Electrochemical Assessment of Anticancer Compounds on the Human Tongue Squamous Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Preparation and Culture Conditions
2.2. Impedance Measurement by ECIS
2.3. Cell Viability Assay
2.4. Annexin V/7-AAD Binding Assay
2.5. Statistical Analysis
3. Results
3.1. Effects of Anticancer Compounds on the Overall Resistance Time Course of SCC-25 Cells
3.2. Effects of Anticancer Compounds on the Morphological Parameters of SCC-25 Cells
3.3. Effects of Anticancer Compounds on the Cellular Micromotion of SCC-25 Cells
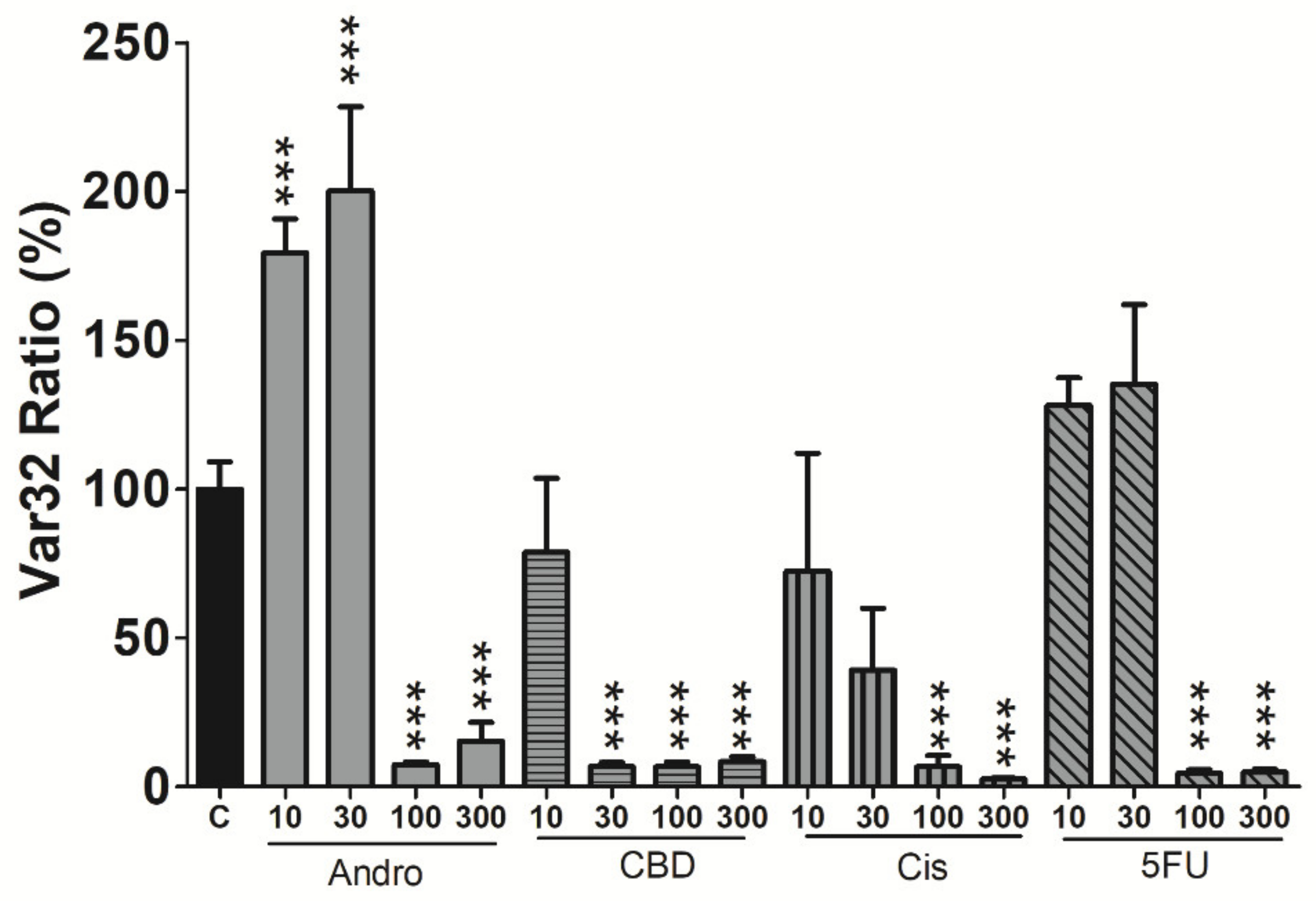
3.4. Effects of Anticancer Compounds on Cell Viability
3.5. Apoptosis Profile of SCC-25 Cells Induced by Drug Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, R.; Oberley, T.D.; Oberley, L.W. Transfection and expression of mnsod cdna decreases tumor malignancy of human oral squamous carcinoma scc-25 cells. Hum. Gene Ther. 1997, 8, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Talamini, R.; Vaccarella, S.; Barbone, F.; Tavani, A.; La Vecchia, C.; Herrero, R.; Munoz, N.; Franceschi, S. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br. J. Cancer 2000, 83, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.M.; Berek, J.S.; Haskell, C.M. Cancer Treatment; W.B. Saunders: Philadelphia, PA, USA, 1980. [Google Scholar]
- Vikram, B.; Strong, E.W.; Shah, J.P.; Spiro, R. Failure at the primary site following multimodality treatment in advanced head and neck cancer. Head Neck Surg. 1984, 6, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanuš, L.O. Cannabidiol—Recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the crosstalk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012, 26, 1535–1548. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, S.E.; Tan, S.H.; Cao, R.; Chen, Y.; Xia, D.; Zhu, X.; Yang, X.F.; Ong, C.N.; Shen, H.M. Andrographolide sensitizes cisplatin-induced apoptosis via suppression of autophagosome-lysosome fusion in human cancer cells. Autophagy 2012, 8, 338–349. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ding, Y.; Lei, Y.; Qi, C.-L.; He, X.-D.; Lan, T.; Li, J.-C.; Gong, P.; Yang, X.; Geng, J.-G. Andrographolide suppress tumor growth by inhibiting tlr4/nf-κb signaling activation in insulinoma. Int. J. Biol. Sci. 2014, 10, 404. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Crippa, J.; Hallak, J.; Moreira, F.; Guimaraes, F. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med Biol. Res. 2006, 39, 421–429. [Google Scholar] [CrossRef]
