Fabrication and Characterisation of Organic EL Devices in the Presence of Cyclodextrin as an Interlayer
Abstract
1. Introduction
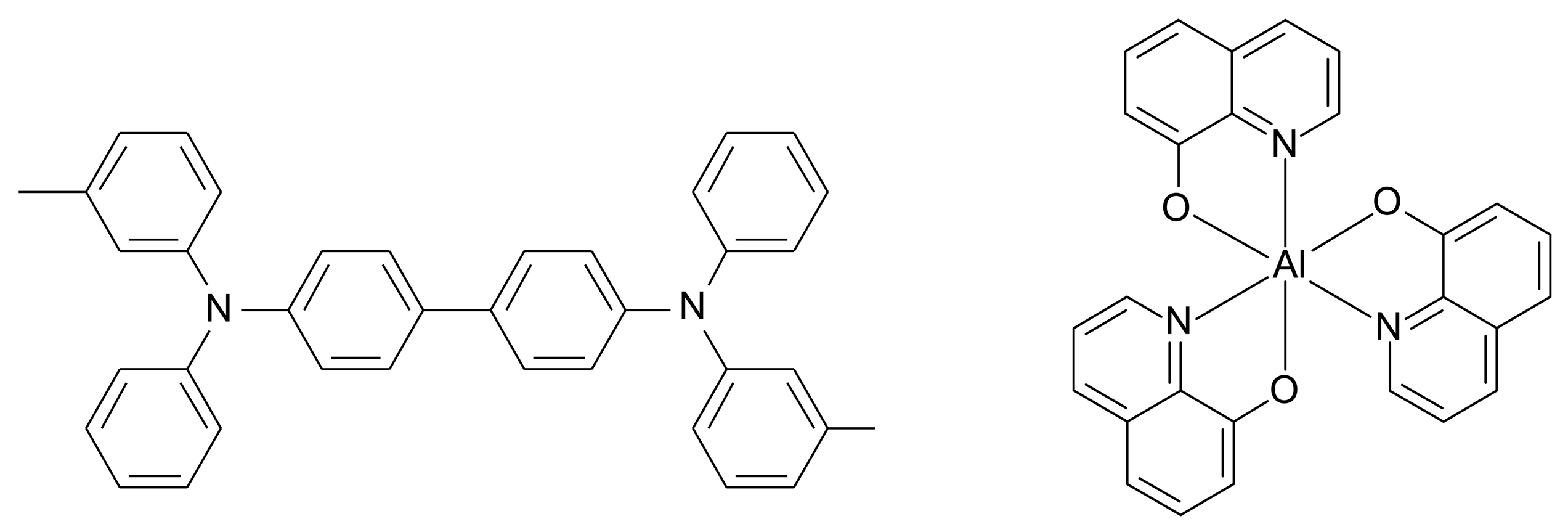
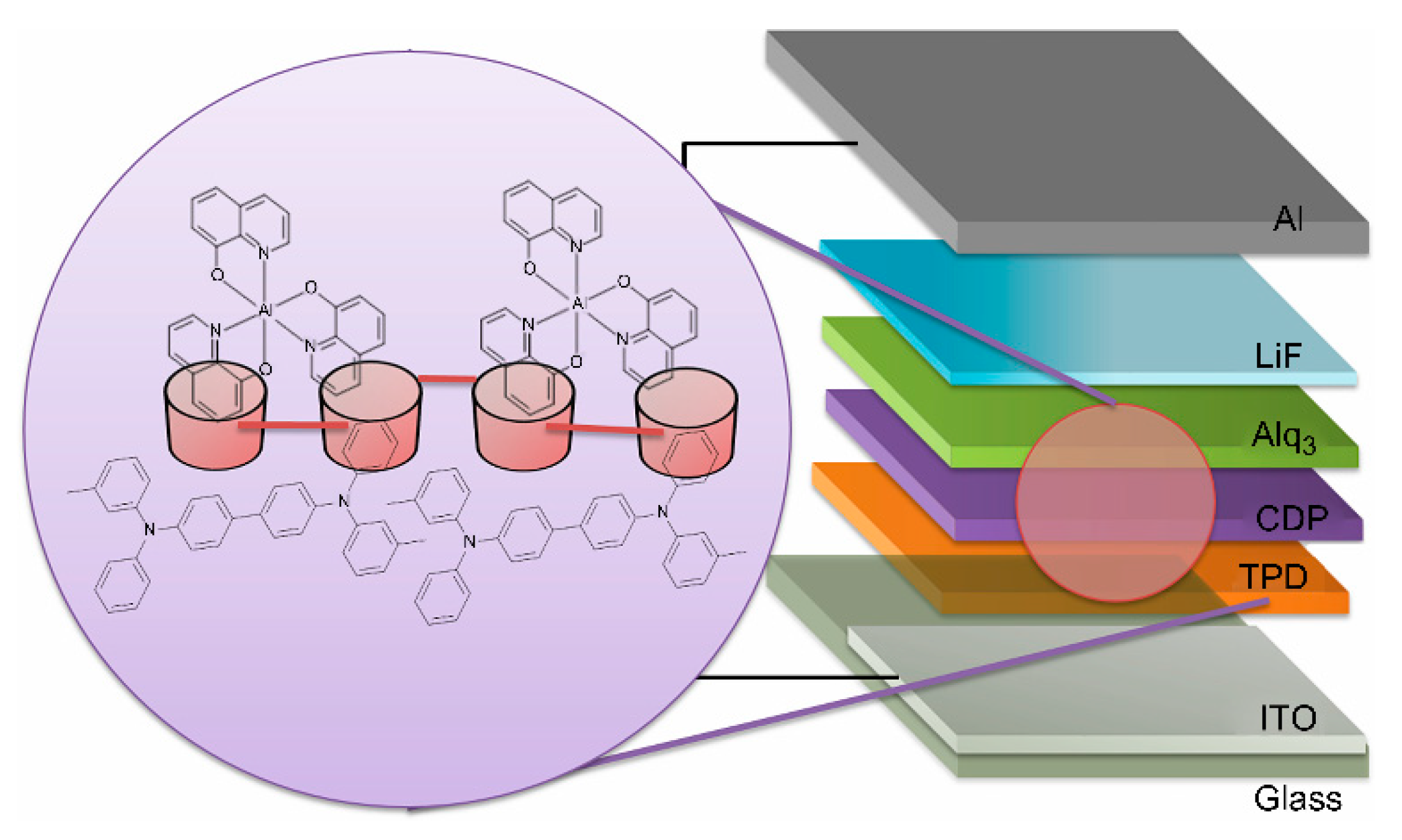
2. Materials and Methods
3. Results and Discussion
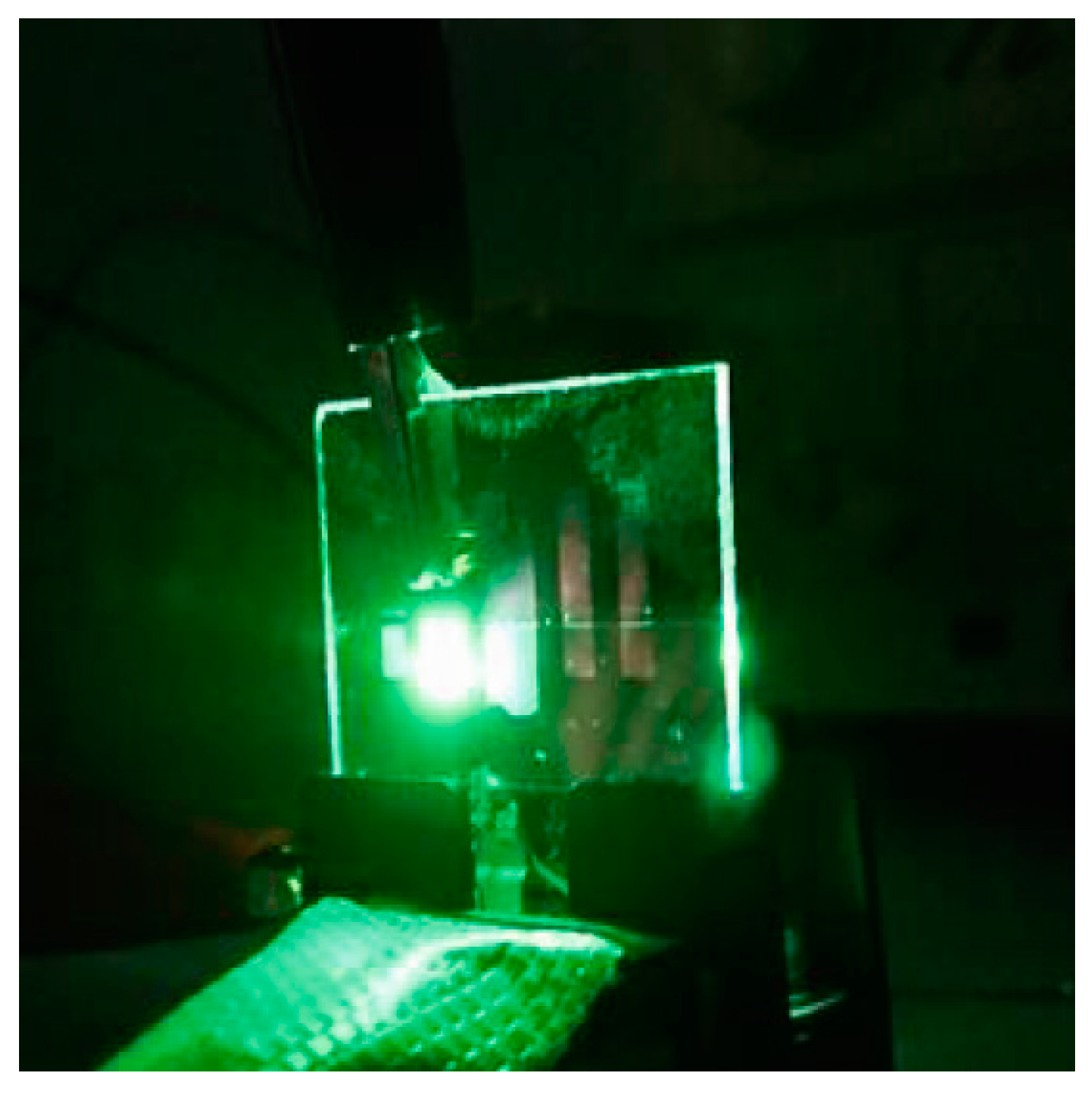
3.1. Characterisation of the OEL Device
3.2. Low Voltage Drive-Type OEL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Li, X.-L.; Nie, H.; Luo, W.; Hu, R.; Qin, A.; Zhao, Z.; Su, S.-J.; Tang, B.Z. Robust Luminescent Materials with Prominent Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence for High-Performance Organic Light-Emitting Diodes. Chem. Mater. 2017, 29, 3623–3631. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Kim, S.; Kim, J.; Lee, J.; Shin, H.; Lee, J.-H.; Park, J. Synthesis and Electroluminescence Properties of Highly Efficient Dual Core Chromophores with Side Groups for Blue Emission. J. Mater. Chem. C 2014, 2, 4737–4747. [Google Scholar] [CrossRef]
- Jhulki, S.; Ghosh, A.; Chow, T.J.; Moorthy, J.N. Twisted Biaryl-amines as Novel Host Materials for Green-emissive Phosphorescent Organic Light-emitting Diodes (PhOLEDs). RSC Adv. 2015, 5, 101169–101176. [Google Scholar] [CrossRef]
