Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review
Abstract
1. Introduction
2. Fundamental Development of LED-Based PAI Technology
3. Preclinical and Clinical Applications of LED-Based PAI
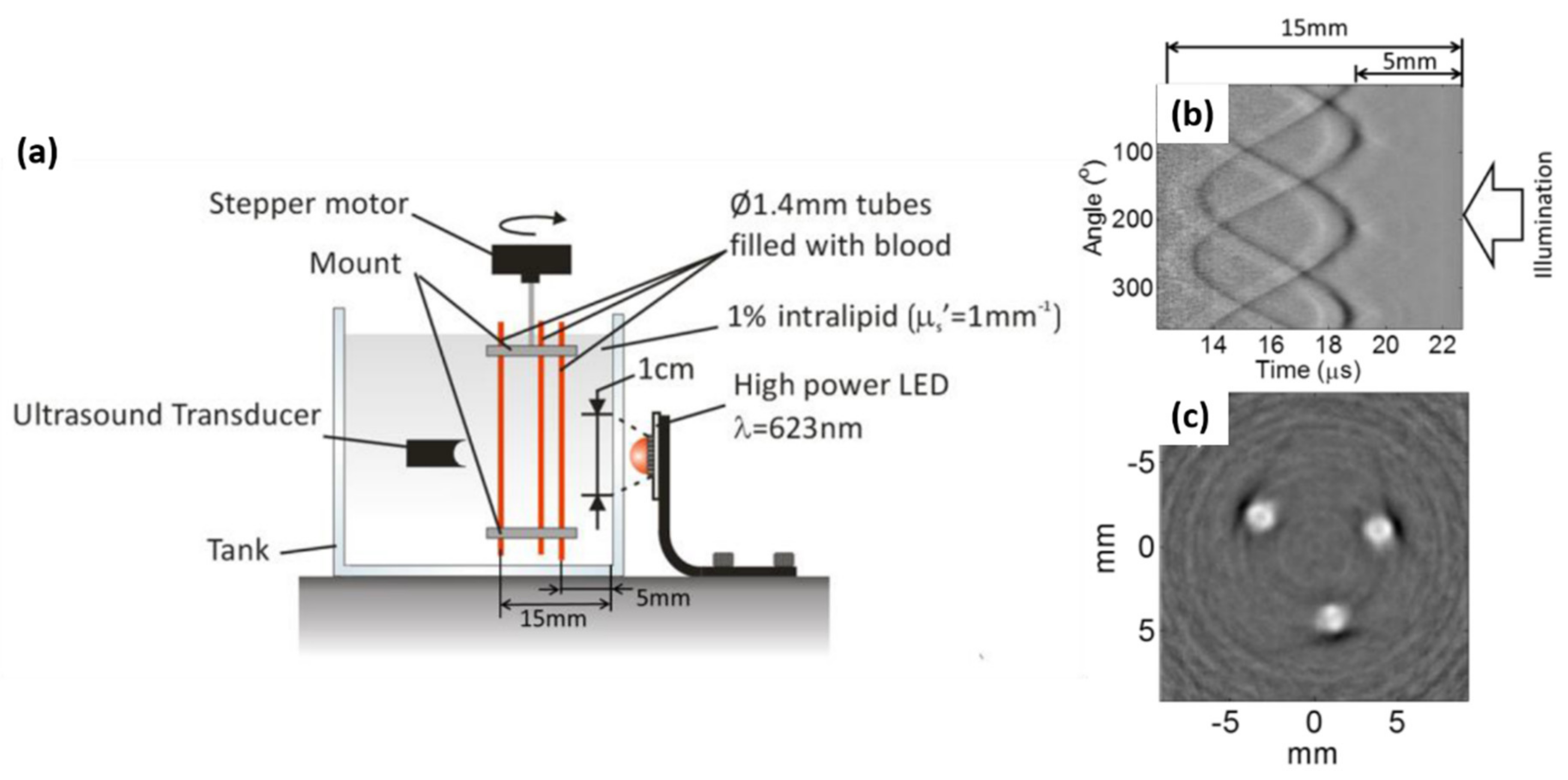
3.1. Phantom and Ex-Vivo Studies
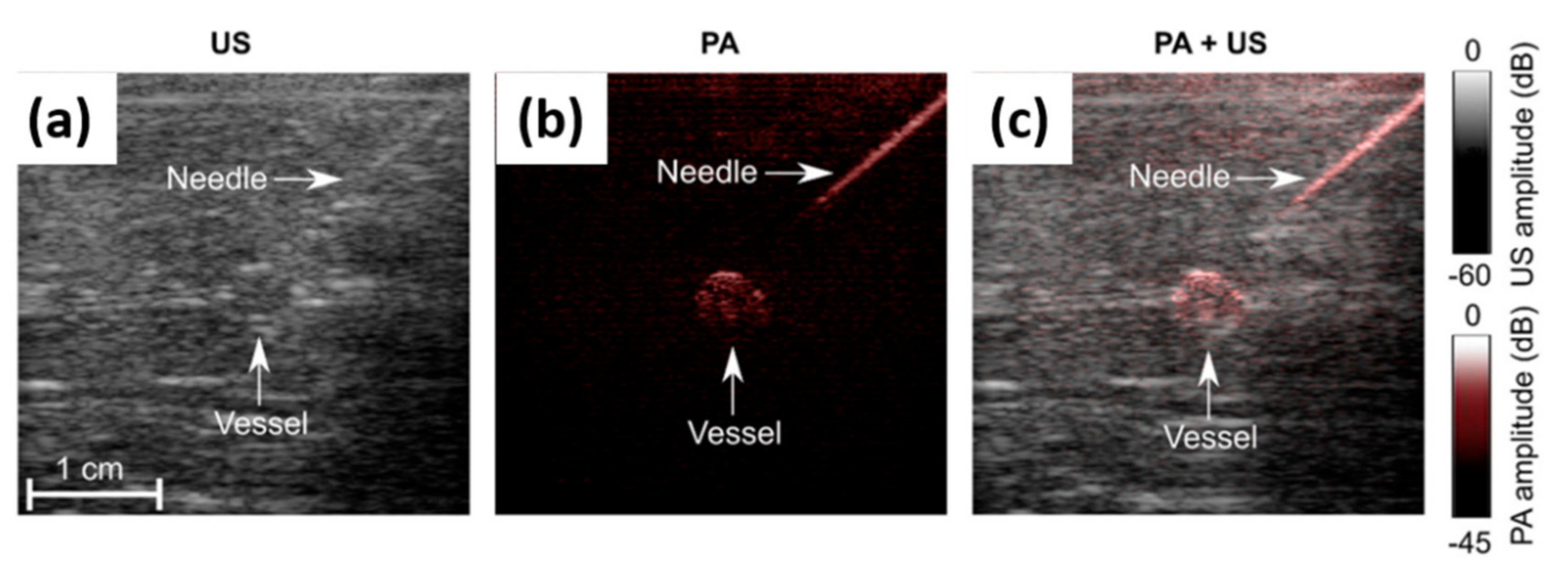
3.1.1. Guidance on Minimally Invasive Procedures with Peripheral Tissue Targets
3.1.2. Imaging of Human Placental Vasculature
3.1.3. Imaging of Intraocular Tumors
3.2. In-Vivo Preclinical Small Animal Imaging Studies
3.2.1. Non-Invasive Monitoring of Angiogenesis
3.2.2. Noninvasive Imaging of Pressure Ulcers
3.2.3. Oxygen Saturation Imaging in Rheumatoid Arthritis Diagnosis
3.2.4. Molecular Imaging: Detection and Monitoring of Reactive Oxygen and Nitrogen Species
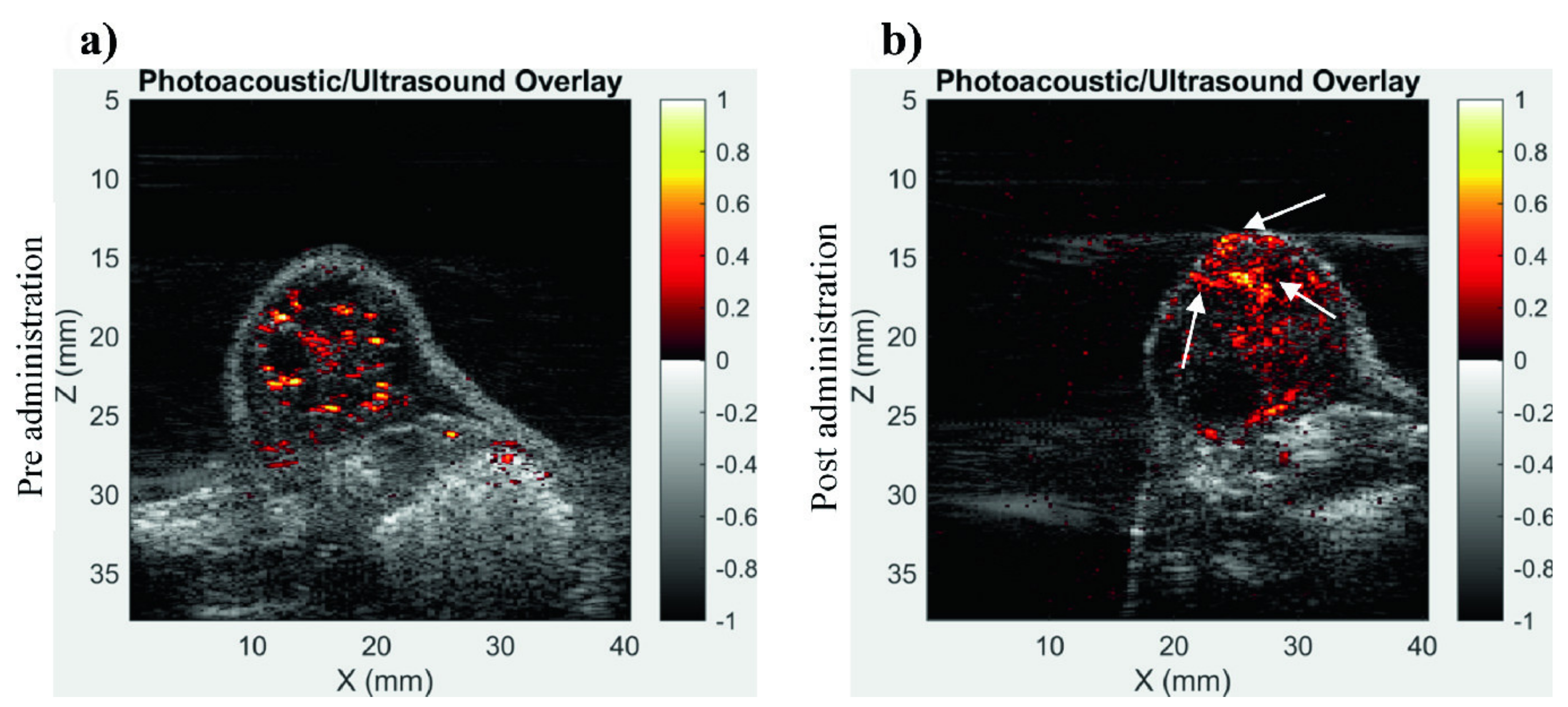
3.2.5. Imaging of Tumor Vasculature Using Contrast Enhancement
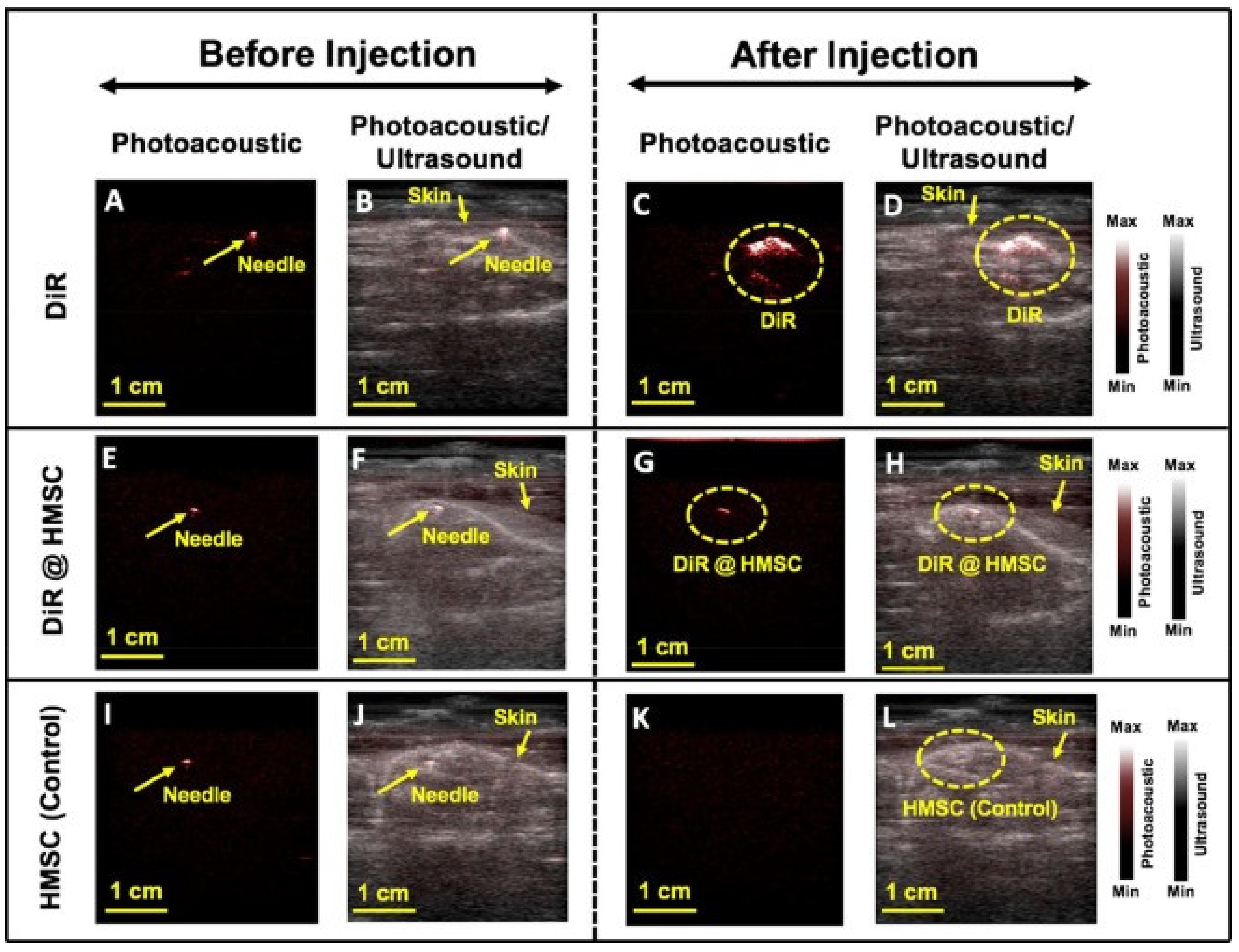
3.2.6. Imaging of Molecular-Labelled Cells
3.3. In-Vivo Human Volunteer Studies
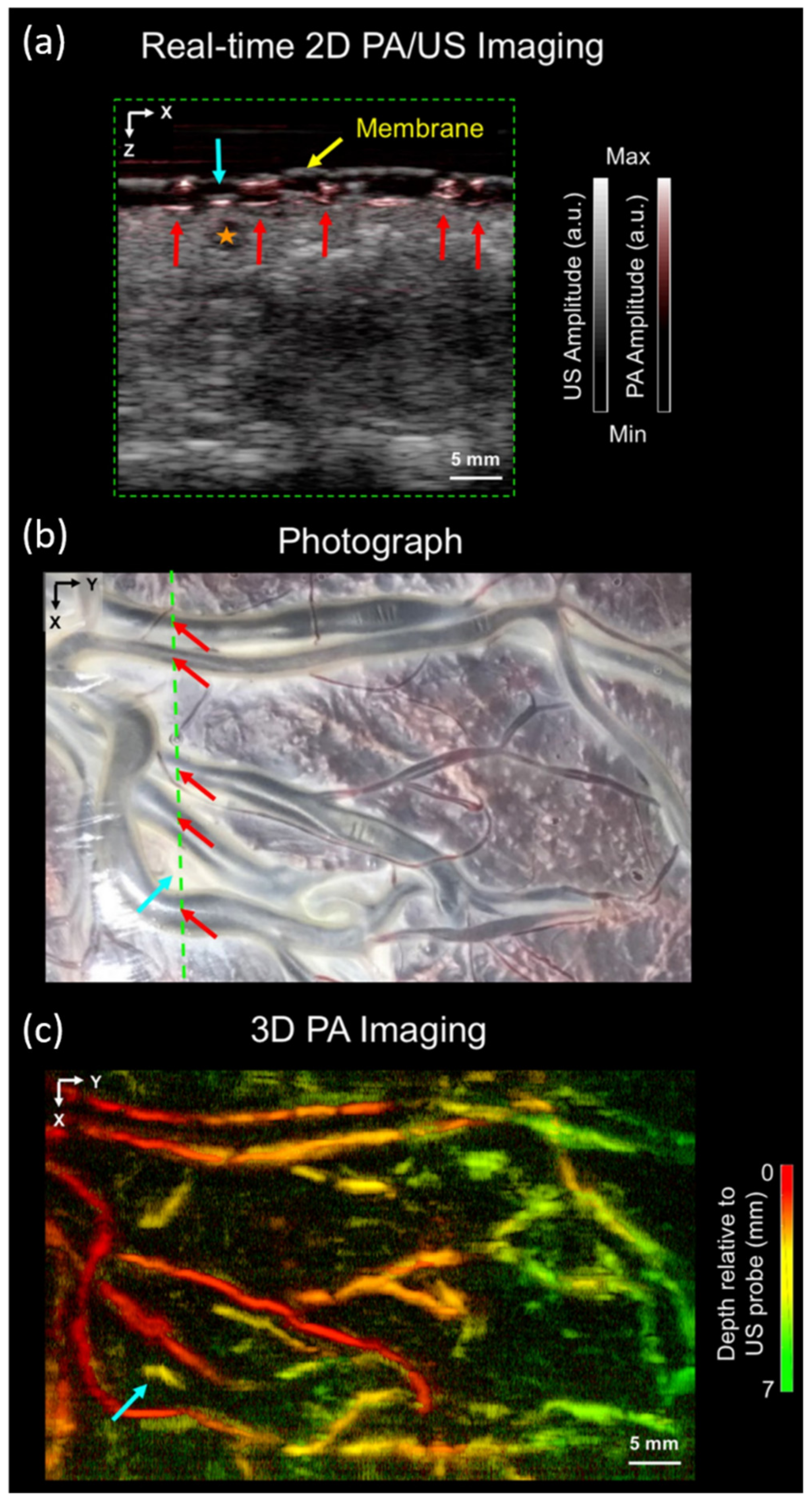
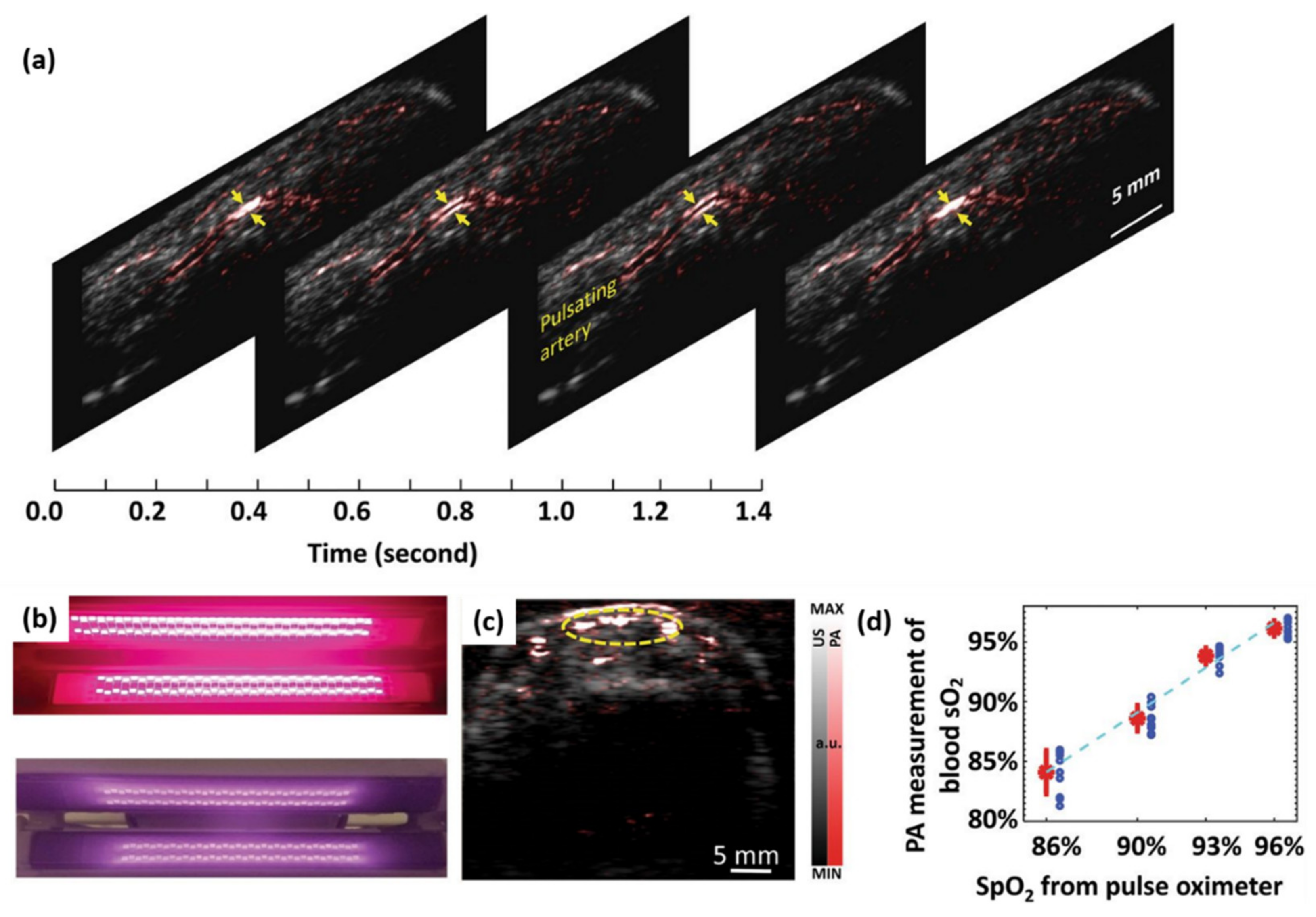
3.3.1. Imaging of Peripheral Microvasculature and Function
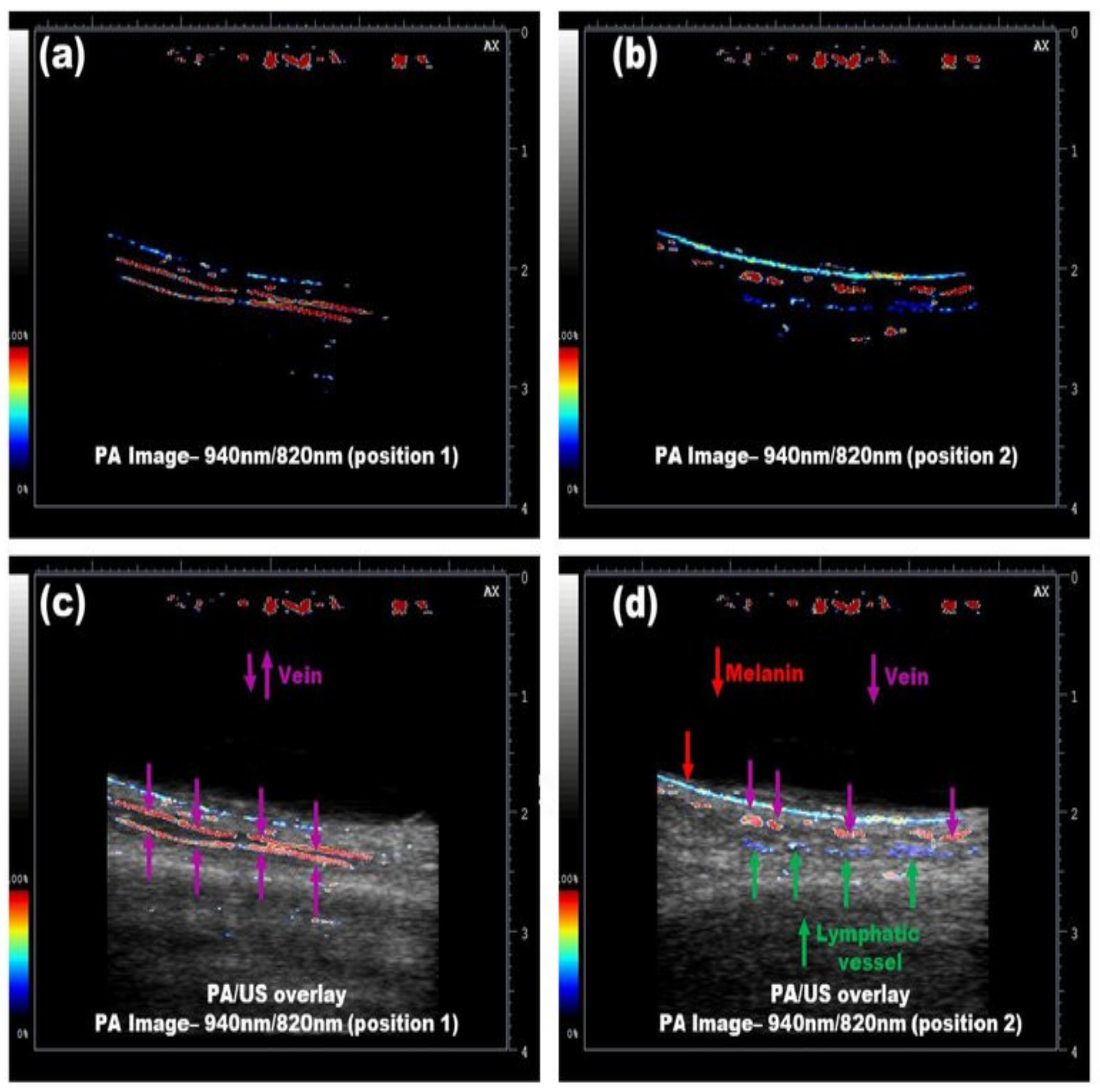
3.3.2. Simultaneous Imaging of Veins and Lymphatic Vessels
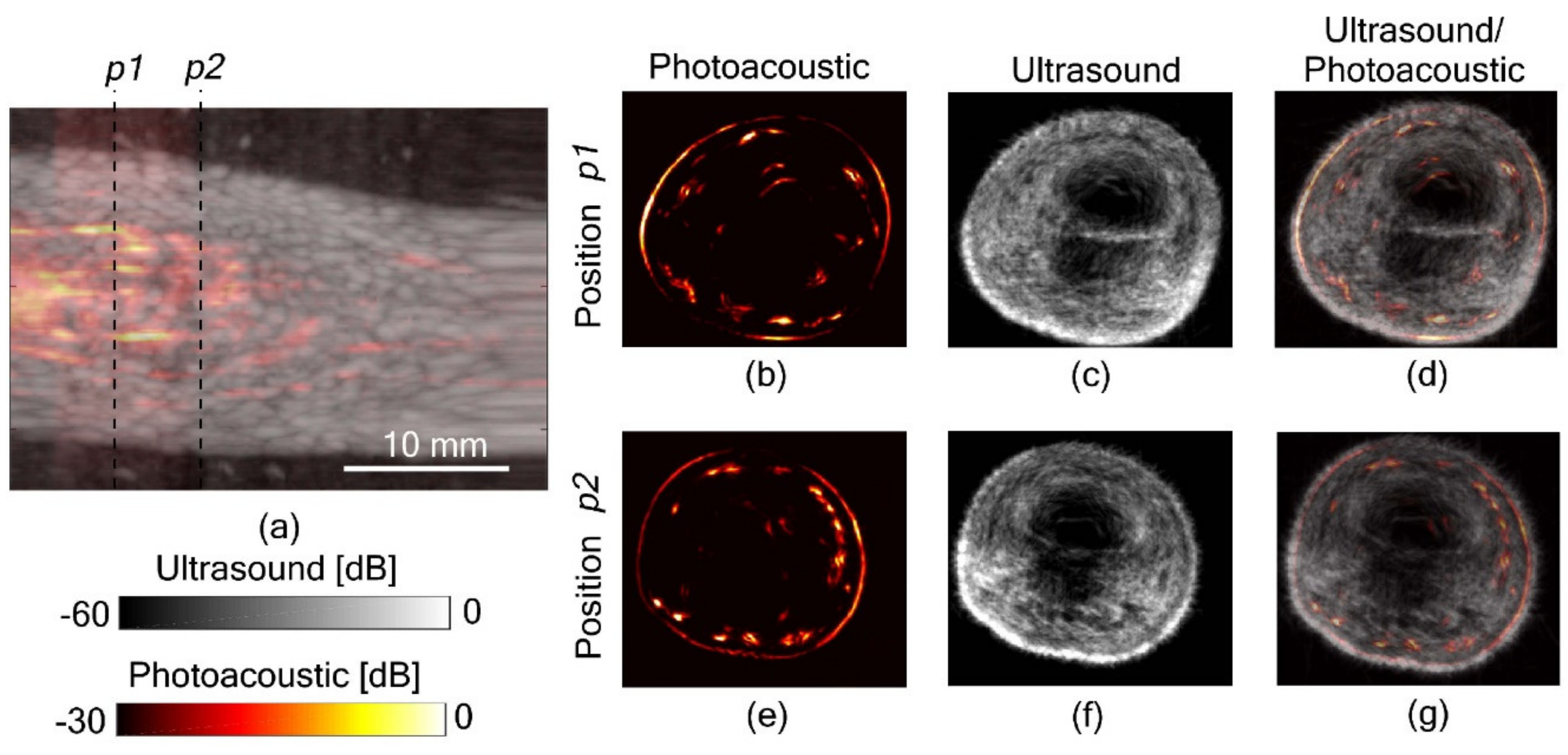
3.3.3. Full View Tomography of Finger Joints
3.4. Clinical Pilot Studies
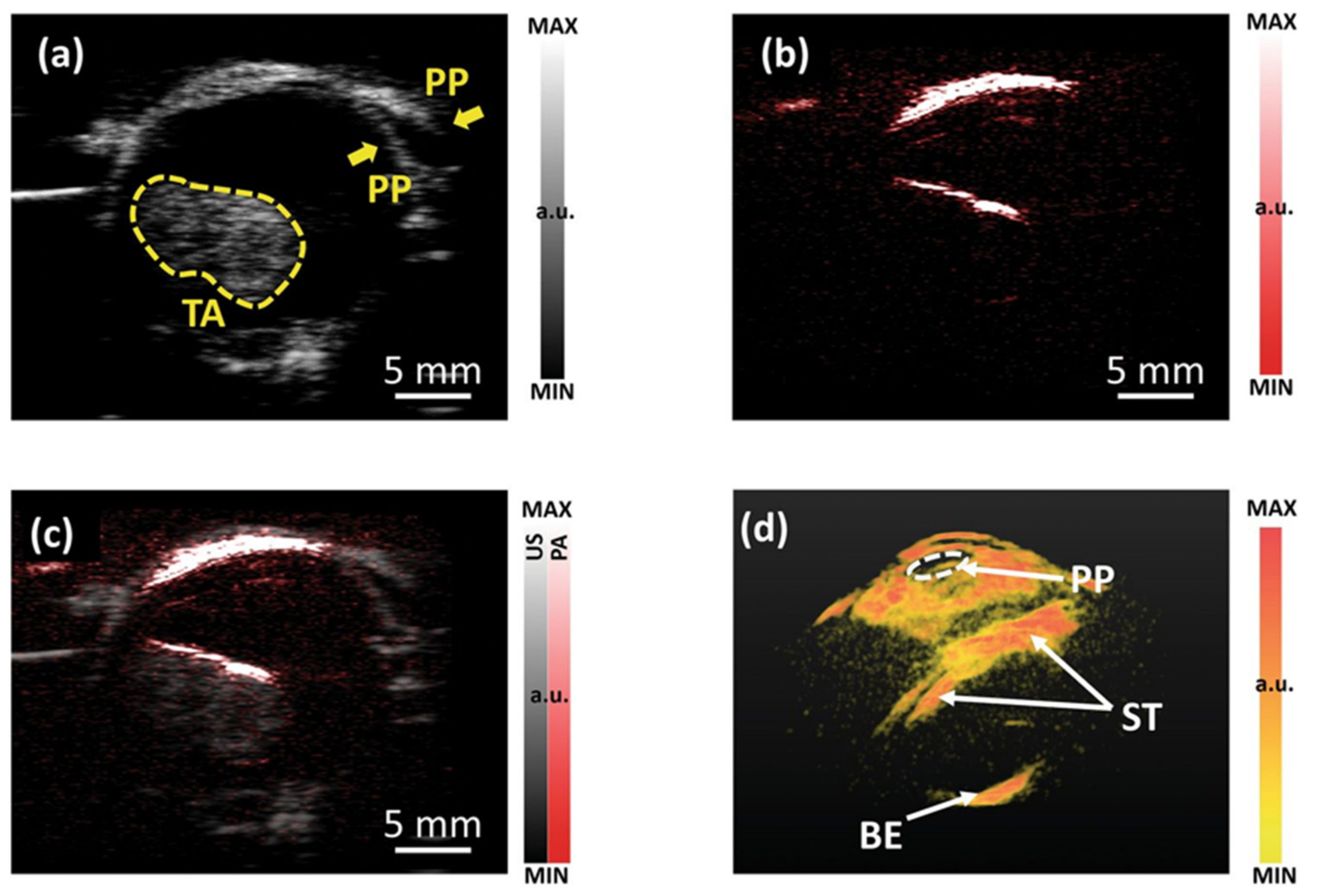
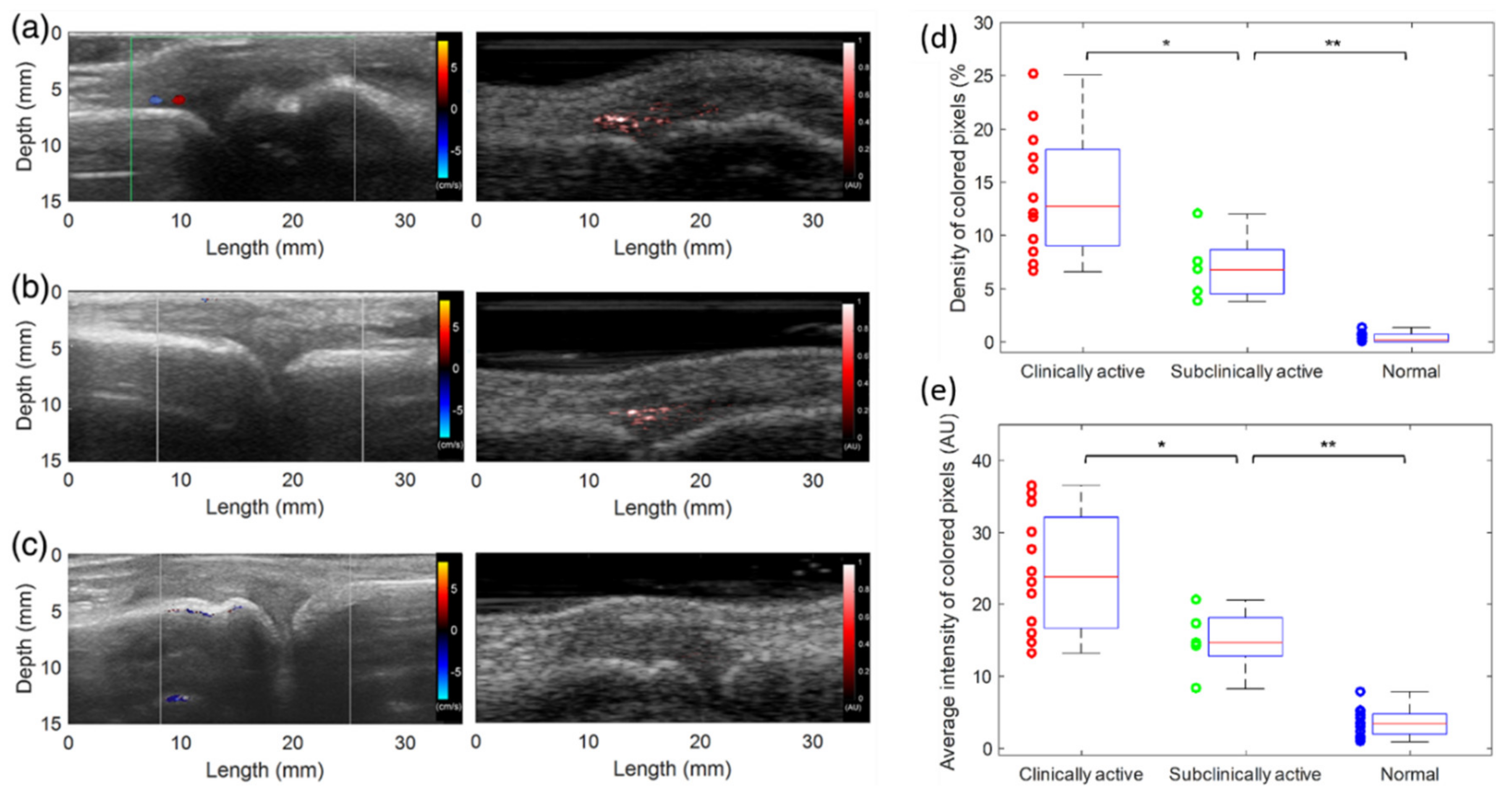
3.4.1. Imaging of Inflammatory Arthritis
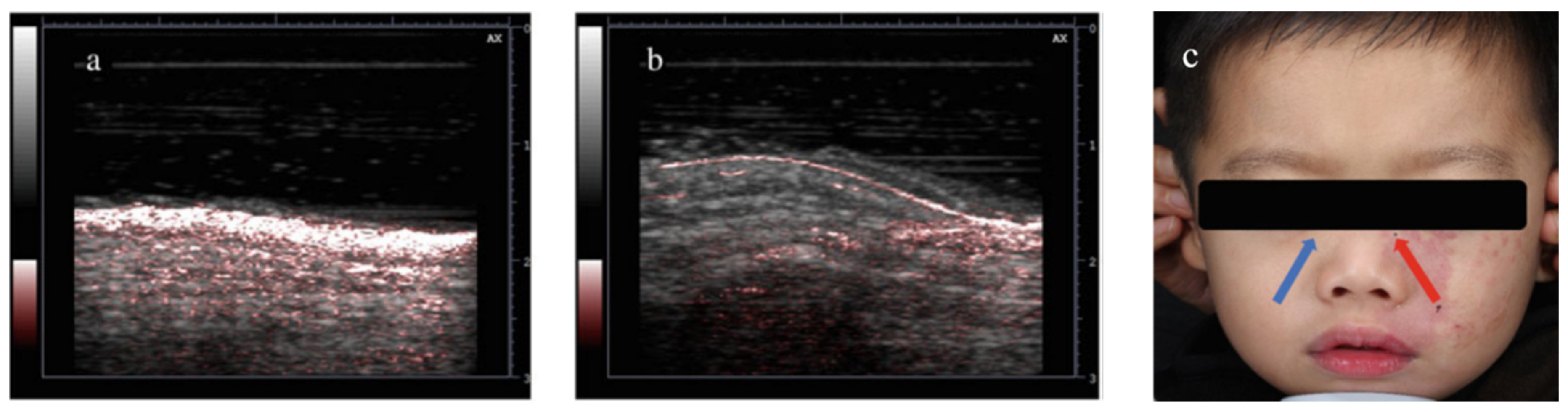
3.4.2. Diagnosis and Treatment Monitoring of Port Wine Stain
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.V.; Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 2012, 335 6075, 1458–1462. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Oladipupo, S.; Hu, S.; Kovalski, J.; Yao, J.; Santeford, A.; Sohn, R.E.; Shohet, R.; Maslov, K.; Wang, L.V.; Arbeit, J.M. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 13264–13269. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, S.S.; Hu, S.; Santeford, A.C.; Yao, J.; Kovalski, J.R.; Shohet, R.V.; Maslov, K.; Wang, L.V.; Arbeit, J.M. Conditional HIF-1 induction produces multistage neovascularization with stage-specific sensitivity to VEGFR inhibitors and myeloid cell independence. Blood J. Am. Soc. Hematol. 2011, 11, 4142–4153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, G.; Yuan, J.; Jo, J.; Gandikota, G.; Demirci, H.; Agano, T.; Sato, N.; Shigeta, Y.; Wang, X. Light emitting diodes based photoacoustic imaging and potential clinical applications. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, X.; Dong, X.; Yuan, J.; Fabiilli, M.L.; Wang, X. LED-Based Photoacoustic Imaging for Monitoring Angiogenesis in Fibrin Scaffolds. Tissue Eng. Part C Methods 2019, 25, 523–531. [Google Scholar] [CrossRef]
- Erpelding, T.N.; Kim, C.; Pramanik, M.; Jankovic, L.; Maslov, K.; Guo, Z.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Sentinel lymph nodes in the rat: noninvasive photoacoustic and US imaging with a clinical US system. Radiology 2010, 256, 102–110. [Google Scholar] [CrossRef]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L. Neurovascular photoacoustic tomography. Front. Neuroenerg. 2010. [Google Scholar] [CrossRef]
