An Algorithm for Choosing the Optimal Number of Muscle Synergies during Walking
Abstract
1. Introduction
2. Materials and Methods
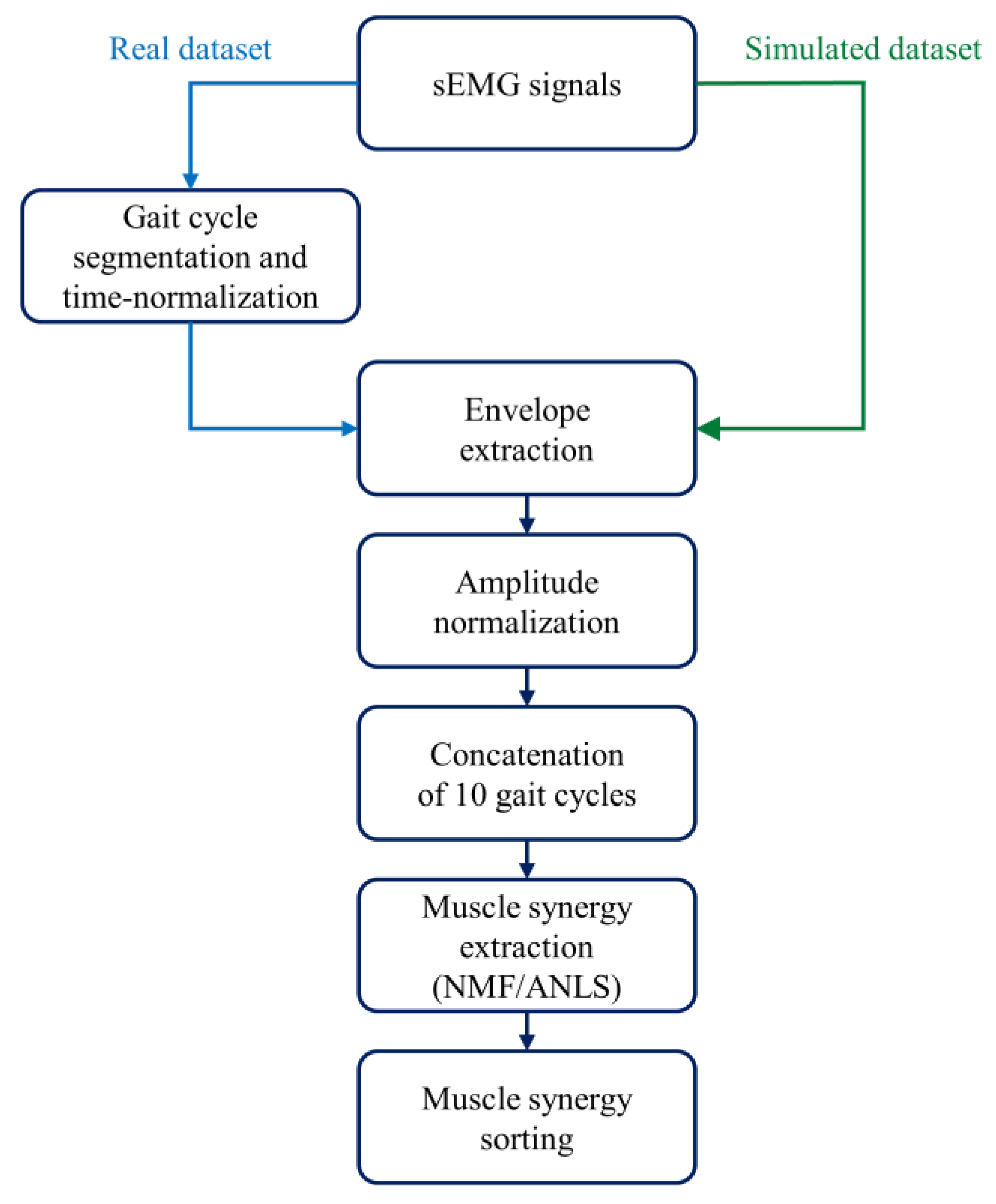
2.1. Real Dataset
2.2. Simulated Dataset
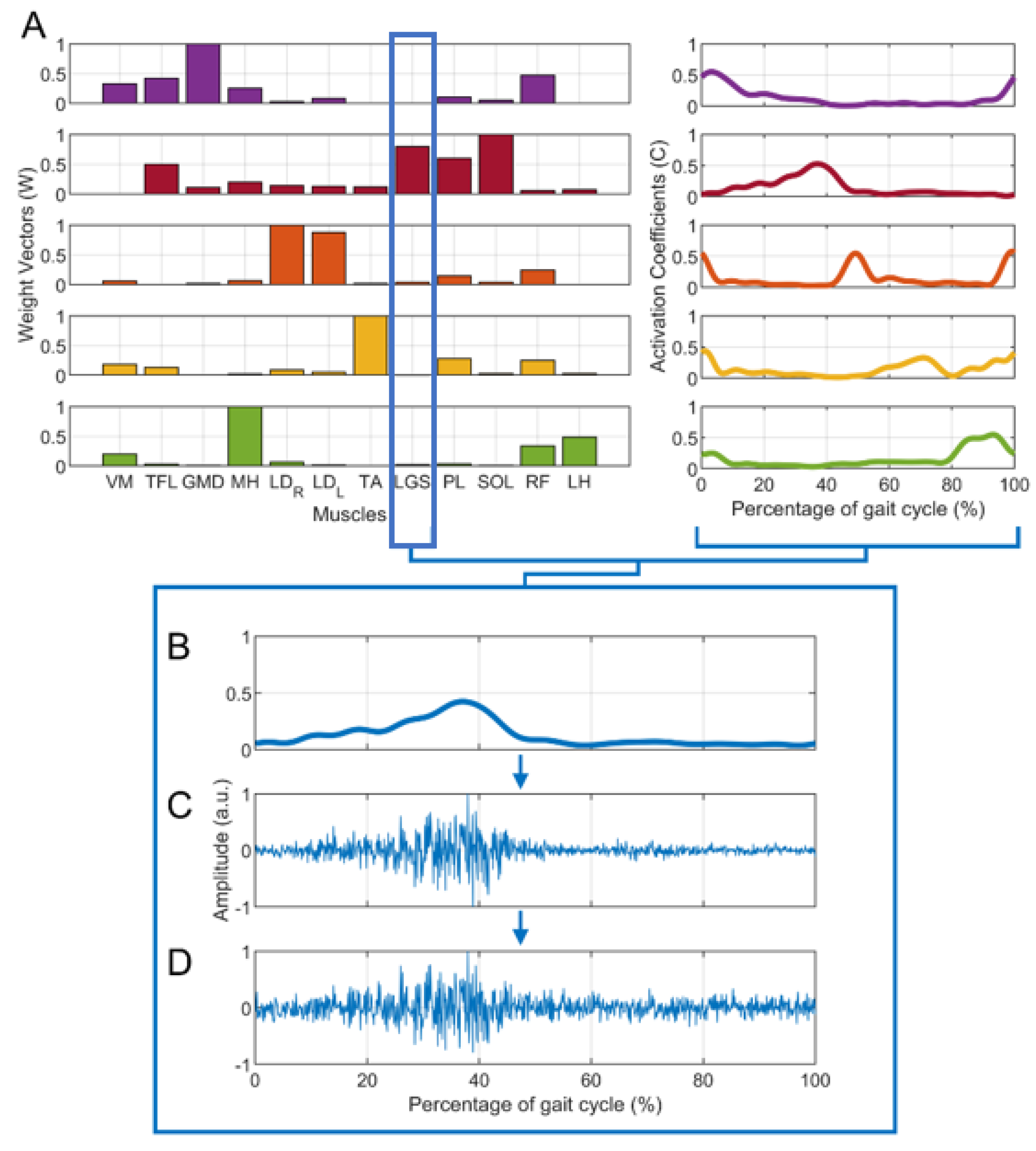
- From the dataset of 20 subjects, 15 subjects were extracted, showing n = 4 (5 subjects), n = 5 (5 subjects), and n = 6 (5 subjects) clearly recognizable muscle synergies, as assessed by expert operators (V.A. and M.G.). Hence, for each subject, activation coefficients () and weight vectors () were obtained. Figure 1A shows an example of muscle synergies (n = 5) representative of a specific subject.
