Gait Analysis under Spatial Navigation Task in Elderly People—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Navigation Task (NT)
2.4. Gait Measurement
2.5. Gait Data Preprocessing
2.6. Gait Indicators
2.7. Data Analysis
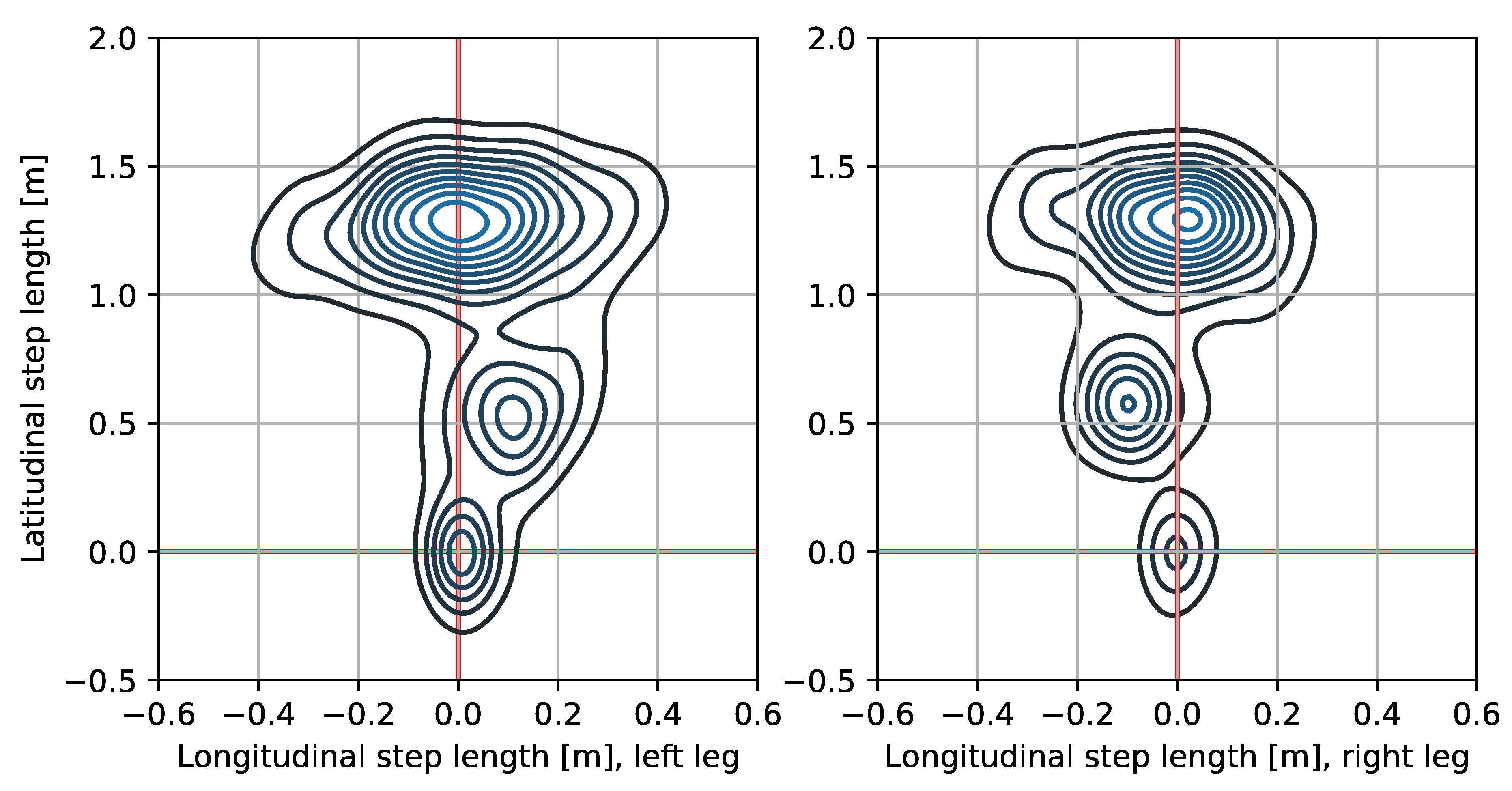
3. Results
4. Discussion
4.1. Gait Variability and Mean Pace
4.2. Task-Related Indicator and Gait Style Change
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorrentino, P.; Lardone, A.; Pesoli, M.; Liparoti, M.; Montuori, S.; Curcio, G.; Sorrentino, G.; Mandolesi, L.; Foti, F. The Development of spatial memory analyzed by means of ecological walking task. Front. Psychol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, T.; Hegarty, M. What determines our navigational abilities? Trends Cogn. Sci. 2010, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Chersi, F.; Burgess, N. The cognitive architecture of spatial navigation: Hippocampal and striatal contributions. Neuron 2015, 88, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Albert, W.; Reinitz, M.T.; Beusmans, J.; Gopal, S. The role of attention in spatial learning during simulated route navigation. Environ. Plan. A 1999, 31, 1459–1472. [Google Scholar] [CrossRef]
- Grön, G.; Wunderlich, A.P.; Spitzer, M.; Tomczak, R.; Riepe, M.W. Brain activation during human navigation: Gender-different neural networks as substrate of performance. Nat. Neurosci. 2000, 3, 404–408. [Google Scholar] [CrossRef]
- Vlček, K. Spatial navigation impairment in healthy aging and Alzheimer’s disease. Clin. Spectr. Alzheimer’s Dis. Charg. Towar. Compr. Diagn. Ther. Strateg. 2011, 5. [Google Scholar] [CrossRef]
- Lester, A.W.; Moffat, S.D.; Wiener, J.M.; Barnes, C.A.; Wolbers, T. The aging navigational system. Neuron 2017, 95, 1019–1035. [Google Scholar] [CrossRef]
- Lithfous, S.; Dufour, A.; Després, O. Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2013, 12, 201–213. [Google Scholar] [CrossRef]
- Zanco, M.; Placido, J.; Marinho, V.; Ferreira, J.V.; de Oliveira, F.; Monteiro-Junior, R.; Barca, M.; Engedal, K.; Laks, J.; Deslandes, A. Spatial navigation in the elderly with Alzheimer’s disease: A cross-sectional study. J. Alzheimer’s Dis. 2018, 66, 1683–1694. [Google Scholar] [CrossRef]
- Cushman, L.A.; Stein, K.; Duffy, C.J. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology 2008, 71, 888–895. [Google Scholar] [CrossRef]
- Weniger, G.; Ruhleder, M.; Lange, C.; Wolf, S.; Irle, E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia 2011, 49, 518–527. [Google Scholar] [CrossRef] [PubMed]
- DeIpolyi, A.; Rankin, K.; Mucke, L.; Miller, B.; Gorno-Tempini, M. Spatial cognition and the human navigation network in AD and MCI. Neurology 2007, 69, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Crucian, G.; Dalrymple-Alford, J.; Dünser, A. Assessing navigation in real and virtual environments: A validation study. Int. J. Disabil. Hum. Dev. 2011, 10, 325–330. [Google Scholar] [CrossRef]
- Carelli, L.; Rusconi, M.L.; Scarabelli, C.; Stampatori, C.; Mattioli, F.; Riva, G. The transfer from survey (map-like) to route representations into Virtual Reality Mazes: Effect of age and cerebral lesion. J. Neuroeng. Rehabil. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Koutakis, P.; Mukherjee, M.; Vallabhajosula, S.; Blanke, D.J.; Stergiou, N. Path integration: Effect of curved path complexity and sensory system on blindfolded walking. Gait Posture 2013, 37, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Skolimowska, J.; Wesierska, M.; Lewandowska, M.; Szymaszek, A.; Szelag, E. Divergent effects of age on performance in spatial associative learning and real idiothetic memory in humans. Behav. Brain Res. 2011, 218, 87–93. [Google Scholar] [CrossRef]