- Hu, S.; Yan, P.; Maslov, K.; Lee, J.-M.; Wang, L.V. Intravital imaging of amyloid plaques in a transgenic mouse model using optical-resolution photoacoustic microscopy. Opt. Lett. 2009, 34, 3899–3901. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rao, B.; Maslov, K.; Wang, L.V. Label-free photoacoustic ophthalmic angiography. Opt. Lett. 2010, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Jiang, M.; Hu, J.; Fawzi, A.; Zhou, Q.; Shung, K.K.; Puliafito, C.A.; Zhang, H.F. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt. Express 2010, 18, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.Z.; Povazay, B.; Laufer, J.; Alex, A.; Hofer, B.; Pedley, B.; Glittenberg, C.; Treeby, B.; Cox, B.; Beard, P. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed. Opt. Express 2011, 2, 2202–2215. [Google Scholar] [CrossRef]
- Favazza, C.P.; Wang, L.V.; Jassim, O.W.; Cornelius, L.A. In vivo photoacoustic microscopy of human cutaneous microvasculature and a nevus. BIOMEDO 2011, 16, 016015. [Google Scholar] [CrossRef]
- Yang, J.-M.; Favazza, C.; Chen, R.; Yao, J.; Cai, X.; Maslov, K.; Zhou, Q.; Shung, K.K.; Wang, L.V. Toward dual-wavelength functional photoacoustic endoscopy: Laser and peripheral optical systems development. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing, San Francisco, CA, USA, 9 February 2012; p. 822316. [Google Scholar]
- Yang, J.-M.; Maslov, K.; Yang, H.-C.; Zhou, Q.; Shung, K.K.; Wang, L.V. Photoacoustic endoscopy. Opt. Lett. 2009, 34, 1591–1593. [Google Scholar] [CrossRef]
- Zhu, Y.; Johnson, L.A.; Huang, Z.; Rubin, J.M.; Yuan, J.; Lei, H.; Ni, J.; Wang, X.; Higgins, P.D.; Xu, G. Identifying intestinal fibrosis and inflammation by spectroscopic photoacoustic imaging: an animal study in vivo. Biomed. Opt. Express 2018, 9, 1590–1600. [Google Scholar] [CrossRef]
- Zhu, Y.; Johnson, L.A.; Rubin, J.M.; Appelman, H.; Ni, L.; Yuan, J.; Wang, X.; Higgins, P.D.; Xu, G. Strain-photoacoustic imaging as a potential tool for characterizing intestinal fibrosis. Gastroenterology 2019, 157, 1196–1198. [Google Scholar] [CrossRef]
- Zhu, Y.; Johnson, L.; Rubin, J.; Wang, X.; Higgins, P.; Xu, G. Characterization of intestinal fibrosis and inflammation with transcutaneous spectroscopic PA imaging (Conference Presentation). Photons Plus Ultrasound Imaging Sens. 2018, 10494. [Google Scholar] [CrossRef]
- Feng, T.; Perosky, J.E.; Kozloff, K.M.; Xu, G.; Cheng, Q.; Du, S.; Yuan, J.; Deng, C.X.; Wang, X. Characterization of bone microstructure using photoacoustic spectrum analysis. Opt. Express 2015, 23, 25217–25224. [Google Scholar] [CrossRef] [PubMed]
- Kaiplavil, S.; Mandelis, A.; Wang, X.; Feng, T. Photothermal tomography for the functional and structural evaluation, and early mineral loss monitoring in bones. Biomed. Opt. Express 2014, 5, 2488–2502. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Kozloff, K.M.; Tian, C.; Perosky, J.E.; Hsiao, Y.-S.; Du, S.; Yuan, J.; Deng, C.X.; Wang, X. Bone assessment via thermal photo-acoustic measurements. Opt. Lett. 2015, 40, 1721–1724. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, T.; Cao, M.; Perosky, J.E.; Kozloff, K.; Cheng, Q.; Yuan, J. Photoacoustic measurement of bone health: A study for clinical feasibility. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016. [Google Scholar]
- Jansen, K.; Van Der Steen, A.F.; van Beusekom, H.M.; Oosterhuis, J.W.; van Soest, G. Intravascular photoacoustic imaging of human coronary atherosclerosis. Opt. Lett. 2011, 36, 597–599. [Google Scholar] [CrossRef]
- Wang, B.; Yantsen, E.; Larson, T.; Karpiouk, A.B.; Sethuraman, S.; Su, J.L.; Sokolov, K.; Emelianov, S.Y. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2009, 9, 2212–2217. [Google Scholar] [CrossRef]
