi, L.; Rusu, A. Capillary Electrophoresis in the Enantioseparation of Modern Antidepressants: An Overview. Biomed. Chromatogr. 2018, 32, e4335. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Zhang, Q.; Huang, Y.; Guo, L.; Fu, Y. Enantioselective Recognition of Penicillamine Enantiomers on Bovine Serum Albumin-Modified Glassy Carbon Electrode. J. Solid State Electrochem. 2013, 17, 627–633. [Google Scholar] [CrossRef]
- Lynch, C.C.; De los Santos, Z.A.; Wolf, C. Chiroptical Sensing of Unprotected Amino Acids, Hydroxy Acids, Amino Alcohols, Amines and Carboxylic Acids with Metal Salts. Chem. Commun. 2019, 55, 6297–6300. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Thungon, P.D.; Estrela, P.; Goswami, P. Development of an Aptamer-Based Field Effect Transistor Biosensor for Quantitative Detection of Plasmodium Falciparum Glutamate Dehydrogenase in Serum Samples. Biosens. Bioelectron. 2019, 123, 30–35. [Google Scholar] [CrossRef]
- Pullano, S.A.; Critello, C.D.; Mahbub, I.; Tasneem, N.T.; Shamsir, S.; Islam, S.K.; Greco, M.; Fiorillo, A.S. EGFET-Based Sensors for Bioanalytical Applications: A Review. Sensors 2018, 18, 4042. [Google Scholar] [CrossRef]
- Batista, P.D.; Mulato, M. ZnO Extended-Gate Field-Effect Transistors as pH Sensors. Appl. Phys. Lett. 2005, 87, 143508. [Google Scholar] [CrossRef]
- Didier, P.; Lobato-Dauzier, N.; Clement, N.; Genot, A.J.; Sasaki, Y.; Leclerc, E.; Minamiki, T.; Sakai, Y.; Fujii, T.; Minami, T. Microfluidic System with Extended-Gate-Type Organic Transistor for Real-Time Glucose Monitoring. Chemelectrochem 2020, 7, 1332–1336. [Google Scholar] [CrossRef]
- Minami, T.; Sasaki, Y.; Minamiki, T.; Wakida, S.-i.; Kurita, R.; Niwa, O.; Tokito, S. Selective Nitrate Detection by an Enzymatic Sensor Based on an Extended-Gate Type Organic Field-Effect Transistor. Biosens. Bioelectron. 2016, 81, 87–91. [Google Scholar] [CrossRef]
- Mulla, M.Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-Modulated Transistor Detects Odorant Binding Protein Chiral Interactions. Nat. Commun. 2015, 6, 6010. [Google Scholar] [CrossRef] [PubMed]
- Iskierko, Z.; Checinska, A.; Sharma, P.S.; Golebiewska, K.; Noworyta, K.; Borowicz, P.; Fronc, K.; Bandi, V.; D’Souza, F.; Kutner, W. Molecularly Imprinted Polymer Based Extended-Gate Field-Effect Transistor Chemosensors for Phenylalanine Enantioselective Sensing. J. Mater. Chem. C 2017, 5, 969–977. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Yan, L. Free-Standing Dried Foam Films of Graphene Oxide for Humidity Sensing. Sens. Actuators B Chem. 2015, 215, 316–322. [Google Scholar] [CrossRef]
- Nayak, J.K.; Jha, R. Graphene-Oxide Coated Ag-Island-Based Inline LSPR Fiber Sensor. IEEE Photonics Technol. Lett. 2018, 30, 1667–1670. [Google Scholar] [CrossRef]