- Mittelstaedt, M.L.; Glasauer, S. Idiothetic navigation in gerbils and humans. Zool. Jb. Physiol 1991, 95, 212. [Google Scholar]
- Allen, G.L.; Kirasic, K.C.; Rashotte, M.A.; Haun, D.B. Aging and path integration skill: Kinesthetic and vestibular contributions to wayfinding. Percept. Psychophys. 2004, 66, 170–179. [Google Scholar] [CrossRef]
- Holtzer, R.; Verghese, J.; Xue, X.; Lipton, R.B. Cognitive processes related to gait velocity: Results from the Einstein Aging Study. Neuropsychology 2006, 20, 215. [Google Scholar] [CrossRef]
- Mielke, M.M.; Roberts, R.O.; Savica, R.; Cha, R.; Drubach, D.I.; Christianson, T.; Pankratz, V.S.; Geda, Y.E.; Machulda, M.M.; Ivnik, R.J.; et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 929–937. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012, 60, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Parihar, R.; Mahoney, J.R.; Verghese, J. Relationship of gait and cognition in the elderly. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Wennberg, A.; Hagen, C.; Edwards, K.; Roberts, R.O.; Hollman, J.H.; Knopman, D.S.; Boeve, B.F.; Machulda, M.M.; Petersen, R.C.; et al. Comparison of gait parameters for predicting cognitive decline: The Mayo Clinic Study of Aging. J. Alzheimer’s Dis. 2017, 55, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Scherder, E.; Eggermont, L.; Swaab, D.; van Heuvelen, M.; Kamsma, Y.; de Greef, M.; van Wijck, R.; Mulder, T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci. Biobehav. Rev. 2007, 31, 485–497. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Yogev, G.; Springer, S.; Simon, E.S.; Giladi, N. Walking is more like catching than tapping: Gait in the elderly as a complex cognitive task. Exp. Brain Res. 2005, 164, 541–548. [Google Scholar] [CrossRef]
- Iersel, M.B.V.; Kessels, R.P.; Bloem, B.R.; Verbeek, A.L.; Olde Rikkert, M.G. Executive functions are associated with gait and balance in community-living elderly people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 1344–1349. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Montero-Odasso, M.; Fantino, B.; Herrmann, F.R.; Allali, G. Gait control: A specific subdomain of executive function? J. Neuroeng. Rehabil. 2012, 9, 12. [Google Scholar] [CrossRef]
- Beurskens, R.; Bock, O. Age-related deficits of dual-task walking: A review. Neural Plast. 2012, 2012. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Berrut, G.; Hommet, C.; Dubost, V.; Assal, F. Gait analysis in demented subjects: Interests and perspectives. Neuropsychiatr. Dis. Treat. 2008, 4, 155. [Google Scholar] [CrossRef]
- Rosso, A.L.; Verghese, J.; Metti, A.L.; Boudreau, R.M.; Aizenstein, H.J.; Kritchevsky, S.; Harris, T.; Yaffe, K.; Satterfield, S.; Studenski, S.; et al. Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology 2017, 89, 336–342. [Google Scholar] [CrossRef]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R.; Xue, X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Priest, A.W.; Salamon, K.B.; Hollman, J.H. Age-related differences in dual task walking: A cross sectional study. J. Neuroeng. Rehabil. 2008, 5, 29. [Google Scholar] [CrossRef]
- Beauchet, O.; Kressig, R.W.; Najafi, E.; Aminian, K.; Dubost, V.; Mourey, F. Age-related decline of gait control under a dual-task condition. J. Am. Geriat. Soc. 2003. [Google Scholar] [CrossRef]