- Hariri, A.; Lemaster, J.; Wang, J.; Jeevarathinam, A.S.; Chao, D.L.; Jokerst, J.V. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics 2018, 9, 10–20. [Google Scholar] [CrossRef]
- Hariri, A.; Jeevarathinam, A.S.; Zhao, E.; Jokerst, J.V. Molecular imaging of oxidative stress sensing using LED-based photoacoustic imaging (Conference Presentation). Photons Plus Ultrasound Imaging Sens. 2019, 10878. [Google Scholar] [CrossRef]
- Hariri, A.; Zhao, E.; Jeevarathinam, A.S.; Lemaster, J.; Zhang, J.; Jokerst, J.V. Molecular imaging of oxidative stress using an LED-based photoacoustic imaging system. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Xia, W.; Maneas, E.; Huynh, N.T.; Singh, M.K.A.; Brown, N.M.; Ourselin, S.; Gilbert-Kawai, E.; West, S.J.; Desjardins, A.E. Imaging of human peripheral blood vessels during cuff occlusion with a compact LED-based photoacoustic and ultrasound system. Photons Plus Ultrasound Imaging Sens. 2019, 10878. [Google Scholar] [CrossRef]
- Jo, J.; Xu, G.; Zhu, Y.; Burton, M.; Sarazin, J.; Schiopu, E.; Gandikota, G.; Wang, X. Detecting joint inflammation by an LED-based photoacoustic imaging system: a feasibility study. BIOMEDO 2018, 23, 110501. [Google Scholar] [CrossRef]
- Xia, W.; Kuniyil Ajith Singh, M.; Maneas, E.; Sato, N.; Shigeta, Y.; Agano, T.; Ourselin, S.; J West, S.; E Desjardins, A. Handheld real-time LED-based photoacoustic and ultrasound imaging system for accurate visualization of clinical metal needles and superficial vasculature to guide minimally invasive procedures. Sensors 2018, 18, 1394. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.J.; Beard, P.C. High power visible light emitting diodes as pulsed excitation sources for biomedical photoacoustics. Biomed. Opt. Express 2016, 7, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Agano, T.; Sato, N.; Nakatsuka, H.; Kitagawa, K.; Hanaoka, T.; Morisono, K.; Shigeta, Y. Attempts to increase penetration of photoacoustic system using LED array light source. Photons Plus Ultrasound Imaging Sens. 2015, 9323. [Google Scholar] [CrossRef]
- Agano, T.; Sato, N.; Nakatsuka, H.; Kitagawa, K.; Hanaoka, T.; Morisono, K.; Shigeta, Y. Comparative experiments of photoacoustic system using laser light source and LED array light source. Photons Plus Ultrasound Imaging Sens. 2015, 9323. [Google Scholar] [CrossRef]
- Allen, T.J.; Beard, P.C. Pulsed near-infrared laser diode excitation system for biomedical photoacoustic imaging. Opt. Lett. 2006, 31, 3462–3464. [Google Scholar] [CrossRef]
- Kolkman, R.G.; Steenbergen, W.; van Leeuwen, T.G. In vivo photoacoustic imaging of blood vessels with a pulsed laser diode. Lasers Med. Sci. 2006, 21, 134–139. [Google Scholar] [CrossRef]
- Daoudi, K.; Van Den Berg, P.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P.; Steenbergen, W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365–26374. [Google Scholar] [CrossRef]
- Kalva, S.K.; Upputuri, P.K.; Pramanik, M. High-speed, low-cost, pulsed-laser-diode-based second-generation desktop photoacoustic tomography system. Opt. Lett. 2019, 44, 81–84. [Google Scholar] [CrossRef]
- Agano, T.; Singh, M.K.A.; Nagaoka, R.; Awazu, K. Effect of light pulse width on frequency characteristics of photoacoustic signal—An experimental study using a pulse-width tunable LED-based photoacoustic imaging system. Int. J. Eng. Technol. 2018, 7, 4300–4303. [Google Scholar]
- Upputuri, P.K.; Pramanik, M. Pulsed laser diode based optoacoustic imaging of biological tissues. Biomed. Phys. Eng. Express 2015, 1, 045010. [Google Scholar] [CrossRef]