- Pereira, D.; Pinto, A.; California, A.; Gomes, J.; Pereira, L. Control of a White Organic Light Emitting Diode Emission Parameters Using a Single Doped RGB Active Layer. Mater. Sci. Eng. B 2016, 211, 156–165. [Google Scholar] [CrossRef]
- Taylor-Shaw, E.; Angioni, E.; Findlay, N.J.; Breig, B.; Inigo, A.R.; Bruckbauer, J.; Wallis, D.J.; Skabara, P.J.; Martin, R.W. Cool to Warm White Light Emission from Hybrid Inorganic/Organic Light-emitting Diodes. J. Mater. Chem. C 2016, 4, 11499–11507. [Google Scholar] [CrossRef]
- Tetsuka, H.; Ebina, T.; Tsunoda, T.; Nanjo, H.; Mizukami, F. Flexible Organic Electroluminescent Devices Based on Transparent Clay Films. Nanotechnology 2007, 18, 355701. [Google Scholar] [CrossRef]
- Tetsuka, H.; Ebina, T.; Tsunoda, T.; Nanjo, H.; Mizukami, F. Fabrication of Flexible Organic Light-emitting Diodes Using Transparent Clay Films as Substrate. Jpn. J. Appl. Phys. 2008, 47, 1894–1896. [Google Scholar] [CrossRef]
- Keum, C.; Murawski, C.; Archer, E.; Kwon, S.; Mischok, A.; Gather, M.C. A Substrateless, Flexible, and Water-resistant Organic Light-emitting Diode. Nat. Commun. 2020, 11, 6250. [Google Scholar] [CrossRef]