- For each group of 5 subjects, data augmentation was performed to obtain 25 “simulated subjects”, considering all the possible combinations of and . In other words, the matrix of weight vectors of the first subject () was combined with the coefficient matrix of every subject in the group (, , … ), and the same was performed for the other weight matrixes (, … ), obtaining 25 sets of muscle synergies. Overall, 25 sets were obtained with n = 4, 25 sets with n = 5, and 25 sets with n = 6, for a total of 75 sets.
- For each set of and , each muscle’s envelope was reconstructed as the product muscle *, where muscle is the weight vector of a specific muscle. Figure 1B provides an example for the LGS muscle.
- For each muscle’s envelope, a simulated sEMG signal (S) was generated by multiplying the envelope by a zero-mean Gaussian process (GS) with standard deviation σ = 1 a.u. (Figure 1C). At this step, no additive noise was superimposed on the signals. This does not mean that there was “no noise”, but rather that additional noise to the noise originally present in the envelope was not introduced.
- Then, different levels of background noise were added to obtain different SNR values (15 dB, 20 dB, 25 dB, and 30 dB), through a zero-mean Gaussian process (GN) with a standard deviation a.u. [21,34]. Figure 1D shows an example in which SNR was equal to 20 dB. The formula below (1) summarizes how each simulated sEMG signal was generated:
2.3. Muscle Synergy Extraction and Sorting
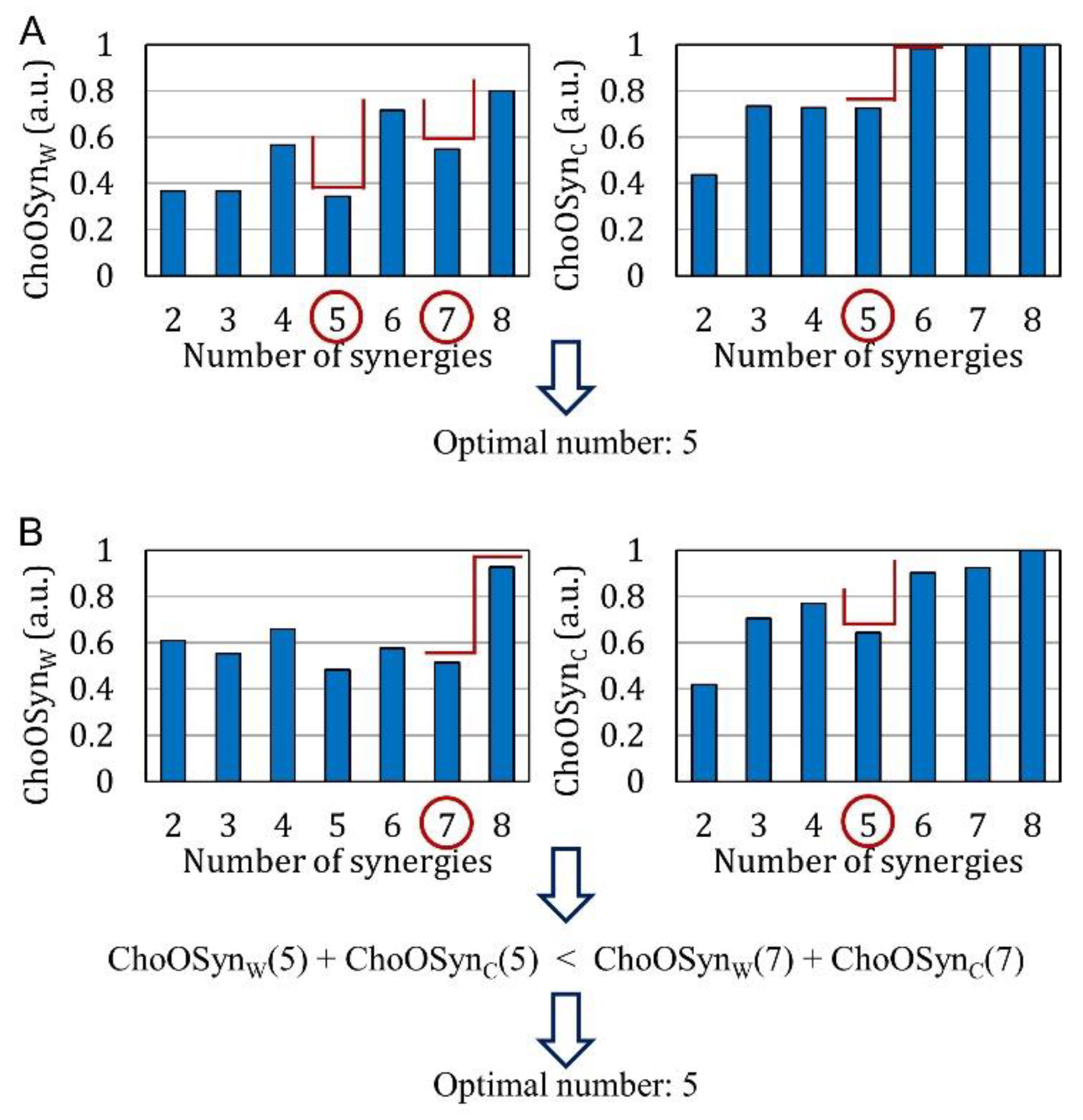
2.4. Choosing the Optimal Number of Synergies (ChoOSyn)
- Low similarity across synergies, to avoid selecting muscle synergies containing redundant information.
2.4.1. Intra-Cluster Variability
2.4.2. Weight Similarity
2.4.3. Coefficient Similarity
2.4.4. ChoOSyn
2.4.5. ChoOSyn Rules
- There is at least a common choice in the selection(s) provided by the two parameters (Figure 3A). In this case, the common number of synergies is selected.
- The two parameters provide a different selection for the number of synergies (Figure 3B). The number is chosen as the one providing the lowest sum of and (i.e., with the lowest similarity and highest consistency).
2.5. VAF-Based Methods
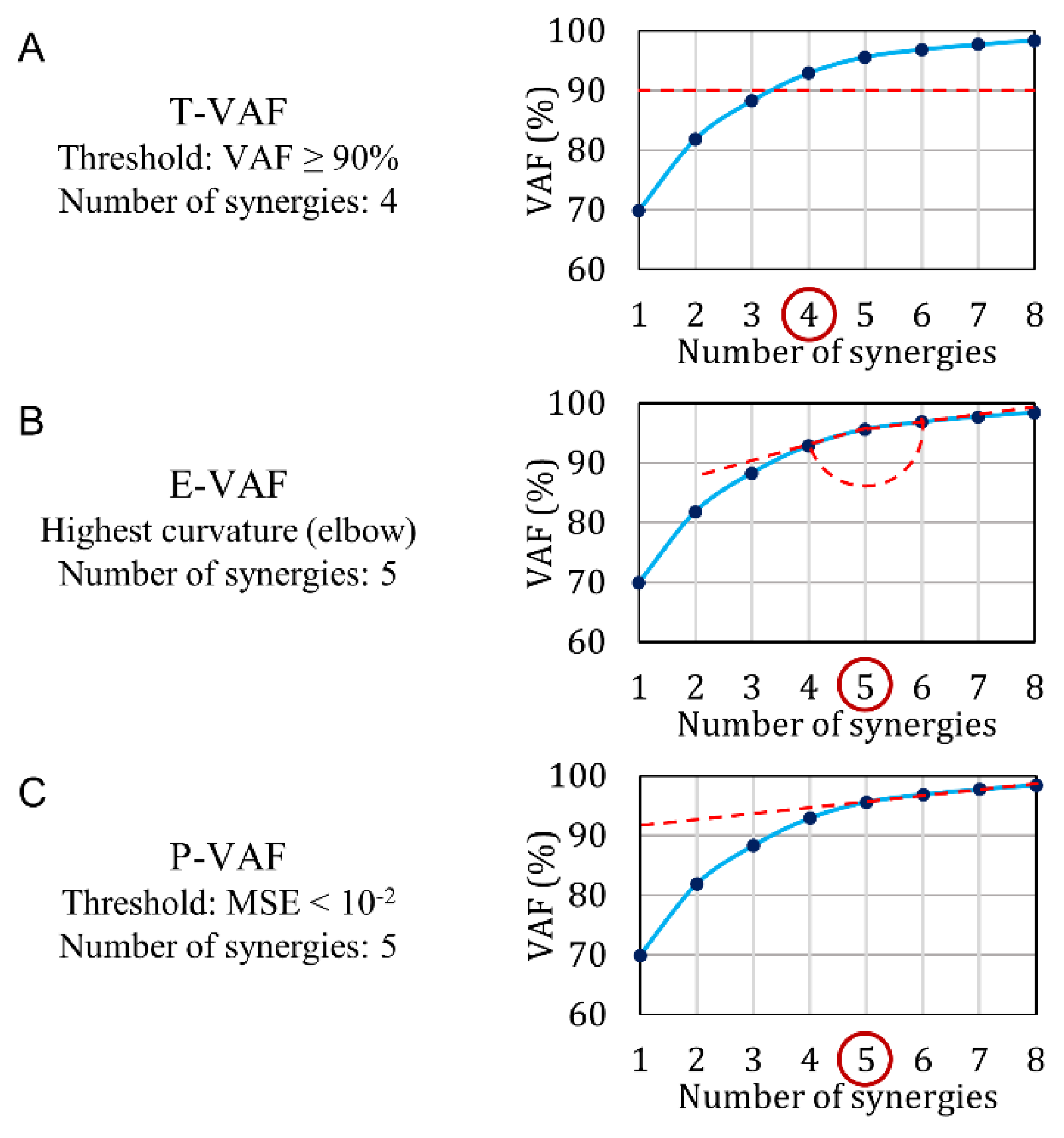
- T-VAF (Threshold VAF) (Figure 4A): this method is the most widely used in the literature [1,2,18,19,20,21,22,23,24,25]. It involves the setting of an arbitrary threshold and the subsequent choice of the first number of synergies with VAF above the threshold. The threshold is commonly set at 90% and less frequently at 95%: therefore, we chose to test both 90% and 95% thresholds.