- Hollman, J.H.; Kovash, F.M.; Kubik, J.J.; Linbo, R.A. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture 2007, 26, 113–119. [Google Scholar] [CrossRef]
- Lipman, P.D. Age and exposure differences in acquisition of route information. Psychol. Aging 1991, 6, 128. [Google Scholar] [CrossRef]
- Iaria, G.; Palermo, L.; Committeri, G.; Barton, J.J. Age differences in the formation and use of cognitive maps. Behav. Brain Res. 2009, 196, 187–191. [Google Scholar] [CrossRef]
- Van der Ham, I.J.; Claessen, M.H. How age relates to spatial navigation performance: Functional and methodological considerations. Ageing Res. Rev. 2020, 58, 101020. [Google Scholar] [CrossRef]
- Meina, M.; Ratajczak, E.; Sadowska, M.; Rykaczewski, K.; Dreszer, J.; Bałaj, B.; Biedugnis, S.; Węgrzyński, W.; Krasuski, A. Heart Rate Variability and Accelerometry as Classification Tools for Monitoring Perceived Stress Levels—A Pilot Study on Firefighters. Sensors 2020, 20, 2834. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Separating the effects of age and walking speed on gait variability. Gait Posture 2008, 27, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Mariani, B.; Hoskovec, C.; Rochat, S.; Büla, C.; Penders, J.; Aminian, K. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 2010, 43, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Soangra, R.; Lockhart, T.E. Dual-task does not increase slip and fall risk in healthy young and older adults during walking. Appl. Bionics Biomech. 2017, 1014784. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Giladi, N.; Peretz, C.; Yogev, G.; Simon, E.S.; Hausdorff, J.M. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 950–957. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.M.; Ebmeier, K.P. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009, 119, 1–8. [Google Scholar] [CrossRef]
- Tuokko, H.; Garrett, D.; McDowell, I.; Silverberg, N.; Kristjansson, B. Cognitive decline in high-functioning older adults: Reserve or ascertainment bias? Aging Ment. Health 2003, 7, 259–270. [Google Scholar] [CrossRef]
- Fabrigoule, C. Do leisure activities protect against Alzheimer’s disease? Lancet Neurol. 2002, 1, 11. [Google Scholar] [CrossRef]
- Zunzunegui, M.V.; Alvarado, B.E.; Del Ser, T.; Otero, A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2003, 58, S93–S100. [Google Scholar] [CrossRef]
- Frenzel, A.; Binder, H.; Walter, N.; Wirkner, K.; Loeffler, M.; Loeffler-Wirth, H. The aging human body shape. NPJ Aging Mech. Dis. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Hölscher, C.; Meilinger, T.; Vrachliotis, G.; Brösamle, M.; Knauff, M. Up the down staircase: Wayfinding strategies in multi-level buildings. J. Environ. Psychol. 2006, 26, 284–299. [Google Scholar] [CrossRef]
- Foxlin, E. Pedestrian tracking with shoe-mounted inertial sensors. IEEE Comput. Graph. Appl. 2005, 25, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Silverman, I.; Choi, J.; Mackewn, A.; Fisher, M.; Moro, J.; Olshansky, E. Evolved mechanisms underlying wayfinding: Further studies on the hunter-gatherer theory of spatial sex differences. Evol. Hum. Behav. 2000, 21, 201–213. [Google Scholar] [CrossRef]
- Kiss, R. Variability of gait characterized by normalized deviation. Acta Bioeng. Biomech. 2010, 12, 19–23. [Google Scholar] [PubMed]
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Owings, T.M.; Grabiner, M.D. Measuring step kinematic variability on an instrumented treadmill: How many steps are enough? J. Biomech. 2003, 36, 1215–1218. [Google Scholar] [CrossRef]