- Gustafsson, G.; Cao, Y.; Treacy, G.M.; Klavetter, F.; Colaneri, N.; Heeger, A.J. Flexible Light-emitting Diodes Made from Soluble Conducting Polymers. Nature 1992, 357, 477–479. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, Y.; Choi, M.-R.; Woo, S.-H.; Bae, S.-H.; Hong, B.H.; Ahn, J.-H.; Lee, T.-W. Extremely Efficient Flexible Organic Light-emitting Diodes with Modified Graphene Anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Niu, X.; Yu, Z.; Pei, Q. Elastomeric Polymer Light-emitting Devices and Displays. Nat. Photonics 2013, 7, 817–824. [Google Scholar] [CrossRef]
- Zhong, C.; Duan, C.; Huang, F.; Wu, H.; Cao, Y. Materials and Devices Toward Fully Solution Processable Organic Light-Emitting Diodes. Chem. Mater. 2011, 23, 326–340. [Google Scholar] [CrossRef]
- Quinton, C.; Thiery, S.; Jeannin, O.; Tondelier, D.; Geffroy, B.; Jacques, E.; Rault-Berthelot, J.; Poriel, C. Electron-Rich 4-Substituted Spirobifluorenes: Toward a New Family of High Triplet Energy Host Materials for High-Efficiency Green and Sky Blue Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2017, 9, 6194–6206. [Google Scholar] [CrossRef]
- Achelle, S.; Rodriguez-Lopez, J.; Katan, C.; Robin-le Guen, F. Luminescence Behavior of Protonated Methoxy-Substituted Diazine Derivatives: Toward White Light Emission. J. Phys. Chem. C 2016, 120, 26986–26995. [Google Scholar] [CrossRef]