- Hampson, A.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−) δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Smith, P.F.; Rosengren, R.J. Cannabinoids in the treatment of cancer. Cancer Lett. 2009, 285, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S. Cannabinoid action induces autophagy-mediated cell death through stimulation of er stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheung, H.-Y.; Zhang, Z.; Chan, G.K.; Fong, W.-F. Andrographolide induces cell cycle arrest at g2/m phase and cell death in hepg2 cells via alteration of reactive oxygen species. Eur. J. Pharmacol. 2007, 568, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-D.; Lin, H.-H.; Chiang, T.-A.; Tsai, L.-Y.; Tsai, S.-M.; Lee, Y.-C.; Chen, J.-H. Andrographolide could inhibit human colorectal carcinoma lovo cells migration and invasion via down-regulation of mmp-7 expression. Chem. Biol. Interact. 2009, 180, 344–352. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chen, C.F.; Chiou, W.F. Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef]
- Abu-Ghefreh, A.A.; Canatan, H.; Ezeamuzie, C.I. In vitro and in vivo anti-inflammatory effects of andrographolide. Int. Immunopharmacol. 2009, 9, 313–318. [Google Scholar] [CrossRef]
- Chen, J.H.; Hsiao, G.; Lee, A.R.; Wu, C.C.; Yen, M.H. Andrographolide suppresses endothelial cell apoptosis via activation of phosphatidyl inositol-3-kinase/akt pathway. Biochem. Pharmacol. 2004, 67, 1337–1345. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Ong, C.N.; Shen, H.M. Critical role of pro-apoptotic bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem. Pharmacol. 2006, 72, 132–144. [Google Scholar] [CrossRef]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Maeno, E.; Ishizaki, Y.; Kanaseki, T.; Hazama, A.; Okada, Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9487–9492. [Google Scholar] [CrossRef] [PubMed]
- Hessler, J.A.; Budor, A.; Putchakayala, K.; Mecke, A.; Rieger, D.; Banaszak Holl, M.M.; Orr, B.G.; Bielinska, A.; Beals, J.; Baker, J., Jr. Atomic force microscopy study of early morphological changes during apoptosis. Langmuir 2005, 21, 9280–9286. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef]
- Lo, C.M.; Keese, C.R.; Giaever, I. Monitoring motion of confluent cells in tissue culture. Exp. Cell Res. 1993, 204, 102–109. [Google Scholar] [CrossRef]
- Balasubramanian, L.; Yip, K.P.; Hsu, T.H.; Lo, C.M. Impedance analysis of renal vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008, 295, C954–C965. [Google Scholar] [CrossRef]