- P-VAF (Plateau VAF) [40] (Figure 4C): this method requires finding the point beyond which the VAF curve reaches a plateau. It uses an arbitrary threshold: the mean-square error obtained by fitting the VAF-curve through a straight line must be smaller than 10−2. Cheung et al. [40] used a threshold equal to 10−5, but in our simulated dataset 10−2 provided the best performance. The first point satisfying this condition is chosen.
2.6. Performance Evaluation
3. Results
3.1. Simulated Data
3.2. Real Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barroso, F.O.; Torricelli, D.; Molina-Rueda, F.; Alguacil-Diego, I.M.; Cano-De-La-Cuerda, R.; Santos, C.; Moreno, J.C.; Miangolarra-Page, J.C.; Pons, J.L. Combining muscle synergies and biomechanical analysis to assess gait in stroke patients. J. Biomech. 2017, 63, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.L.; Pai, M.M.; McGuirk, T.E.; Fregly, B.J.; Patten, C. Methodological Choices in Muscle Synergy Analysis Impact Differentiation of Physiological Characteristics Following Stroke. Front. Comput. Neurosci. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle Synergies in Parkinson’s Disease. Sensors 2020, 20, 3209. [Google Scholar] [CrossRef] [PubMed]
- Feeney, D.F.; Capobianco, R.A.; Montgomery, J.R.; Morreale, J.; Grabowski, A.M.; Enoka, R.M. Individuals with sacroiliac joint dysfunction display asymmetrical gait and a depressed synergy between muscles providing sacroiliac joint force closure when walking. J. Electromyogr. Kinesiol. 2018, 43, 95–103. [Google Scholar] [CrossRef]
- Mileti, I.; Zampogna, A.; Taborri, J.; Martelli, F.; Rossi, S.; Del Prete, Z.; Paoloni, M.; Suppa, A.; Palermo, E. Parkinson’s disease and Levodopa effects on muscle synergies in postural perturbation. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019. [Google Scholar] [CrossRef]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Dopaminergic modulation of multi-muscle synergies in postural tasks performed by patients with Parkinson’s disease. J. Electromyogr. Kinesiol. 