- Wang, T.; Nandy, S.; Salehi, H.S.; Kumavor, P.D.; Zhu, Q. A low-cost photoacoustic microscopy system with a laser diode excitation. Biomed. Opt. Express 2014, 5, 3053–3058. [Google Scholar] [CrossRef]
- Allen, T.J.; Beard, P.C. Light emitting diodes as an excitation source for biomedical photoacoustics. Photons Plus Ultrasound Imaging Sens. 2013, 8581. [Google Scholar] [CrossRef]
- Allen, T.J. High-Power Light Emitting Diodes; An Alternative Excitation Source for Photoacoustic Tomography. In LED-Based Photoacoustic Imaging; Springer: Berlin/Heidelberg, Germany, 2020; pp. 23–43. [Google Scholar]
- Willert, C.; Stasicki, B.; Klinner, J.; Moessner, S. Pulsed operation of high-power light emitting diodes for imaging flow velocimetry. Meas. Sci. Technol. 2010, 21, 075402. [Google Scholar] [CrossRef]
- Hansen, R.S. Using high-power light emitting diodes for photoacoustic imaging. Ultrason. Imaging Tomogr. Ther. 2011, 7968. [Google Scholar] [CrossRef]
- Ikuta, T.; Shimizu, R. The dynamic response of magnetic domain walls to applied fields in yttrium orthoferrite, observed by a stroboscopic technique. J. Phys. D Appl. Phys. 1974, 7, 726. [Google Scholar] [CrossRef]
- McFarlane, W. An inexpensive nanosecond light pulser for use in photomultiplier system testing. Rev. Sci. Instrum. 1974, 45, 286–289. [Google Scholar] [CrossRef]
- Araki, T.; Misawa, H. Light emitting diode-based nanosecond ultraviolet light source for fluorescence lifetime measurements. Rev. Sci. Instrum. 1995, 66, 5469–5472. [Google Scholar] [CrossRef]
- Araki, T.; Fujisawa, Y.; Hashimoto, M. An ultraviolet nanosecond light pulse generator using a light emitting diode for test of photodetectors. Rev. Sci. Instrum. 1997, 68, 1365–1368. [Google Scholar] [CrossRef]
- Chaney, A.; Sundararajan, R. Simple MOSFET-based high-voltage nanosecond pulse circuit. IEEE Trans. Plasma Sci. 2004, 32, 1919–1924. [Google Scholar] [CrossRef]
- Agano, T.; Sato, N. Photoacoustic Imaging System using LED light source. In Proceedings of the 2016 Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 5–10 June 2016; pp. 1–2. [Google Scholar]
- Narouze, S.N. Atlas of Ultrasound-Guided Procedures in Interventional Pain Management; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627. [Google Scholar] [CrossRef]
- Kim, C.; Erpelding, T.N.; Maslov, K.I.; Jankovic, L.; Akers, W.J.; Song, L.; Achilefu, S.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes. BIOMEDO 2010, 15, 046010. [Google Scholar] [CrossRef]
- Su, J.L.; Karpiouk, A.B.; Wang, B.; Emelianov, S.Y. Photoacoustic imaging of clinical metal needles in tissue. BIOMEDO 2010, 15, 021309. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.; Deprest, J.; Vercauteren, T.; Ourselin, S.; David, A.L. Computer-assisted surgical planning and intraoperative guidance in fetal surgery: a systematic review. Prenat. Diagn. 2015, 35, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Deprest, J.; Hecher, K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am. J. Obstet. Gynecol. 2013, 208, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Guiot, C.; Gaglioti, P.; Oberto, M.; Piccoli, E.; Rosato, R.; Todros, T. Is three-dimensional power Doppler ultrasound useful in the assessment of placental perfusion in normal and growth-restricted pregnancies? Ultrasound Obstet. Gynecol. 2008, 31, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Razansky, D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794. [Google Scholar] [CrossRef]
