Wrist-Based Photoplethysmography Assessment of Heart Rate and Heart Rate Variability: Validation of WHOOP
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Overview
2.3. Sleep Stage Identification
2.4. Heart Rate and Heart Rate Variability Calculation
2.5. Statistical Analysis
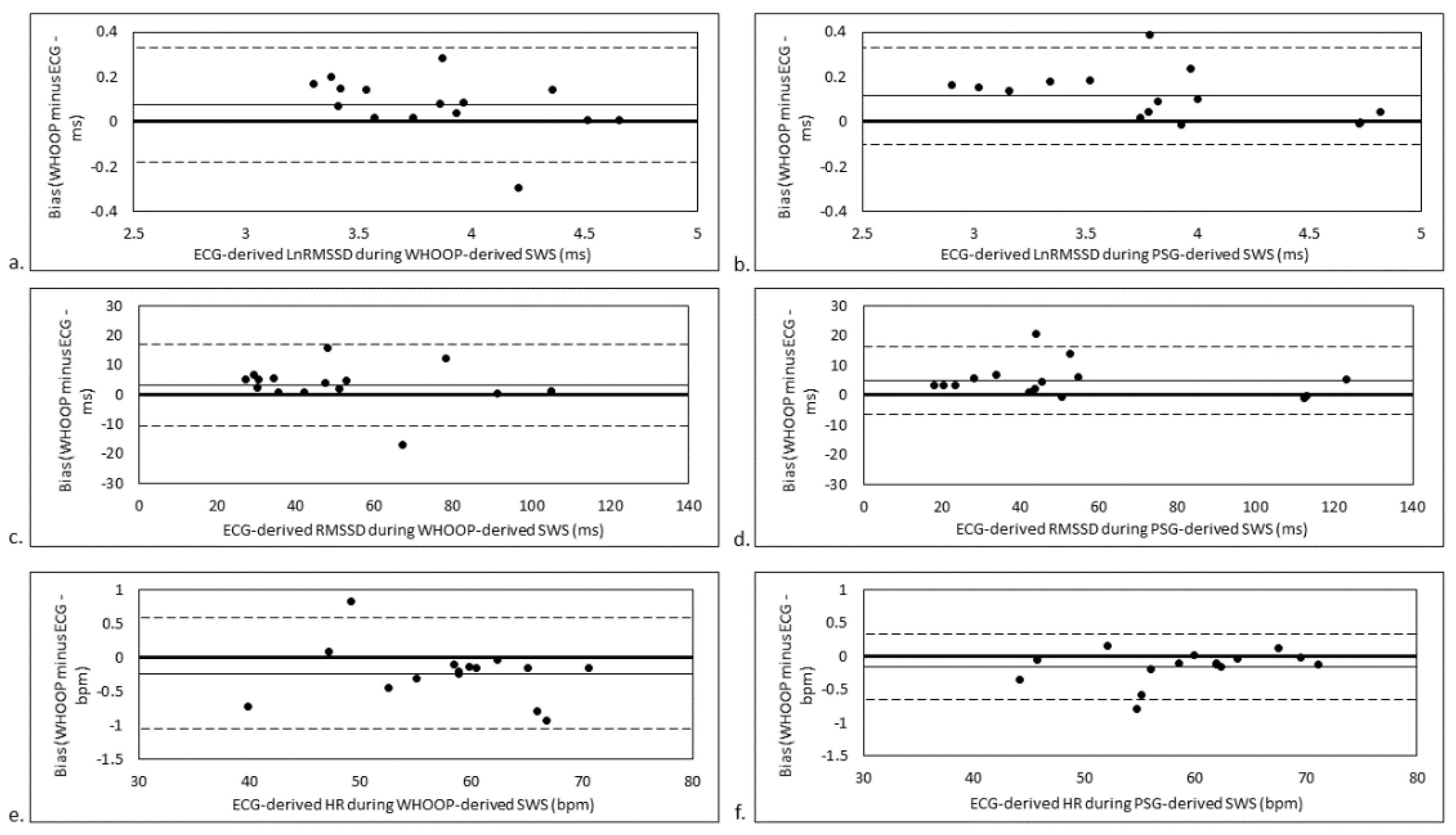
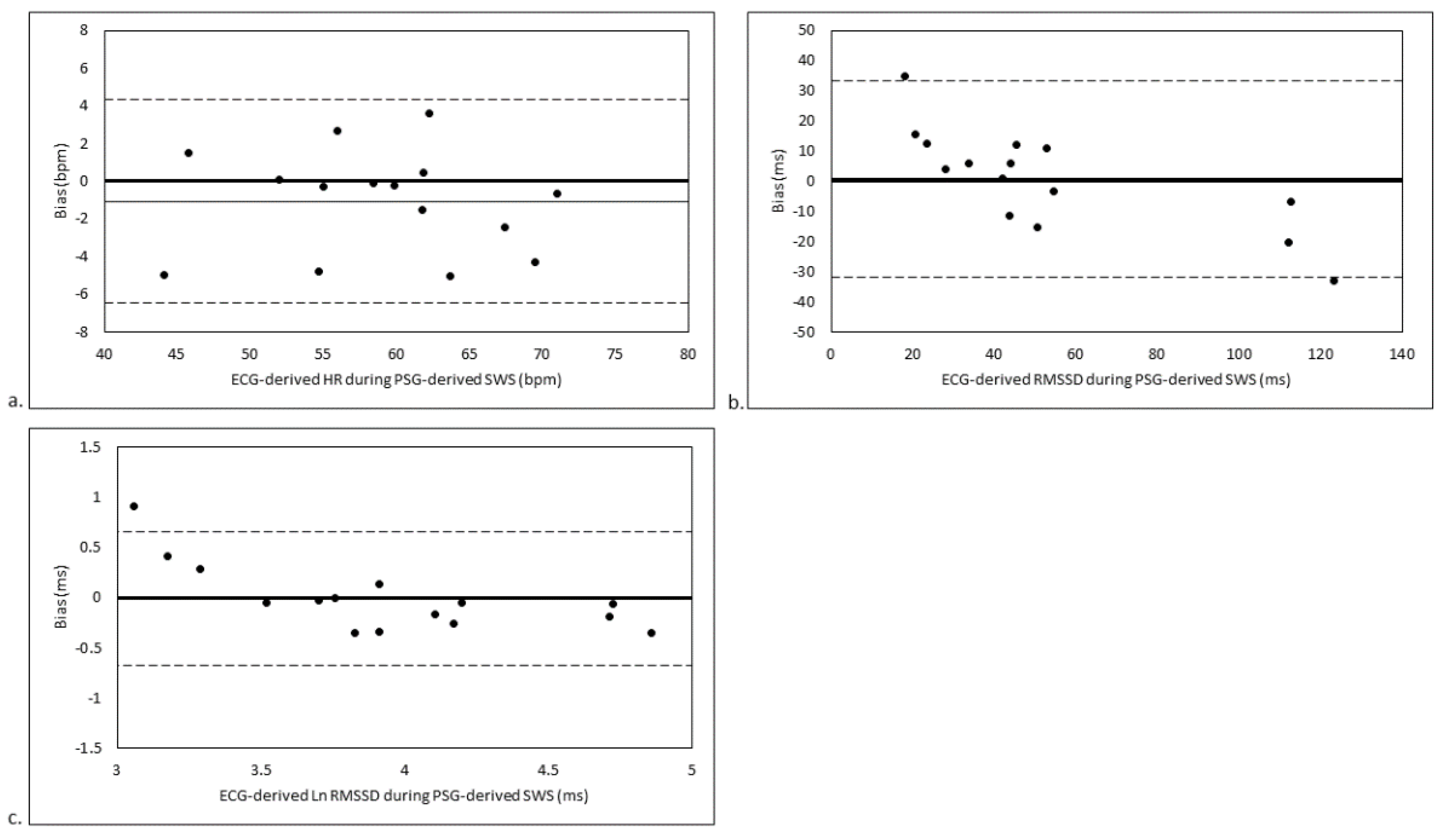
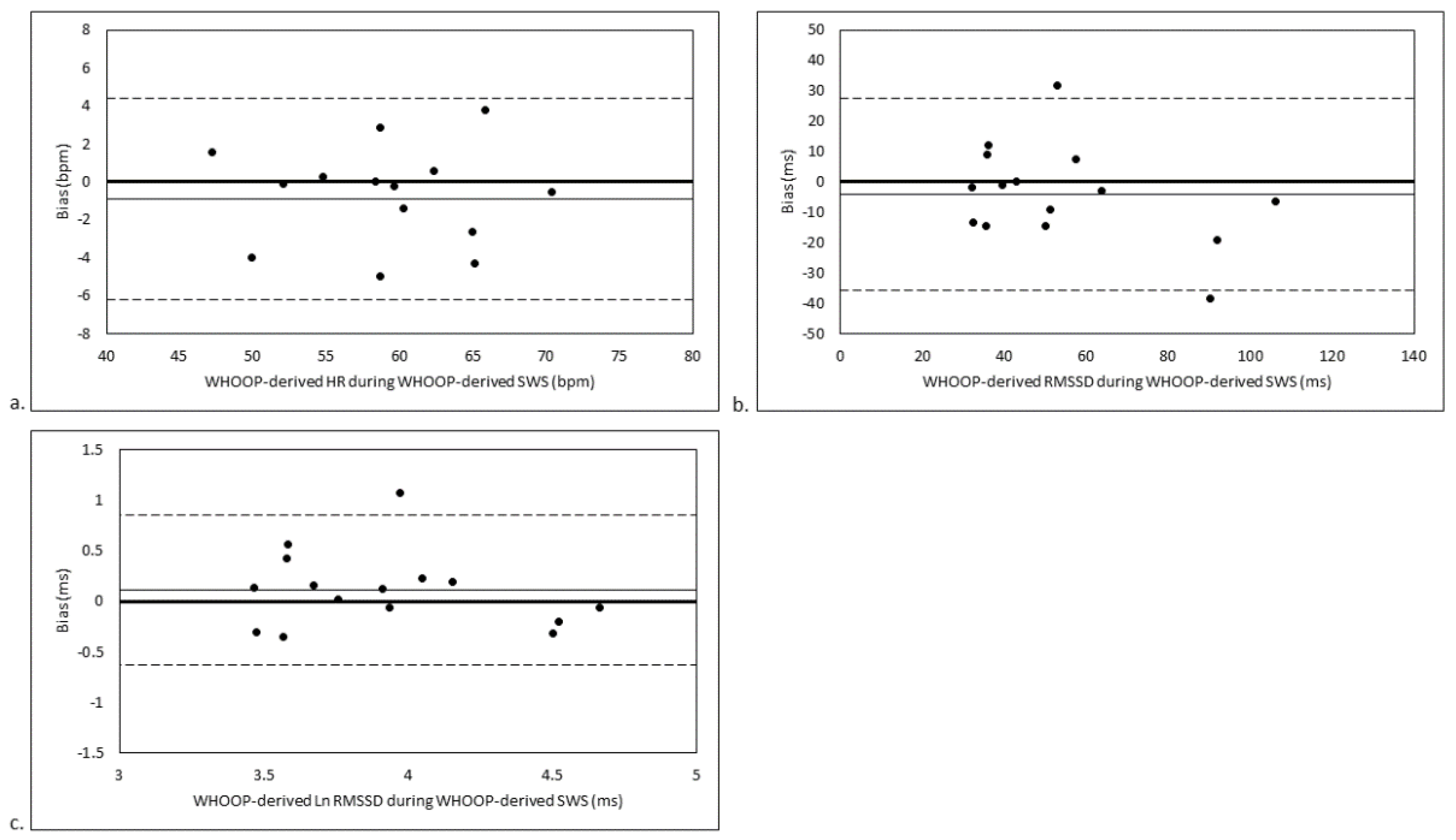
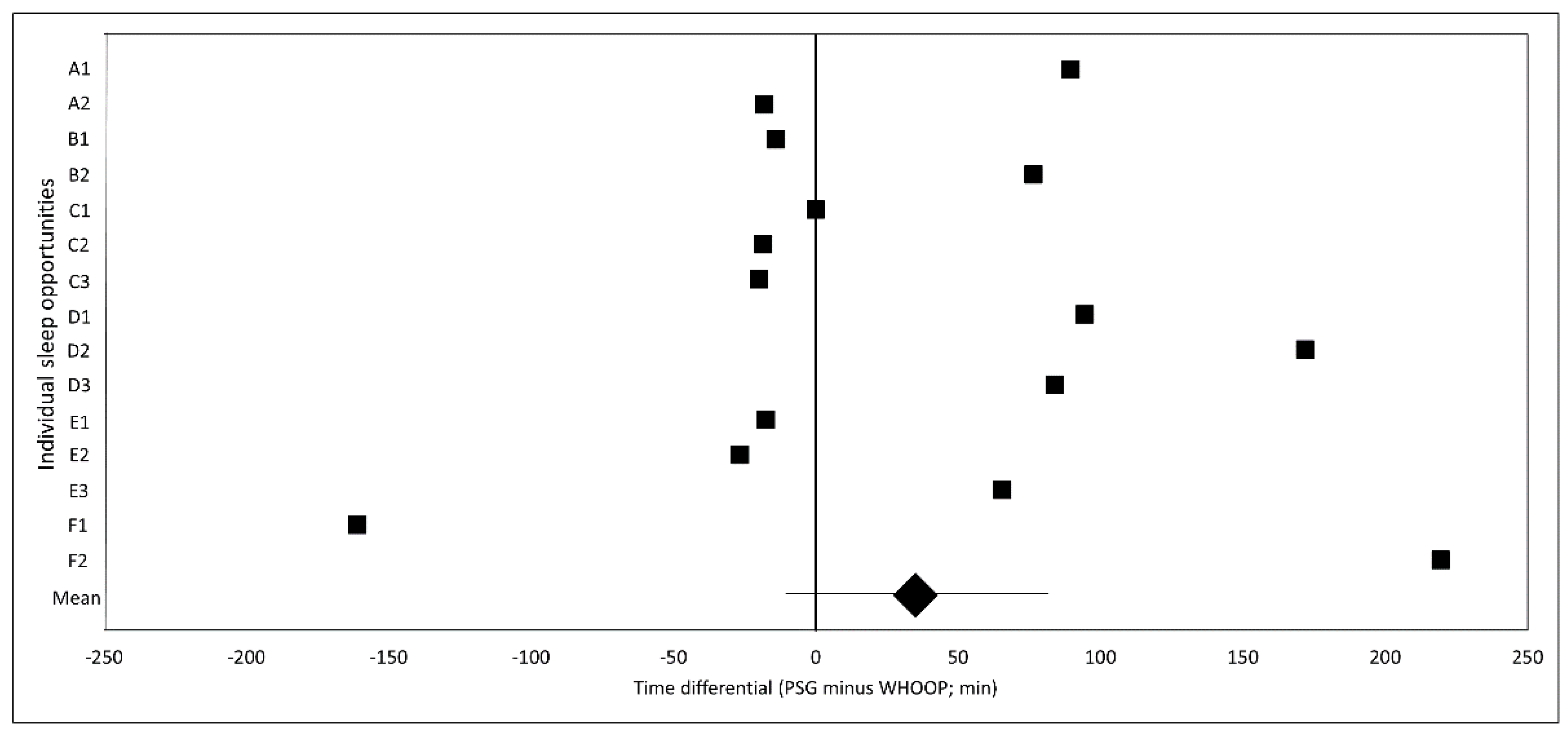
3. Results
3.1. Filter Analysis
3.2. Analytical Method Analysis
3.3. SWS Stage-Matched Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achten, J.; Jeukendrup, A.E. Heart rate monitoring: Applications and limitations. Sports Med. 2003, 33, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Borresen, J.; Lambert, M.I. Autonomic control of heart rate during and after exercise: Measurements and implications for monitoring training status. Sports Med. 2008, 38, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Fuller, J.T.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. Monitoring athletic training status through autonomic heart rate regulation: A systematic review and meta-analysis. Sports Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front Physiol. 2014, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, R.; Virtanen, P.K. Heart rate monitors: State of the art. J. Sports Sci. 1998, 16, S3–S7. [Google Scholar] [CrossRef]
- Plews, D.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.; Laursen, P. Comparison of heart-rate-variability recording with smartphone photoplethysmography, Polar H7 chest strap, and electrocardiography. Int. J. Sport Physiol. Perf. 2017, 12, 1324–1328. [Google Scholar] [CrossRef]