- Nagai, Y.; Sasabe, H.; Takahashi, J.; Onuma, N.; Ito, T.; Ohisa, S.; Kido, J. Highly Efficient, Deep-red Organic Lght-emitting Devices Using Energy Transfer from Exciplexes. J. Mater. Chem. C 2017, 5, 527–530. [Google Scholar] [CrossRef]
- Chapran, M.; Angioni, E.; Findlay, N.J.; Breig, B.; Cherpak, V.; Stakhira, P.; Tuttle, T.; Volyniuk, D.; Grazulevicius, J.V.; Nastishin, Y.A.; et al. An Ambipolar BODIPY Derivative for a White Exciplex OLED and Cholesteric Liquid Crystal Laser Toward Multifunctional Devices. ACS Appl. Mater. Interfaces 2017, 9, 4750–4757. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.L.; Dias, F.B.; Monkman, A.P. Investigation of the Mechanisms Giving Rise to TADF in Exciplex States. J. Phys. Chem. C 2016, 120, 18259–18267. [Google Scholar] [CrossRef]
- Stolz, S.; Petzoldt, M.; Kotadiya, N.; Roedlmeier, T.; Eckstein, R.; Freudenberg, J.; Bunz, U.H.F.; Lemmer, U.; Mankel, E.; Hamburger, M.; et al. One-step Additive Crosslinking of Conjugated Polyelectrolyte Interlayers: Improved Lifetime and Performance of Solution-processed OLEDs. J. Mater. Chem. C 2016, 4, 11150–11156. [Google Scholar] [CrossRef]