2017, 33, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Grazioso, S.; Caporaso, T.; Palomba, A.; Nardella, S.; Ostuni, B.; Panariello, D.; Di Gironimo, G.; Lanzotti, A. Assessment of upper limb muscle synergies for industrial overhead tasks: A preliminary study. In Proceedings of the 2019 IEEE International Workshop on Metrology for Industry 4.0 and IoT, Naples, Italy, 4–6 June 2019; pp. 89–92. [Google Scholar] [CrossRef]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of Muscle Synergy Outcomes in Clinics, Robotics, and Sports: A Systematic Review. Appl. Bionics Biomech. 2018, 2018, 1–19. [Google Scholar] [CrossRef]
- Santos, P.; Vaz, J.; Correia, P.; Valamatos, M.; Veloso, A.; Pezarat-Correia, P. Intermuscular Coordination in the Power Clean Exercise: Comparison between Olympic Weightlifters and Untrained Individuals—A Preliminary Study. Sensors 2021, 21, 1904. [Google Scholar] [CrossRef]
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Tresch, M.C.; Cheung, V.C.K.; D’Avella, A. Matrix Factorization Algorithms for the Identification of Muscle Synergies: Evaluation on Simulated and Experimental Data Sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nat. Cell Biol. 1999, 401, 788–791. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, E.; De Marchis, C.; Pedrocchi, A.; Ferrigno, G.; Monticone, M.; Schmid, M.; D’Alessio, T.; Conforto, S.; Ferrante, S. Neuro-Mechanics of Recumbent Leg Cycling in Post-Acute Stroke Patients. Ann. Biomed. Eng. 2016, 44, 3238–3251. [Google Scholar] [CrossRef]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.-K.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef]
- Sawers, A.; Allen, J.L.; Ting, L.H. Long-term training modifies the modular structure and organization of walking balance control. J. Neurophysiol. 2015, 114, 3359–3373. [Google Scholar] [CrossRef]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef]
- Rodriguez, K.L.; Roemmich, R.T.; Cam, B.; Fregly, B.J.; Hass, C.J. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 2013, 124, 1390–1397. [Google Scholar] [CrossRef]
- Pale, U.; Atzori, M.; Müller, H.; Scano, A. Variability of Muscle Synergies in Hand Grasps: Analysis of Intra- and Inter-Session Data. Sensors 2020, 20, 4297. [Google Scholar] [CrossRef]
- Ghislieri, M.; Agostini, V.; Knaflitz, M. The effect of signal-to-noise ratio on muscle synergy extraction. In Proceedings of the 2018 IEEE Life Sciences Conference, LSC 2018, Montreal, QC, Canada, 28–30 October 2018; pp. 227–230. [Google Scholar] [CrossRef]
- Ghislieri, M.; Agostini, V.; Knaflitz, M. Muscle Synergies Extracted Using Principal Activations: Improvement of Robustness and Interpretability. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 453–460. [Google Scholar] [CrossRef]