- Lo, C.M.; Keese, C.R.; Giaever, I. Ph changes in pulsed CO2 incubators cause periodic changes in cell morphology. Exp. Cell Res. 1994, 213, 391–397. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Malik, A.B.; Del Vecchio, P.J.; Keese, C.R.; Giaever, I. Electrical method for detection of endothelial cell shape change in real time: Assessment of endothelial barrier function. Proc. Natl. Acad. Sci. USA 1992, 89, 7919–7923. [Google Scholar] [CrossRef]
- Reddy, L.; Wang, H.S.; Keese, C.R.; Giaever, I.; Smith, T.J. Assessment of rapid morphological changes associated with elevated camp levels in human orbital fibroblasts. Exp. Cell Res. 1998, 245, 360–367. [Google Scholar] [CrossRef]
- Arndt, S.; Seebach, J.; Psathaki, K.; Galla, H.J.; Wegener, J. Bioelectrical impedance assay to monitor changes in cell shape during apoptosis. Biosens. Bioelectron. 2004, 19, 583–594. [Google Scholar] [CrossRef]
- Lee, K.-C.; Chang, H.-H.; Chung, Y.-H.; Lee, T.-Y. Andrographolide acts as an anti-inflammatory agent in lps-stimulated raw264. 7 macrophages by inhibiting stat3-mediated suppression of the nf-κb pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, H.H.; Hsu, C.H.; Wang, C.J.; Chiang, T.A.; Chen, J.H. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer a549 cells via down-regulation of pi3k/akt signaling pathway. Eur. J. Pharmacol. 2010, 632, 23–32. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Tung, T.-H.; Huang, C.-C.; Lin, S.-Y.; Chao, S.-C.; Chiu, S.-P.; Lee, S.-P.; Lo, C.-M. Electrochemical Assessment of Anticancer Compounds on the Human Tongue Squamous Carcinoma Cells. Sensors 2020, 20, 2632. https://doi.org/10.3390/s20092632
Huang C-C, Tung T-H, Huang C-C, Lin S-Y, Chao S-C, Chiu S-P, Lee S-P, Lo C-M. Electrochemical Assessment of Anticancer Compounds on the Human Tongue Squamous Carcinoma Cells. Sensors. 2020; 20(9):2632. https://doi.org/10.3390/s20092632
Chicago/Turabian StyleHuang, Chun-Chung, Tse-Hua Tung, Chien-Chu Huang, Shao-Yi Lin, Shih-Chi Chao, Sheng-Po Chiu, Shiao-Pieng Lee, and Chun-Min Lo. 2020. "Electrochemical Assessment of Anticancer Compounds on the Human Tongue Squamous Carcinoma Cells" Sensors 20, no. 9: 2632. https://doi.org/10.3390/s20092632
APA StyleHuang, C.-C., Tung, T.-H., Huang, C.-C., Lin, S.-Y., Chao, S.-C., Chiu, S.-P., Lee, S.-P., & Lo, C.-M. (2020). Electrochemical Assessment of Anticancer Compounds on the Human Tongue Squamous Carcinoma Cells. Sensors, 20(9), 2632. https://doi.org/10.3390/s20092632