- Maneas, E.; Xia, W.; Singh, M.K.A.; Sato, N.; Agano, T.; Ourselin, S.; West, S.J.; David, A.L.; Vercauteren, T.; Desjardins, A.E. Human placental vasculature imaging using an LED-based photoacoustic/ultrasound imaging system. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 19 February 2018. [Google Scholar]
- Xia, W.; Maneas, E.; Nikitichev, D.I.; Mosse, C.A.; Dos Santos, G.S.; Vercauteren, T.; David, A.L.; Deprest, J.; Ourselin, S.; Beard, P.C. Interventional photoacoustic imaging of the human placenta with ultrasonic tracking for minimally invasive fetal surgeries. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Xia, W.; Nikitichev, D.I.; Mari, J.M.; West, S.J.; Pratt, R.; David, A.L.; Ourselin, S.; Beard, P.C.; Desjardins, A.E. Performance characteristics of an interventional multispectral photoacoustic imaging system for guiding minimally invasive procedures. BIOMEDO 2015, 20, 086005. [Google Scholar] [CrossRef]
- Maneas, E.; Aughwane, R.; Huynh, N.; Xia, W.; Ansari, R.; Kuniyil Ajith Singh, M.; Hutchinson, J.C.; Sebire, N.J.; Arthurs, O.J.; Deprest, J. Photoacoustic imaging of the human placental vasculature. J. Biophotonics 2019, e201900167. [Google Scholar] [CrossRef]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emelianov, S. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef]
- Krishna, V.; Singh, A.; Sharma, P.; Iwakuma, N.; Wang, Q.; Zhang, Q.; Knapik, J.; Jiang, H.; Grobmyer, S.R.; Koopman, B. Polyhydroxy fullerenes for non-invasive cancer imaging and therapy. Small 2010, 6, 2236–2241. [Google Scholar] [CrossRef]
- Kircher, M.F.; De La Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-L.; Oh, J.-T.; Xie, X.; Ku, G.; Wang, W.; Li, C.; Lungu, G.; Stoica, G.; Wang, L.V. Simultaneous molecular and hypoxia imaging of brain tumors in vivo using spectroscopic photoacoustic tomography. Proc. IEEE 2008, 96, 481–489. [Google Scholar]
- Levi, J.; Kothapalli, S.-R.; Bohndiek, S.; Yoon, J.-K.; Dragulescu-Andrasi, A.; Nielsen, C.; Tisma, A.; Bodapati, S.; Gowrishankar, G.; Yan, X. Molecular photoacoustic imaging of follicular thyroid carcinoma. Clin. Cancer Res. 2013, 19, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Tomori, A.; Hotta, K.; Morita, S.; Kominato, K.; Tanaka, M.; Miyata, Y. Endoscopic submucosal dissection of early esophageal cancer. Clin. Gastroenterol. Hepatol. 2005, 3, S67–S70. [Google Scholar] [CrossRef]
- Polednak, A.P.; Flannery, J.T. Brain, other central nervous system, and eye cancer. Cancer 1995, 75, 330–337. [Google Scholar] [CrossRef]
- Xu, G.; Xue, Y.; Özkurt, Z.G.; Slimani, N.; Hu, Z.; Wang, X.; Xia, K.; Ma, T.; Zhou, Q.; Demirci, H. Photoacoustic imaging features of intraocular tumors: Retinoblastoma and uveal melanoma. PLoS ONE 2017, 12, e0170752. [Google Scholar] [CrossRef]
- Draijer, M.; Hondebrink, E.; van Leeuwen, T.; Steenbergen, W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med. Sci. 2009, 24, 639. [Google Scholar] [CrossRef]
- Briers, J.D. Laser speckle contrast imaging for measuring blood flow. Opt. Appl. 2007, 37, 139–152. [Google Scholar]
- Dunn, A.K. Laser speckle contrast imaging of cerebral blood flow. Ann. Biomed. Eng. 2012, 40, 367–377. [Google Scholar] [CrossRef]
- Hariri, A.; Chen, F.; Moore, C.; Jokerst, J.V. Noninvasive staging of pressure ulcers using photoacoustic imaging. Wound Repair Regen. 2019, 27, 488–496. [Google Scholar] [CrossRef]