- Malik, P.; Bhushan, R. Thin Layer Chromatographic Resolution of Some beta-adrenolytics and a beta 2-Agonist Using Bovine Serum Albumin as Chiral Additive in Stationary Phase. J. Chromatogr. Sci. 2018, 56, 92–98. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, C.; Zhao, Y.; Wang, C.; Maihemuti, M.; Sun, C.; Qi, Y.; Peng, J.; Guo, X.; Zhang, Z.; et al. Evaluation of Chiral Separation Based on Bovine Serum Albumin-Conjugated Carbon Nanotubes as Stationary Phase in Capillary Electrochromatography. Electrophoresis 2020, 41, 1253–1260. [Google Scholar] [CrossRef]
- Ye, Q.; Guo, L.; Wu, D.; Yang, B.; Tao, Y.; Deng, L.; Kong, Y. Covalent Functionalization of Bovine Serum Albumin with Graphene Quantum Dots for Stereospecific Molecular Recognition. Anal. Chem. 2019, 91, 11864–11871. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.V.; Buranaprapuk, A.; Sze, H.C. Large Chiral Discrimination of a Molecular Probe by Bovine Serum Albumin. Chem. Commun. 2001, 297–298. [Google Scholar] [CrossRef]
- Wang, F.; Huang, W.; Dai, Z. Spectroscopic Investigation of the Interaction between Riboflavin and Bovine Serum Albumin. J. Mol. Struct. 2008, 875, 509–514. [Google Scholar] [CrossRef]
- Du, D.; Wang, L.; Shao, Y.; Wang, J.; Engelhard, M.H.; Lin, Y. Functionalized Graphene Oxide as a Nanocarrier in a Multienzyme Labeling Amplification Strategy for Ultrasensitive Electrochemical Immunoassay of Phosphorylated p53 (S392). Anal. Chem. 2011, 83, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-S.; Chen, S.-Y.; Huang, X.-J.; Wang, L.-S.; Wang, H.-Y.; Wang, J.-F. Simple Approach for Ultrasensitive Electrochemical Immunoassay of Clostridium Difficile Toxin B Detection. Biosens. Bioelectron. 2014, 53, 238–244. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Li, R.; Liu, L.; Wang, J. Bovine Serum Albumin-Coated Graphene Oxide for Effective Adsorption of Uranium (VI) from Aqueous Solutions. Ind. Eng. Chem. Res. 2017, 56, 3588–3598. [Google Scholar] [CrossRef]
- Emadi, F.; Amini, A.; Gholami, A.; Ghasemi, Y. Functionalized Graphene Oxide with Chitosan for Protein Nanocarriers to Protect against Enzymatic Cleavage and Retain Collagenase Activity. Sci. Rep. 2017, 7, 42258. [Google Scholar] [CrossRef]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced Graphene Oxide Nanosheet for Chemo-photothermal Therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef]
- Chiu, N.-F.; Kuo, C.-T.; Chen, C.-Y. High-Affinity Carboxyl-Graphene Oxide-Based SPR Aptasensor for the Detection of hCG Protein in Clinical Serum Samples. Int. J. Nanomed. 2019, 14, 4833–4847. [Google Scholar] [CrossRef]
- Guo, C.; Sun, H.; Zhao, X.S. Myoglobin within Graphene Oxide Sheets and Nafion Composite Films as Highly Sensitive Biosensor. Sens. Actuators B Chem. 2012, 164, 82–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Zhu, L.; Luo, Z.; Tang, H. Fabrication of Molecular Imprinted Polymer Sensor for Chlortetracycline Based on Controlled Electrochemical Reduction of Graphene Oxide. Sens. Actuators B Chem. 2013, 185, 438–444. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. A Novel Electrochemical Sensor for the Analysis of beta-Agonists: The poly(Acid Chrome Blue K)/Graphene Oxide-Nafion/Glassy Carbon Electrode. J. Hazard. Mater. 2013, 260, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Sabatani, E.; Cohen-Boulakia, J.; Bruening, M.; Rubinstein, I. Thioaromatic Monolayers on Gold: A New Family of Self-Assembling Monolayers. Langmuir 1993, 9, 2974–2981. [Google Scholar] [CrossRef]