- Shimada, H.; Kim, H.; Yoshida, H.; Suzukawa, M.; Makizako, H.; Yoshida, Y.; Saito, K.; Suzuki, T. Relationship between age-associated changes of gait and falls and life-space in elderly people. J. Phys. Ther. Sci. 2010, 22, 419–424. [Google Scholar] [CrossRef]
- Meina, M.; Janusz, A.; Rykaczewski, K.; Ślęzak, D.; Celmer, B.; Krasuski, A. Tagging firefighter activities at the emergency scene: Summary of AAIA’15 data mining competition at Knowledge Pit. In Proceedings of the IEEE 2015 Federated Conference on Computer Science and Information Systems (FedCSIS), Lodz, Poland, 13–16 September 2015; pp. 367–373. [Google Scholar]
- Rubner, Y.; Tomasi, C. Perceptual Metrics for Image Database Navigation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 594. [Google Scholar]
- Pintea, S.; Moldovan, R. The receiver-operating characteristic (ROC) analysis: Fundamentals and applications in clinical psychology. J. Cogn. Behav. Psychother. 2009, 9, 49–66. [Google Scholar]
- Streiner, D.L.; Cairney, J. What’s under the ROC? An introduction to receiver operating characteristics curves. Can. J. Psychiatry 2007, 52, 121–128. [Google Scholar] [CrossRef]
- Stepankova, K.; Pastalkova, E.; Kalova, E.; Kalina, M.; Bures, J. A battery of tests for quantitative examination of idiothetic and allothetic place navigation modes in humans. Behav. Brain Res. 2003, 147, 95–105. [Google Scholar] [CrossRef]
- Granacher, U.; Wolf, I.; Wehrle, A.; Bridenbaugh, S.; Kressig, R.W. Effects of muscle fatigue on gait characteristics under single and dual-task conditions in young and older adults. J. Neuroeng. Rehabil. 2010, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.P.; Federspiel, C. The effects of cognitive training on gait speed and stride variability in old adults: Findings from a pilot study. Aging Clin. Exp. Res. 2014, 26, 635–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beauchet, O.; Dubost, V.; Gonthier, R.; Kressig, R.W. Dual-Task-Related Gait Changes in. Gerontology 2005, 51, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Berlin, J.E.; VanSwearingen, J.M.; Newman, A.B.; Studenski, S.A. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J. Neuroeng. Rehabil. 2005, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Mbourou, G.A.; Lajoie, Y.; Teasdale, N. Step length variability at gait initiation in elderly fallers and non-fallers, and young adults. Gerontology 2003, 49, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Howe, T.; Greenland, J.; Simpson, L.; Rochester, L. Gait variability in older adults: A structured review of testing protocol and clinimetric properties. Gait Posture 2011, 34, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.; Isaacs, B. Characteristics of the gait in old people who fall. Int. Rehabil. Med. 1980, 2, 177–180. [Google Scholar] [CrossRef]
- Kerrigan, D.C.; Todd, M.K.; Della Croce, U.; Lipsitz, L.A.; Collins, J.J. Biomechanical gait alterations independent of speed in the healthy elderly: Evidence for specific limiting impairments. Arch. Phys. Med. Rehabil. 1998, 79, 317–322. [Google Scholar] [CrossRef]
- Morio, Y.; Izawa, K.P.; Omori, Y.; Katata, H.; Ishiyama, D.; Koyama, S.; Yamano, Y. The relationship between walking speed and step length in older aged patients. Diseases 2019, 7, 17. [Google Scholar] [CrossRef]
- Judge, J.O.; Ounpuu, S.; Davis, R.B., III. Effects of age on the biomechanics and physiology of gait. Clin. Geriatr. Med. 1996, 12, 659–678. [Google Scholar] [CrossRef]