- Støve, M.P.; Haucke, E.; Nymann, M.L.; Sigurdsson, T.; Larsen, B.T. Accuracy of the wearable activity tracker Garmin Forerunner 235 for the assessment of heart rate during rest and activity. J. Sports Sci. 2019, 37, 895–901. [Google Scholar] [CrossRef]
- Hertzman, A.B. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am. J. Physiol. 1938, 124, 328–340. [Google Scholar] [CrossRef]
- Horton, F.J.; Stergiou, S.P.; Fung, S.T.; Katz, S.L. Comparison of Polar M600 optical heart rate and ECG heart rate during exercise. Med. Sci. Sports Exerc. 2017, 49, 2600–2607. [Google Scholar] [CrossRef]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R. Accuracy of PurePulse photoplethysmography technology of Fitbit Charge 2 for assessment of heart rate during sleep. Chronobiol. Int. 2019, 36, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, N.; Jaryal, A.; Santhosh, J.; Deepak, K.K.; Anand, S. Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. J. Med. Eng. Technol. 2008, 32, 479–484. [Google Scholar] [CrossRef]
- Heathers, J.A.J. Smartphone-enabled pulse rate variability: An alternative methodology for the collection of heart rate variability in psychophysiological research. Int. J. Psychophysiol. 2013, 89, 297–304. [Google Scholar] [CrossRef]
- Gil, E.; Orini, M.; Bailón, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas. 2010, 31, 1271–1290. [Google Scholar] [CrossRef] [PubMed]