- Chiu, N.-F.; Chen, C.-C.; Yang, C.-D.; Kao, Y.-S.; Wu, W.-R. Enhanced Plasmonic Biosensors of Hybrid Gold Nanoparticle-Graphene Oxide-Based Label-Free Immunoassay. Nanoscale Res. Lett. 2018, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Zhang, J.; Li, W. Chitosan/Graphene Oxide Nanocomposite-Based Electrochemical Sensor for ppb Level Detection of Melamine. J. Electrochem. Soc. 2019, 166, B1364–B1369. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmandy, R.; van der Zalm, J.; Chen, A. Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef]
- Lu, P.-P.; Shang, D.-S.; Yang, C.-S.; Sun, Y. An Organic Synaptic Transistor with Nafion Electrolyte. J. Phys. D Appl. Phys. 2020, 53, 485102. [Google Scholar] [CrossRef]
- Levitsky, I.A.; Kolobov, I.G.; Euler, W.B. Field Effect of Carbon Nanotubes with an Ionomeric Polymer. Phys. Status Solidi B 2007, 244, 2666–2672. [Google Scholar] [CrossRef]
- Wang, H.; Sun, N.; Xu, X.; Wang, S.; Kang, W.; Zhuang, X.; Yin, Y.; Cheng, B. Adenosine Triphosphate@Graphene Oxide Proton Channels for Proton Exchange Membranes Constructed via Electrostatic Layer-by-Layer Deposition. J. Membr. Sci. 2021, 620, 118880. [Google Scholar] [CrossRef]
- Chen, C.-P.; Ganguly, A.; Lu, C.-Y.; Chen, T.-Y.; Kuo, C.-C.; Chen, R.-S.; Tu, W.-H.; Fischer, W.B.; Chen, K.-H.; Chen, L.-C. Ultrasensitive in Situ Label-Free DNA Detection Using a GaN Nanowire-Based Extended-Gate Field-Effect-Transistor Sensor. Anal. Chem. 2011, 83, 1938–1943. [Google Scholar] [CrossRef]


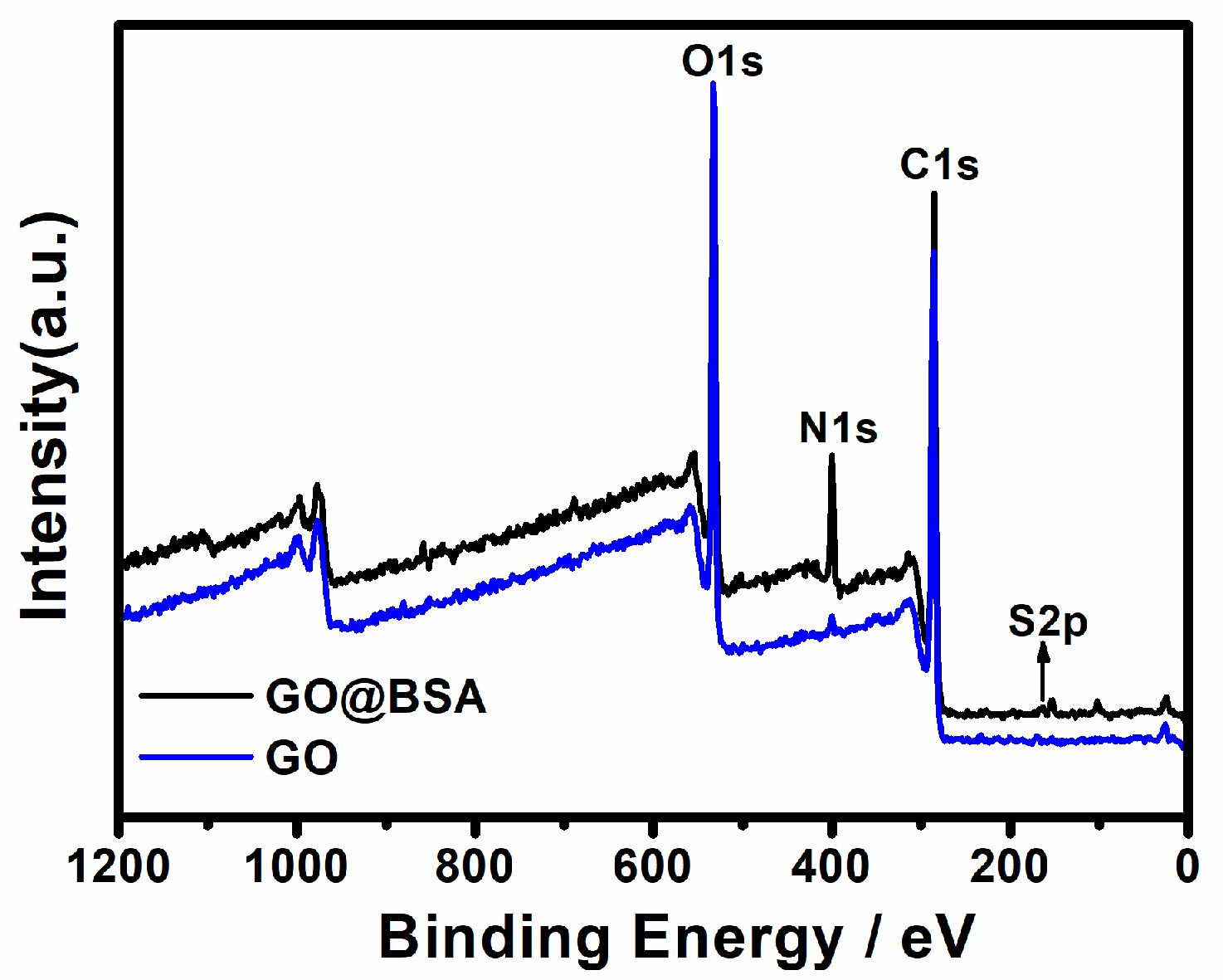



| C | N | O | S | |
|---|---|---|---|---|
| GO | 68.7% | 1.41% | 29.27% | 0.62% |
| GO@BSA | 66.09% | 9.84% | 23.74% | 0.32% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Ma, X.; Xiao, Y.; Wang, Y. Construction and Application of Graphene Oxide-Bovine Serum Albumin Modified Extended Gate Field Effect Transistor Chiral Sensor. Sensors 2021, 21, 3921. https://doi.org/10.3390/s21113921
Li L, Ma X, Xiao Y, Wang Y. Construction and Application of Graphene Oxide-Bovine Serum Albumin Modified Extended Gate Field Effect Transistor Chiral Sensor. Sensors. 2021; 21(11):3921. https://doi.org/10.3390/s21113921
Chicago/Turabian StyleLi, Le, Xiaofei Ma, Yin Xiao, and Yong Wang. 2021. "Construction and Application of Graphene Oxide-Bovine Serum Albumin Modified Extended Gate Field Effect Transistor Chiral Sensor" Sensors 21, no. 11: 3921. https://doi.org/10.3390/s21113921
APA StyleLi, L., Ma, X., Xiao, Y., & Wang, Y. (2021). Construction and Application of Graphene Oxide-Bovine Serum Albumin Modified Extended Gate Field Effect Transistor Chiral Sensor. Sensors, 21(11), 3921. https://doi.org/10.3390/s21113921