- Joseph, F.K.; Xavierselvan, M.; Singh, M.K.A.; Mallidi, S.; van der Laken, C.; van de Loo, F.; Steenbergen, W. LED-based photoacoustic imaging for early detection of joint inflammation in rodents: Towards achieving 3Rs in rheumatoid arthritis research. Photons Plus Ultrasound Imaging Sens. 2020, 11240. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Pu, K.; Cui, L.; Uetrecht, J.P.; Rao, J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 2014, 32, 373. [Google Scholar] [CrossRef] [PubMed]
- Xavierselvan, M.; Mallidi, S. LED-Based Functional Photoacoustics—Portable and Affordable Solution for Preclinical Cancer Imaging. In LED-Based Photoacoustic Imaging: From Bench to Bedside; Singh, M.K.A., Ed.; Springer Singapore: Singapore, 2020; pp. 303–319. [Google Scholar]
- Jokerst, J.V.; Thangaraj, M.; Kempen, P.J.; Sinclair, R.; Gambhir, S.S. Photoacoustic imaging of mesenchymal stem cells in living mice via silica-coated gold nanorods. ACS Nano 2012, 6, 5920–5930. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Ricles, L.M.; Suggs, L.J.; Emelianov, S.Y. In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS ONE 2012, 7, e37267. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Ku, G.; Wang, L.V.; Stoica, G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. BIOMEDO 2006, 11, 024015. [Google Scholar] [CrossRef]
- Yang, S.; Xing, D.; Zhou, Q.; Xiang, L.; Lao, Y. Functional imaging of cerebrovascular activities in small animals using high-resolution photoacoustic tomography. Med. Phys. 2007, 34, 3294–3301. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Agano, T.; Sato, N.; Shigeta, Y.; Uemura, T. Real-time in vivo imaging of human lymphatic system using an LED-based photoacoustic/ultrasound imaging system. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 19 February 2018. [Google Scholar]
- Joseph, F.K.; Boink, Y.; Dantuma, M.; Singh, M.K.A.; Manohar, S.; Steenbergen, W. Tomographic imaging with an ultrasound andLED-based photoacoustic system. Biomed. Opt. Express 2020, 11, 2152–2165. [Google Scholar] [CrossRef]
- Francis, K.J.; Boink, Y.E.; Dantuma, M.; Singh, M.K.A.; Manohar, S.; Steenbergen, W. Light Emitting Diodes Based Photoacoustic and Ultrasound Tomography: Imaging Aspects and Applications. In LED-Based Photoacoustic Imaging; Springer: Berlin/Heidelberg, Germany, 2020; pp. 245–266. [Google Scholar]
- Agrawal, S.; Fadden, C.; Dangi, A.; Yang, X.; Albahrani, H.; Frings, N.; Heidari Zadi, S.; Kothapalli, S.-R. Light-Emitting-Diode-Based Multispectral Photoacoustic Computed Tomography System. Sensors 2019, 19, 4861. [Google Scholar] [CrossRef]
- Wang, X.; Chamberland, D.L.; Carson, P.L.; Fowlkes, J.B.; Bude, R.O.; Jamadar, D.A.; Roessler, B.J. Imaging of joints with laser-based photoacoustic tomography: An animal study. Med. Phys. 2006, 33, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Appl. Phys. Lett. 2007, 90, 053901. [Google Scholar] [CrossRef]
- Sun, Y.; Sobel, E.; Jiang, H. Quantitative three-dimensional photoacoustic tomography of the finger joints: an in vivo study. J. Biomed. Opt. 2009, 14, 064002. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Rajian, J.R.; Girish, G.; Kaplan, M.J.; Fowlkes, J.B.; Carson, P.L.; Wang, X. Photoacoustic and ultrasound dual-modality imaging of human peripheral joints. J. Biomed. Opt. 2013, 18, 10502. [Google Scholar] [CrossRef] [PubMed]