- Lee, B.M.; Yoo, S.I.; Kang, J.S.; Yoon, J.A.; Kim, W.Y.; Mascher, P. Hybrid Blue Organic Light Emitting Diodes with Fluorescent and Phosphorescent Emitters along with an Interlayer. Sci. Adv. Mater. 2016, 8, 301–306. [Google Scholar] [CrossRef]
- Guo, K.; Chen, C.; Sun, C.; Peng, C.; Yang, L.; Cai, M.; Zhang, X.; Wei, B. Use of Space Interlayer in Phosphorescent Organic Light-emitting Diodes to Improve Efficiency and Reduce Efficiency Roll-off. J. Phys. D Appl. Phys. 2016, 49, 235105. [Google Scholar] [CrossRef]
- Yu, T.; Xu, J.; Liu, L.; Ren, Z.; Yang, W.; Yan, S.; Ma, Y. Electrochemically Deposited Interlayer between PEDOT:PSS and Phosphorescent Emitting Layer for Multilayer Solution-processed Phosphorescent OLEDs. J. Mater. Chem. C 2016, 4, 9509–9515. [Google Scholar] [CrossRef]
- He, X.; Wu, Z.; Xue, Y.; Gao, Z.; Yang, X. Fabrication of Interlayer β-CD/g-C3N4 and MoS2 for Highly Enhanced Photodegradation of Glyphosate under Simulated Sunlight Irradiation. RSC Adv. 2019, 9, 4635–4643. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Zhang, W.; Diao, G. Preparation and Characterization Water-soluble Inclusion Complexes of Imidacloprid-β-cyclodextrin Polymer and Their Electrochemical Behavior. J. Electroanal. Chem. 2013, 696, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, T.; Cheng, C.; Wang, Q.; Lin, S.; Liu, C.; Han, X. Research Progress on Synthesis and Application of Cyclodextrin Polymers. Molecules 2021, 26, 1090. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Umeda, T.; Oonishi, N.; Hara, M. Application of a Noncarboxylated Dye Compound in a Dye-sensitized Solar Cell Containing a Cyclodextrin Layer. Int. J. Photoenergy 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Takeshita, T.; Umeda, T.; Hara, M. Fabrication of a Dye-sensitized Solar Cell Containing a Noncarboxylated Spiropyran-derived Photomerocyanine with Cyclodextrin. J. Photochem. Photobiol. A Chem. 2017, 333, 87–91. [Google Scholar] [CrossRef]
- Hara, M.; Takeshita, T.; Umeda, T. Effect of Cyclodextrin Cavity Size on the Photovoltaic Performance of Unanchored Ruthenium(II) Polypyridine Complex-containing Dye-sensitized Solar Cells. J. Photonics Energy 2020, 10, 045503. [Google Scholar] [CrossRef]

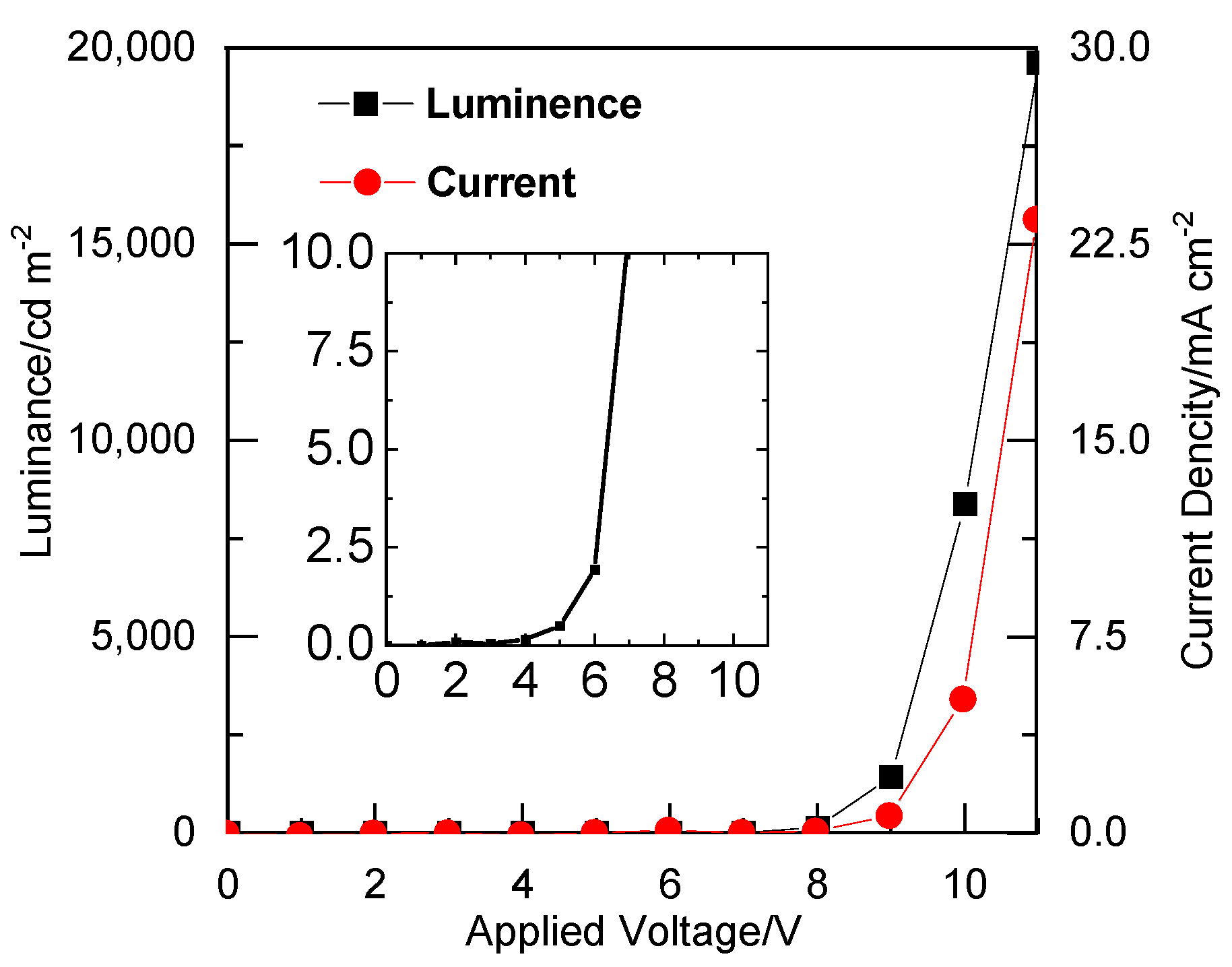

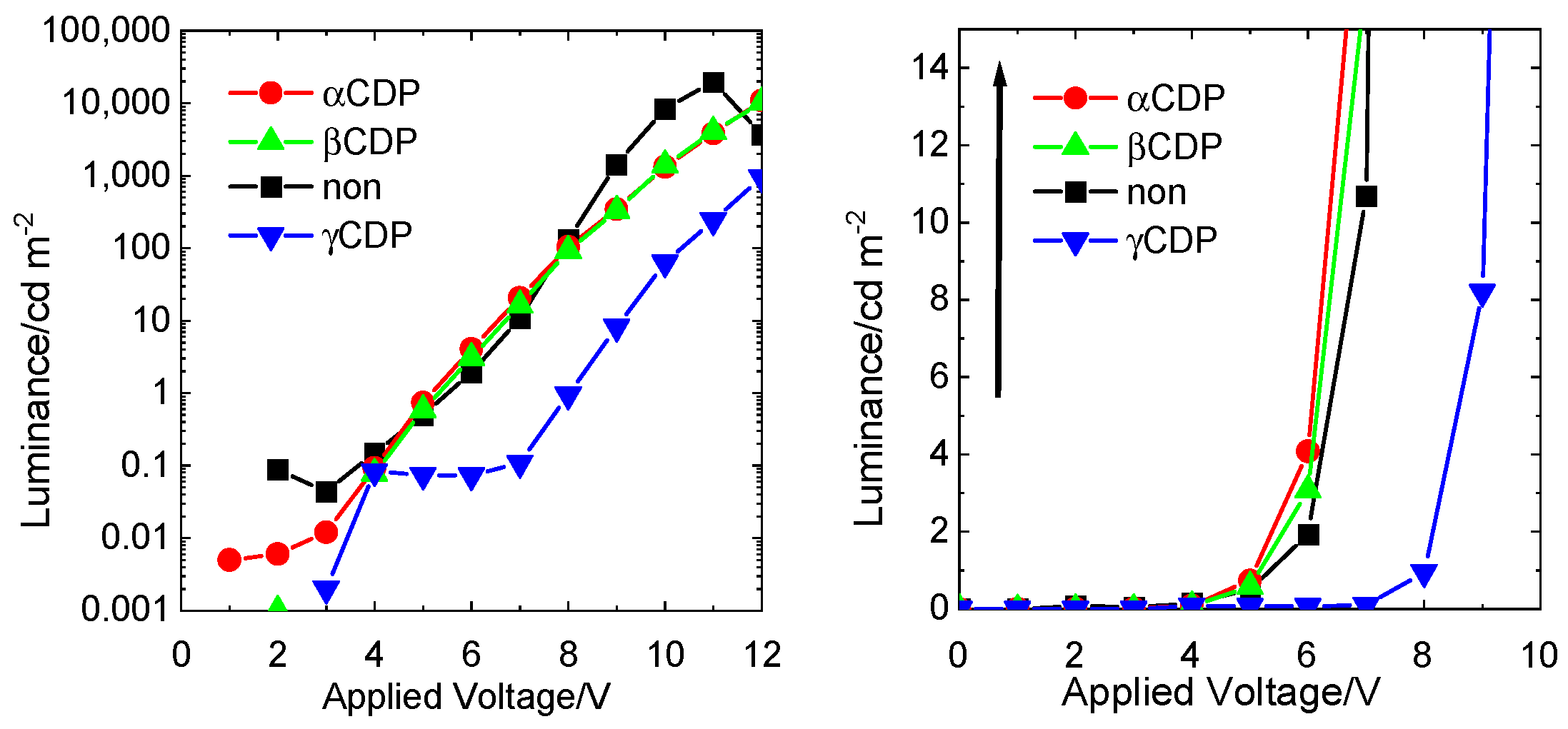
| CDP | Applied Voltage (V) | Luminance (cd m−2) | Current Density (mA cm−2) |
|---|---|---|---|