- Pernice, R.; Javorka, M.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Busacca, A.; Faes, L. Comparison of short-term heart rate variability indexes evaluated through electrocardiographic and continuous blood pressure monitoring. Med. Biol. Eng. Comput. 2019, 57, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Charlot, K.; Cornolo, J.; Brugniaux, J.V.; Richalet, J.P.; Pichon, A. Interchangeability between heart rate and photoplethysmography variabilities during sympathetic stimulations. Physiol. Meas. 2009, 30, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Vescio, B.; Salsone, M.; Gambardella, A.; Quattrone, A. Comparison between electrocardiographic and earlobe pulse photoplethysmographic detection for evaluating heart rate variability in healthy subjects in short- and long-term recordings. Sensors 2018, 18, 844. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Validity of the ithlete™ smart phone application for determining ultra-short-term heart rate variability. J. Hum. Kinet. 2013, 39, 85–92. [Google Scholar] [CrossRef] [PubMed]
- WHOOP. Experience WHOOP Recovery. Available online: https://www.whoop.com/experience/ (accessed on 30 September 2019).
- Miller, D.J.; Lastella, M.; Scanlan, A.T.; Bellenger, C.; Halson, S.L.; Roach, G.D.; Sargent, C. A validation of the WHOOP strap against polysomnography to assess sleep. J. Sports Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Halson, S. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44 (Suppl. S1), 13–23. [Google Scholar] [CrossRef]