- Piotrowski, A.; Cole, J. Clinical measures of balance and functional assessment in elderly persons. Aust. J. Physiother. 1994, 40, 183–188. [Google Scholar] [CrossRef][Green Version]
- Espy, D.D.; Yang, F.; Bhatt, T.; Pai, Y.C. Independent influence of gait speed and step length on stability and fall risk. Gait Posture 2010, 32, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Larish, D.D.; Martin, P.E.; Mungiole, M. Characteristic patterns of gait in the healthy old. Ann. N. Y. Acad. Sci. 1988, 515, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Molinero, A.; Herrero-Larrea, A.; Miñarro, A.; Narvaiza, L.; Gálvez-Barrón, C.; León, N.G.; Valldosera, E.; de Mingo, E.; Macho, O.; Aivar, D.; et al. The spatial parameters of gait and their association with falls, functional decline and death in older adults: A prospective study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tolman, E.C. Cognitive maps in rats and men. Psychol. Rev. 1948, 55, 189. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Krishnan, S. Statistical analysis of gait rhythm in patients with Parkinson’s disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 18, 150–158. [Google Scholar]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef]
| Elderly Participants (n = 13) | Young Participants (n = 16) | |
|---|---|---|
| Age years, mean (SD) | 69.1 (5.4) | 21.5 (2.2) |
| Education years, mean (SD) | 12.8 (2.7) | 13.6 (2.1) |
| Height centimeters, mean (SD) | 160.4 (6.0) | 167.0 (5.2) |
| Weight kilograms, mean (SD) | 68.3 (7.3) | 62.5 (10.3) |
| Indicator | Gait Parameters | Coefficients |
|---|---|---|
| Gait Variability | SD of step length (MP) | −0.49 |
| SD of step length (LP) | −0.45 | |
| SD of step time (DP) | −0.35 | |
| SD of step time (MP) | −0.35 | |
| SD of step time (LP) | −0.30 | |
| Mean Pace | Mean of step length (MP) | −0.61 |
| Mean of step length (LP) | −0.46 | |
| Mean of step length (DP) | −0.45 | |
| Task-Related | step count (MP) | 0.80 |
| step count (DP) | 0.48 | |
| step count (LP) | 0.33 | |
| Gait Style Change | FL-PDF phase substraction (LP-MP) | 0.87 |
| FL-PDF phase substraction (MP-DP) | 0.40 |
| Indicator | Area Under Curve | Confidence Interval | |
|---|---|---|---|
| Lower | Upper | ||
| Gait Style Change | 0.91 | 0.81 | 1.0 |
| Task-Related | 0.83 | 0.70 | 0.95 |
| Mean Pace | 0.63 | 0.47 | 0.81 |
| Gait Variability | 0.51 | 0.34 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk, N.; Szmytke, M.; Meina, M.; Lewandowska, M.; Stępniak, J.; Bałaj, B.; Dreszer, J. Gait Analysis under Spatial Navigation Task in Elderly People—A Pilot Study. Sensors 2021, 21, 270. https://doi.org/10.3390/s21010270
Pawlaczyk N, Szmytke M, Meina M, Lewandowska M, Stępniak J, Bałaj B, Dreszer J. Gait Analysis under Spatial Navigation Task in Elderly People—A Pilot Study. Sensors. 2021; 21(1):270. https://doi.org/10.3390/s21010270
Chicago/Turabian StylePawlaczyk, Natalia, Magdalena Szmytke, Michał Meina, Monika Lewandowska, Justyna Stępniak, Bibianna Bałaj, and Joanna Dreszer. 2021. "Gait Analysis under Spatial Navigation Task in Elderly People—A Pilot Study" Sensors 21, no. 1: 270. https://doi.org/10.3390/s21010270
APA StylePawlaczyk, N., Szmytke, M., Meina, M., Lewandowska, M., Stępniak, J., Bałaj, B., & Dreszer, J. (2021). Gait Analysis under Spatial Navigation Task in Elderly People—A Pilot Study. Sensors, 21(1), 270. https://doi.org/10.3390/s21010270