- Rimini, D.; Agostini, V.; Knaflitz, M. Intra-Subject Consistency during Locomotion: Similarity in Shared and Subject-Specific Muscle Synergies. Front. Hum. Neurosci. 2017, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Rimini, D.; Agostini, V.; Knaflitz, M. Evaluation of muscle synergies stability in human locomotion: A comparison between normal and fast walking speed. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017. [Google Scholar] [CrossRef]
- Sy, H.V.N.; Nambu, I.; Wada, Y. The adjustment of muscle synergy recruitment by controlling muscle contraction during the reaching movement. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics, SMC 2016, Budapest, Hungary, 9–12 October 2016; pp. 756–761. [Google Scholar] [CrossRef]
- Hirashima, M.; Oya, T. How does the brain solve muscle redundancy? Filling the gap between optimization and muscle synergy hypotheses. Neurosci. Res. 2016, 104, 80–87. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kaneko, N.; Ogawa, T.; Kawashima, N.; Watanabe, K.; Nakazawa, K. Cortical Correlates of Locomotor Muscle Synergy Activation in Humans: An Electroencephalographic Decoding Study. iScience 2019, 15, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bulea, T.C.; Damiano, D.L. Novel Methods to Enhance Precision and Reliability in Muscle Synergy Identification during Walking. Front. Hum. Neurosci. 2016, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Delis, I.; Berret, B.; Pozzo, T.; Panzeri, S. Quantitative evaluation of muscle synergy models: A single-trial task decoding approach. Front. Comput. Neurosci. 2013, 7, 1–21. [Google Scholar] [CrossRef]
- Delis, I.; Hilt, P.M.; Pozzo, T.; Panzeri, S.; Berret, B. Deciphering the functional role of spatial and temporal muscle synergies in whole-body movements. Sci. Rep. 2018, 8, 8391. [Google Scholar] [CrossRef]
- Agostini, V.; Ghislieri, M.; Rosati, S.; Balestra, G.; Knaflitz, M. Surface Electromyography Applied to Gait Analysis: How to Improve Its Impact in Clinics? Front. Neurol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Soomro, M.H.; Conforto, S.; Giunta, G.; Ranaldi, S.; De Marchis, C. Comparison of Initialization Techniques for the Accurate Extraction of Muscle Synergies from Myoelectric Signals via Nonnegative Matrix Factorization. Appl. Bionics Biomech. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Ranaldi, S.; De Marchis, C.; Rinaldi, M.; Conforto, S. The effect of Non-Negative Matrix Factorization initialization on the accurate identification of muscle synergies with correlated activation signals. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Agostini, V.; Knaflitz, M. An Algorithm for the Estimation of the Signal-To-Noise Ratio in Surface Myoelectric Signals Generated During Cyclic Movements. IEEE Trans. Biomed. Eng. 2011, 59, 219–225. [Google Scholar] [CrossRef]
- Ghislieri, M.; Knaflitz, M.; Labanca, L.; Barone, G.; Bragonzoni, L.; Benedetti, M.G.; Agostini, V. Muscle Synergy Assessment During Single-Leg Stance. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2914–2922. [Google Scholar] [CrossRef]
- Torricelli, D.; Barroso, F.; Coscia, M.; Alessandro, C.; Lunardini, F.; Esteban, E.B.; d’Avella, A. Muscle Synergies in Clinical Practice: Theoretical and Practical Implications. In Emerging Therapies in Neurorehabilitation II; Springer: Cham, Switzerland, 2016; Volume 10, pp. 251–272. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Egizzi, L.; Efarina, D.; Kersting, U.G. Motor modules of human locomotion: Influence of EMG averaging, concatenation, and number of step cycles. Front. Hum. Neurosci. 2014, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H. Nonnegative Matrix Factorization Based on Alternating Nonnegativity Constrained Least Squares and Active Set Method. SIAM J. Matrix Anal. Appl. 2008, 30, 713–730. [Google Scholar] [CrossRef]
- D’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; D’Avella, A.; Tresch, M.C.; Bizzi, E. Central and Sensory Contributions to the Activation and Organization of Muscle Synergies during Natural Motor Behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Scalona, E.; Taborri, J.; Del Prete, Z.; Palermo, E.; Rossi, S. EMG factorization during walking: Does digital filtering influence the accuracy in the evaluation of the muscle synergy number? In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018. [Google Scholar] [CrossRef]
- Agostini, V.; Lanotte, M.; Carlone, M.; Campagnoli, M.; Azzolin, I.; Scarafia, R.; Massazza, G.; Knaflitz, M. Instrumented Gait Analysis for an Objective Pre-/Postassessment of Tap Test in Normal Pressure Hydrocephalus. Arch. Phys. Med. Rehabil. 2015, 96, 1235–1241. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Agostini, V.; Knaflitz, M.; Gasparroni, V.; Boschi, M.; Piperno, R. Self-reported gait unsteadiness in mildly impaired neurological patients: An objective assessment through statistical gait analysis. J. Neuroeng. Rehabil. 2012, 9, 64. [Google Scholar] [CrossRef]