- Shapiro, C.M.; Bortz, R.; Mitchell, D.; Bartel, P.; Jooste, P. Slow-wave sleep: A recovery period after exercise. Science 1981, 214, 1253–1254. [Google Scholar] [CrossRef]
- Dijk, D.J. Slow-wave sleep deficiency and enhancement: Implications for insomnia and its management. World J. Biol. Psychiatry 2010, 11, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Al Haddad, H.; Laursen, P.; Chollet, D.; Ahmaidi, S.; Buchheit, M. Reliability of resting and postexercise heart rate measures. Int. J. Sports Med. 2011, 32, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Howells, D. Effects of varying training load on heart rate variability and running performance among an Olympic rugby sevens team. J. Sci. Med. Sport 2019, 22, 222–226. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Le Meur, Y.; Hausswirth, C.; Kilding, A.E.; Buchheit, M. Monitoring training with heart rate-variability: How much compliance is needed for valid assessment? Int. J. Sport Physiol. Perf. 2014, 9, 783. [Google Scholar] [CrossRef]
- Chen, Y.S.; Clemente, F.M.; Bezerra, P.; Lu, Y. Ultra-short-term and short-term heart rate variability recording during training camps and an international tournament in U-20 national futsal players. Int. J. Environ. Res. Public Health 2020, 17, 775. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.Y.; Antunes, P.; Nunes, C.; Costa, J.A.; Esco, M.R.; Travassos, B. Heart rate variability changes from traditional vs. ultra–short-term recordings in relation to preseason training load and performance in Futsal players. J. Strength Cond Res. 2020, 34, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Esco, M.R. Smartphone-derived heart-rate variability and training load in a women’s soccer team. Int. J. Sports Physiol. Perform. 2015, 10, 994–1000. [Google Scholar] [CrossRef]