- Van Es, P.; Biswas, S.K.; Moens, H.J.B.; Steenbergen, W.; Manohar, S. Initial results of finger imaging using photoacoustic computed tomography. J. Biomed. Opt. 2014, 19, 060501. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sobel, E.S.; Jiang, H. First assessment of three-dimensional quantitative photoacoustic tomography for in vivo detection of osteoarthritis in the finger joints. Med. Phys. 2011, 38, 4009–4017. [Google Scholar] [CrossRef]
- Rajian, J.R.; Girish, G.; Wang, X. Photoacoustic tomography to identify inflammatory arthritis. J. Biomed. Opt. 2012, 17, 96011–96013. [Google Scholar] [CrossRef]
- Rajian, J.R.; Shao, X.; Chamberland, D.L.; Wang, X. Characterization and treatment monitoring of inflammatory arthritis by photoacoustic imaging: a study on adjuvant-induced arthritis rat model. Biomed. Opt. Express 2013, 4, 900–908. [Google Scholar] [CrossRef]
- Beziere, N.; von Schacky, C.; Kosanke, Y.; Kimm, M.; Nunes, A.; Licha, K.; Aichler, M.; Walch, A.; Rummeny, E.J.; Ntziachristos, V.; et al. Optoacoustic imaging and staging of inflammation in a murine model of arthritis. Arthritis Rheumatol. 2014, 66, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xu, G.; Cao, M.; Marquardt, A.; Francis, S.; Gandikota, G.; Wang, X. A Functional Study of Human Inflammatory Arthritis Using Photoacoustic Imaging. Sci. Rep. 2017, 7, 15026. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, J.; Wen, L.; Shen, S.; Zhang, Y.; Wang, P.; Cheng, Q.; Wang, X.; Wang, X. A novel light emitting diodes based photoacoustic evaluation method for port wine stain and its clinical trial (Conference Presentation). Photons Plus Ultrasound Imaging Sens. 2019, 10878. [Google Scholar] [CrossRef]
- Cheng, Q.; Qian, M.; Wang, X.; Zhang, H.; Wang, P.; Wen, L.; Pan, J.; Gao, Y.; Wu, S.; Zhang, M. Diagnosis and Treatment Monitoring of Port-Wine Stain Using LED-Based Photoacoustics: Theoretical Aspects and First In-Human Clinical Pilot Study. In LED-Based Photoacoustic Imaging: From Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2020; pp. 351–377. [Google Scholar]
- Agano, T.; Sato, N.; Awazu, K. LED-based photoacoustic imaging system: Why it achieves the same signal to noise ratio as solid-state-laser-based system: A review. Photons Plus Ultrasound Imaging Sens. 2020, 11240. [Google Scholar] [CrossRef]
- Anas, E.M.A.; Zhang, H.K.; Kang, J.; Boctor, E. Enabling fast and high quality LED photoacoustic imaging: a recurrent neural networks based approach. Biomed. Opt. Express 2018, 9, 3852–3866. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarzadeh, M.; Hariri, A.; Moore, C.; Jokerst, J.V. The double-stage delay-multiply-and-sum image reconstruction method improves imaging quality in a led-based photoacoustic array scanner. Photoacoustics 2018, 12, 22–29. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Sivasubramanian, K.; Sato, N.; Ichihashi, F.; Sankai, Y.; Xing, L. Deep learning-enhanced LED-based photoacoustic imaging. Photons Plus Ultrasound Imaging Sens. 2020, 11240. [Google Scholar] [CrossRef]
- Dai, X.; Yang, H.; Jiang, H. In vivo photoacoustic imaging of vasculature with a low-cost miniature light emitting diode excitation. Opt. Lett. 2017, 42, 1456–1459. [Google Scholar] [CrossRef]
- Dai, X.; Yang, H.; Jiang, H. Low-cost high-power light emitting diodes for photoacoustic imaging. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2017, San Francisco, CA, USA, 29 January–1 February 2017; p. 100644. [Google Scholar]
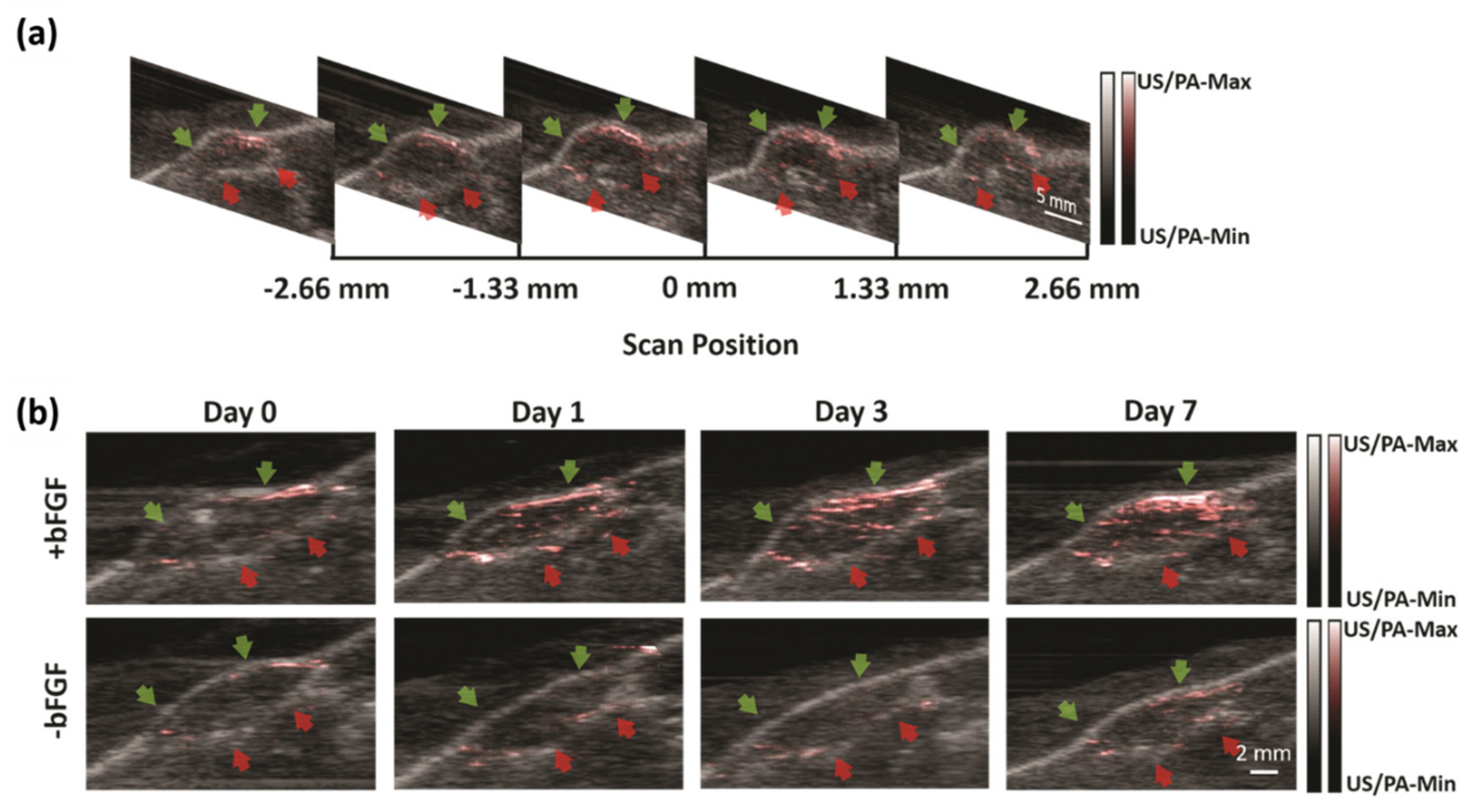
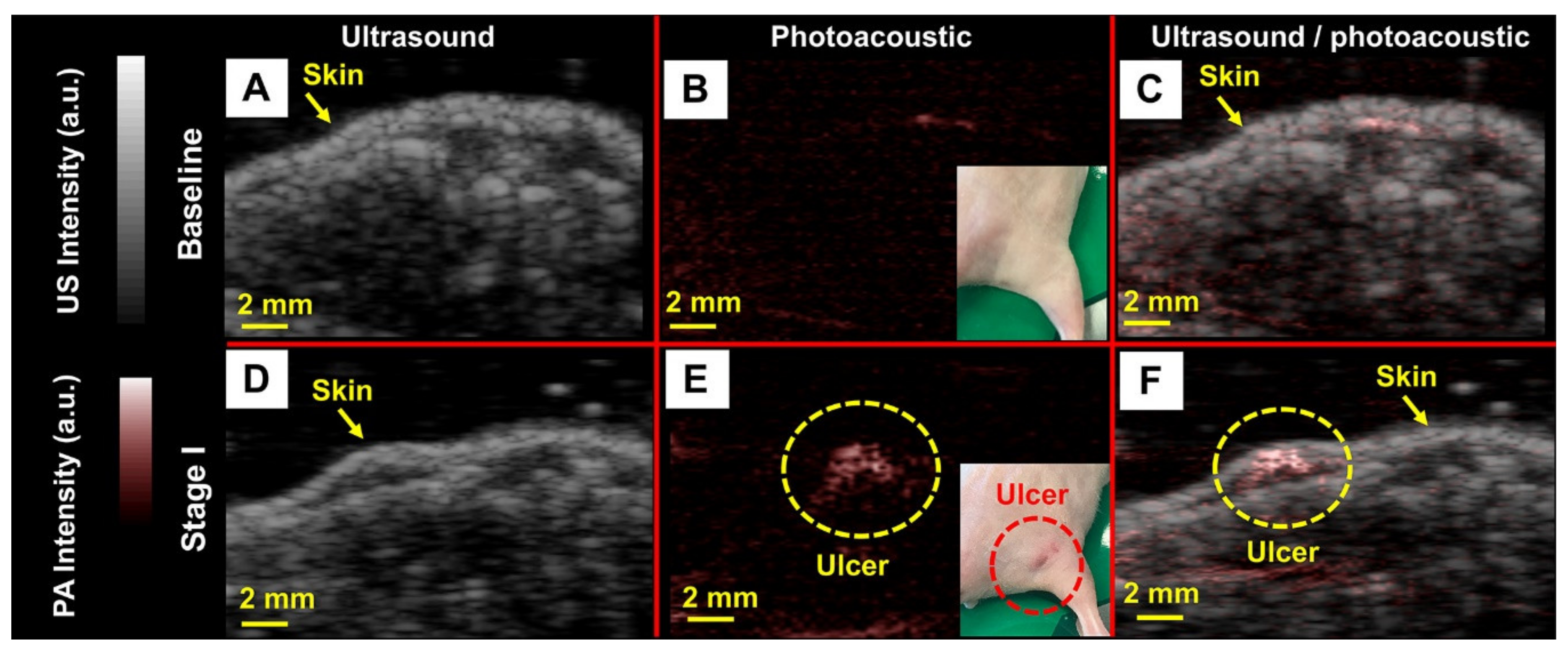
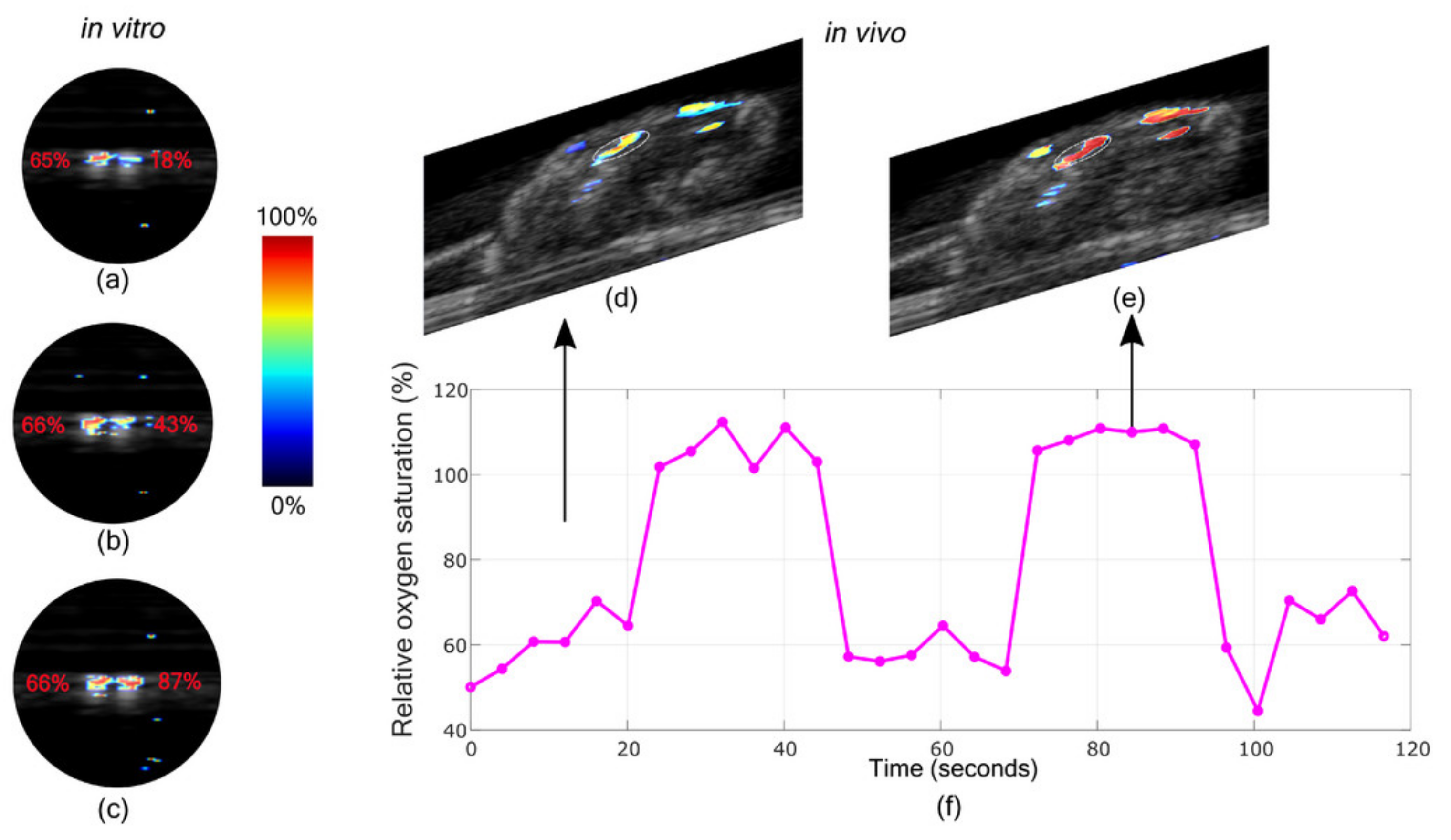
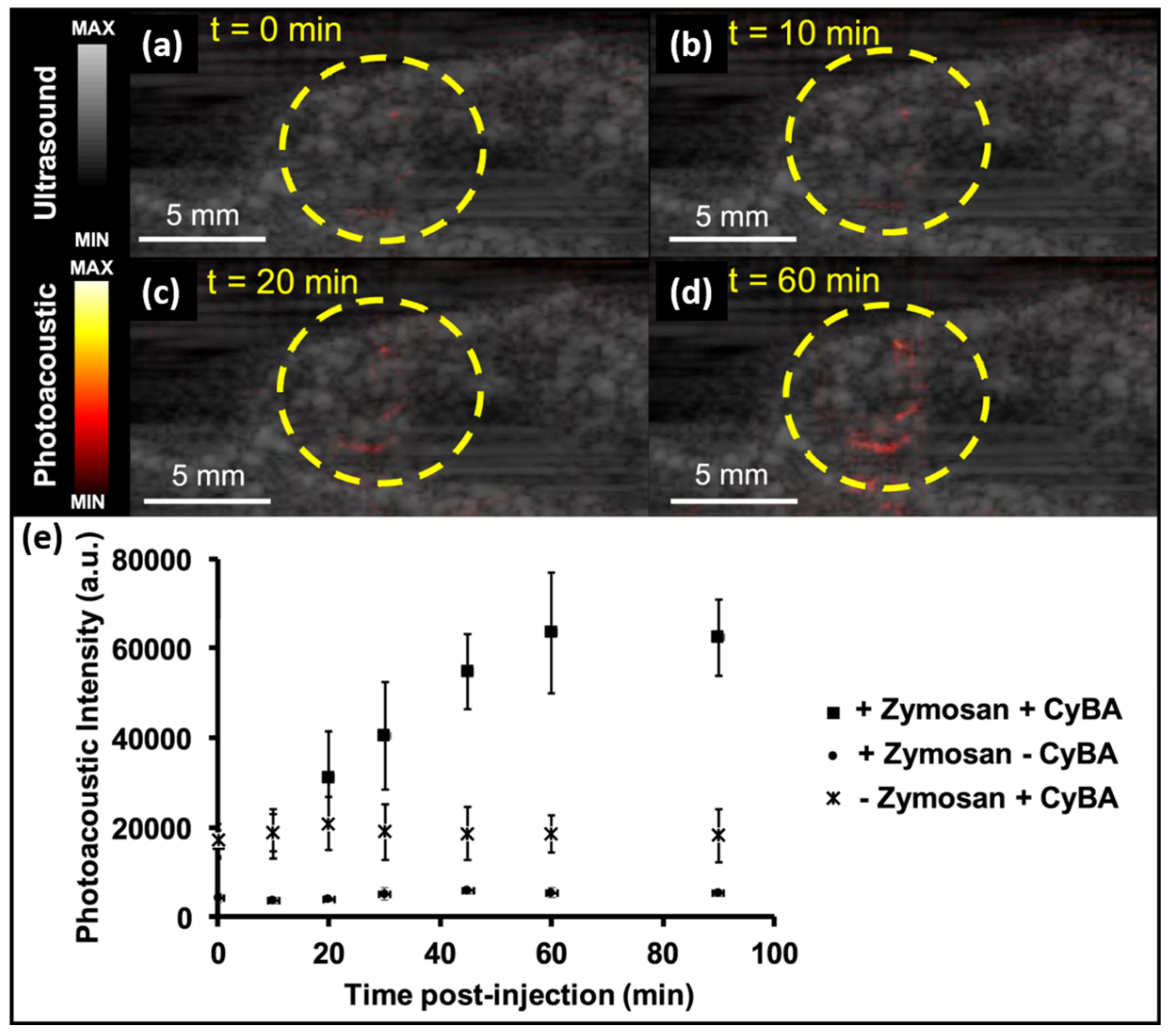