- Agostini, V.; Nascimbeni, A.; Gaffuri, A.; Knaflitz, M. Multiple gait patterns within the same Winters class in children with hemiplegic cerebral palsy. Clin. Biomech. 2015, 30, 908–914. [Google Scholar] [CrossRef]

| Fraction of Correctly Classified | T-VAF (90%) | T-VAF (95%) | E-VAF | P-VAF | ChoOSyn |
| No noise | 2/75 | 36/75 | 75/75 | 75/75 | 73/75 |
| SNR = 30 dB | 0/75 | 24/75 | 75/75 | 75/75 | 73/75 |
| SNR = 25 dB | 0/75 | 18/75 | 75/75 | 75/75 | 74/75 |
| SNR = 20 dB | 0/75 | 9/75 | 74/75 | 72/75 | 72/75 |
| SNR = 15 dB | 0/75 | 0/75 | 65/75 | 73/75 | 63/75 |
| ME 1 | T-VAF (90%) | T-VAF (95%) | E-VAF | P-VAF | ChoOSyn |
| No noise | −1.29 | −0.52 | 0.00 | 0.00 | −0.03 |
| SNR = 30 dB | −1.48 | −0.68 | 0.00 | 0.00 | −0.03 |
| SNR = 25 dB | −1.57 | −0.79 | 0.00 | 0.00 | −0.01 |
| SNR = 20 dB | −2.11 | −1.04 | 0.01 | 0.04 | −0.05 |
| SNR = 15 dB | −3.07 | −1.93 | −0.15 | 0.03 | 0.04 |
| RMSE 1 | T-VAF (90%) | T-VAF (95%) | E-VAF | P-VAF | ChoOSyn |
| No noise | 1.39 | 0.72 | 0.00 | 0.00 | 0.16 |
| SNR = 30 dB | 1.56 | 0.82 | 0.00 | 0.00 | 0.16 |
| SNR = 25 dB | 1.65 | 0.92 | 0.00 | 0.00 | 0.12 |
| SNR = 20 dB | 2.19 | 1.17 | 0.12 | 0.20 | 0.28 |
| SNR = 15 dB | 3.15 | 2.00 | 0.53 | 0.16 | 0.53 |
| T-VAF (90%) | T-VAF (95%) | E-VAF | P-VAF | ChoOSyn | |
|---|---|---|---|---|---|
| Fraction of correctly classified | 8/20 | 7/20 | 12/20 | 6/20 | 17/20 |
| ME 1 | −0.90 | 0.55 | 0.70 | 0.90 | 0.20 |
| RMSE 1 | 1.30 | 0.98 | 1.18 | 1.18 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballarini, R.; Ghislieri, M.; Knaflitz, M.; Agostini, V. An Algorithm for Choosing the Optimal Number of Muscle Synergies during Walking. Sensors 2021, 21, 3311. https://doi.org/10.3390/s21103311
Ballarini R, Ghislieri M, Knaflitz M, Agostini V. An Algorithm for Choosing the Optimal Number of Muscle Synergies during Walking. Sensors. 2021; 21(10):3311. https://doi.org/10.3390/s21103311
Chicago/Turabian StyleBallarini, Riccardo, Marco Ghislieri, Marco Knaflitz, and Valentina Agostini. 2021. "An Algorithm for Choosing the Optimal Number of Muscle Synergies during Walking" Sensors 21, no. 10: 3311. https://doi.org/10.3390/s21103311
APA StyleBallarini, R., Ghislieri, M., Knaflitz, M., & Agostini, V. (2021). An Algorithm for Choosing the Optimal Number of Muscle Synergies during Walking. Sensors, 21(10), 3311. https://doi.org/10.3390/s21103311