- Costa, J.A.; Brito, J.; Nakamura, F.Y.; Oliveira, E.M.; Rebelo, A.N. Effects of late-night training on “slow-wave sleep episode” and hour-by-hour-derived nocturnal cardiac autonomic activity in female soccer players. Int. J. Sports Physiol. Perform. 2018, 13, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Drinnan, M.J.; Allen, J.; Murray, A. Relation between heart rate and pulse transit time during paced respiration. Physiol. Meas. 2001, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Ahlstrom, C.; Lanne, T.; Ask, P. Pulse wave transit time for monitoring respiration rate. Med. Biol. Eng. Comput. 2006, 44, 471–478. [Google Scholar] [CrossRef]
- Porta, A.; Gasperi, C.; Nollo, G.; Lucini, D.; Pizzinelli, P.; Antolini, R.; Pagani, M. Global versus local linear beat-to-beat analysis of the relationship between arterial pressure and pulse transit time during dynamic exercise. Med. Biol. Eng. Comput. 2006, 44, 331–337. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-nerve activity during sleep in nrmal subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Penev, P.D.; Schneider, R.; Van Cauter, E. Estimating cardiac autonomic activity during sleep: Impedance cardiography, spectral analysis, and Poincaré plots. Clin. Neurophysiol. 2004, 115, 19–28. [Google Scholar] [CrossRef]