| Energy (mJ) | PRR (Hz) | Pulse Width (ns) | Cost * | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|
| Laser | 5 ~120 | ~10 | <10 | $70–200 K | Powerful, ~5 cm penetration depth, tunable wavelength | Bulky size, eye protection and laser safe rooms needed |
| LD | 0.5–2.5 | ~1 K−6 K | 30–200 | ~$10–25 K | Integration in a handheld probe feasible, high PRR | Limited penetration depth, eye protection and laser safe rooms needed, wavelength tuning not possible |
| LED | 0.2 | ~200–16 K | 30–100 | $10–15 K | Integration in a handheld probe feasible, high PRR, no need for laser-safe rooms or eye-safety goggles | Limited penetration depth, wavelength tuning not possible |
| Year | Authors | Pulse Width (ns) | Peak Current (A) | Pulse Energy (mJ) | Repetition Rate (Hz) | Wavelength (nm) |
|---|---|---|---|---|---|---|
| 2011 | Hansen et al. [46] | 60 | 40 | 0.0004 | 200 | 627 |
| 2013 | Allen et al. [43] | 500 | 200 | 0.0022 | 200 | 623 |
| 2016 | Allen et al. [33] | 200 | 50 | 0.0009 | 500 | 623 |
| 2016–2017 | Agano et al. [34,35,52] | 70 * | 15–20 | 0.15–0.2 ** | 4000 | 850 |
| 2018 | Zhu et al. [6] | 70 * | 20 | 0.2 ** | 4000–16,000 | 850 |
| Target | Application | Depth (mm) | Contrast Agent | Wavelength (nm) | |
|---|---|---|---|---|---|
| Medical needles, Vasculature | Guidance of minimally invasive procedures with peripheral tissue targets [32] | Phantom and ex vivo studies | 38 | N/A | 850 |
| Vasculature | Imaging of human placental vasculature [64] | 7 | N/A | 850 | |
| Tumor | Imaging of intraocular tumors [6] | 10 | N/A | 850 | |
| Vasculature | Non-invasive monitoring of angiogenesis [7] | Animal in vivo | 10 | N/A | 850 |
| Ulcer | Noninvasive imaging of pressure ulcers [76] | 10 | N/A | 690 | |
| Oxygen saturation | Oxygen saturation imaging in Rheumatoid arthritis [77] | 5 | N/A | 750/850 | |
| Molecular | Detection and monitoring of reactive oxygen and nitrogen species [29] | 10 | CyBA | 850 | |
| Tumor/Contrast agents | Imaging of tumor using contrast enhancement [79] | 10 | NC | 850 | |
| Cells/ Contrast agents | Imaging of molecular-labelled cells [27] | 10 | DiR | 850 | |
| Vasculature | Imaging of peripheral microvasculature and function [6] | Healthy human in vivo | 10 | N/A | 690/850 |
| Vasculature | Simultaneous imaging of veins and lymphatic vessels [86] | 10 | ICG | 940/820 | |
| Finger joints | Full view tomography of finger joints [87] | 5 | N/A | 850 | |
| Finger joints | Imaging of inflammatory arthritis [31] | Patient in vivo | 5 | N/A | 850 |
| Skin | Imaging of port wine stain [101] | 10 | N/A | 850 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Feng, T.; Cheng, Q.; Wang, X.; Du, S.; Sato, N.; Yuan, J.; Kuniyil Ajith Singh, M. Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors 2020, 20, 2484. https://doi.org/10.3390/s20092484
Zhu Y, Feng T, Cheng Q, Wang X, Du S, Sato N, Yuan J, Kuniyil Ajith Singh M. Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors. 2020; 20(9):2484. https://doi.org/10.3390/s20092484
Chicago/Turabian StyleZhu, Yunhao, Ting Feng, Qian Cheng, Xueding Wang, Sidan Du, Naoto Sato, Jie Yuan, and Mithun Kuniyil Ajith Singh. 2020. "Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review" Sensors 20, no. 9: 2484. https://doi.org/10.3390/s20092484
APA StyleZhu, Y., Feng, T., Cheng, Q., Wang, X., Du, S., Sato, N., Yuan, J., & Kuniyil Ajith Singh, M. (2020). Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors, 20(9), 2484. https://doi.org/10.3390/s20092484