| Non | 11 | 19,620 | 100 |
| αCDP | 12 | 10,880 | 103 |
| βCDP | 12 | 10,330 | 205 |
| γCDP | 14 | 4886 | 101 |
| CDP | Applied Voltage (V) | Luminance (cd m−2) | Current Density (mA cm−2) |
|---|---|---|---|
| Non | 6 | 1.9 | 0.11 |
| αCDP | 6 | 8.4 | 0.02 |
| βCDP | 6 | 3.1 | 0.77 |
| γCDP | 6 | 7.3 × 10−3 | 7.4 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, M.; Umeda, T.; Kurata, H. Fabrication and Characterisation of Organic EL Devices in the Presence of Cyclodextrin as an Interlayer. Sensors 2021, 21, 3666. https://doi.org/10.3390/s21113666
Hara M, Umeda T, Kurata H. Fabrication and Characterisation of Organic EL Devices in the Presence of Cyclodextrin as an Interlayer. Sensors. 2021; 21(11):3666. https://doi.org/10.3390/s21113666
Chicago/Turabian StyleHara, Michihiro, Takao Umeda, and Hiroyuki Kurata. 2021. "Fabrication and Characterisation of Organic EL Devices in the Presence of Cyclodextrin as an Interlayer" Sensors 21, no. 11: 3666. https://doi.org/10.3390/s21113666
APA StyleHara, M., Umeda, T., & Kurata, H. (2021). Fabrication and Characterisation of Organic EL Devices in the Presence of Cyclodextrin as an Interlayer. Sensors, 21(11), 3666. https://doi.org/10.3390/s21113666