- Gronfier, C.; Simon, C.; Piquard, F.; Ehrhart, J.; Brandenberger, G. Neuroendocrine processes underlying ultradian sleep regulation in man. J. Clin. Endocrinol. Metab. 1999, 84, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, R.; Cerutti, S.; Viardot, G.; Werner, S.; Bianchi, A.M. Modulation of the sympatho-vagal balance during sleep: Frequency domain study of heart rate variability and respiration. Front. Physiol. 2012, 3, 45. [Google Scholar] [CrossRef] [PubMed]
| HR/HRV Derived by… | |||
|---|---|---|---|
| WHOOP | ECG | ||
| SWS period derived by… | WHOOP | 1,3,4 | 1 |
| PSG | 2,4 | 2,3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellenger, C.R.; Miller, D.J.; Halson, S.L.; Roach, G.D.; Sargent, C. Wrist-Based Photoplethysmography Assessment of Heart Rate and Heart Rate Variability: Validation of WHOOP. Sensors 2021, 21, 3571. https://doi.org/10.3390/s21103571
Bellenger CR, Miller DJ, Halson SL, Roach GD, Sargent C. Wrist-Based Photoplethysmography Assessment of Heart Rate and Heart Rate Variability: Validation of WHOOP. Sensors. 2021; 21(10):3571. https://doi.org/10.3390/s21103571
Chicago/Turabian StyleBellenger, Clint R., Dean J. Miller, Shona L. Halson, Gregory D. Roach, and Charli Sargent. 2021. "Wrist-Based Photoplethysmography Assessment of Heart Rate and Heart Rate Variability: Validation of WHOOP" Sensors 21, no. 10: 3571. https://doi.org/10.3390/s21103571
APA StyleBellenger, C. R., Miller, D. J., Halson, S. L., Roach, G. D., & Sargent, C. (2021). Wrist-Based Photoplethysmography Assessment of Heart Rate and Heart Rate Variability: Validation of WHOOP. Sensors, 21(10), 3571. https://doi.org/10.3390/s21103571







