Development of an Aptamer Based Luminescent Optical Fiber Sensor for the Continuous Monitoring of Hg2+ in Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents for the Fabrication of the Sensor
2.2. Taper Fabrication
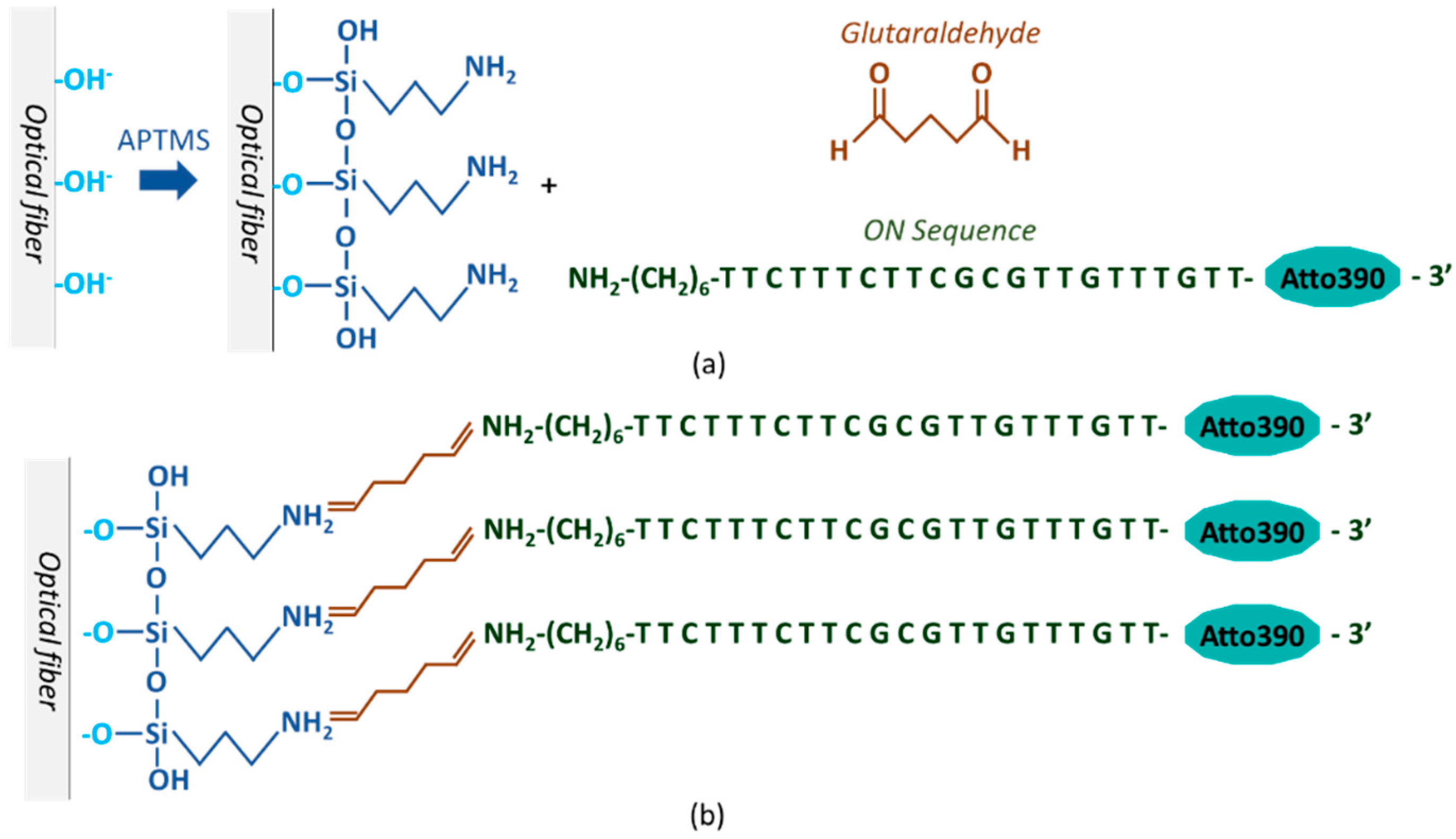
2.3. Immobilization of the ON Sequence onto the Optical Fiber
2.4. Preparation of the Samples for Hg2+ Analysis
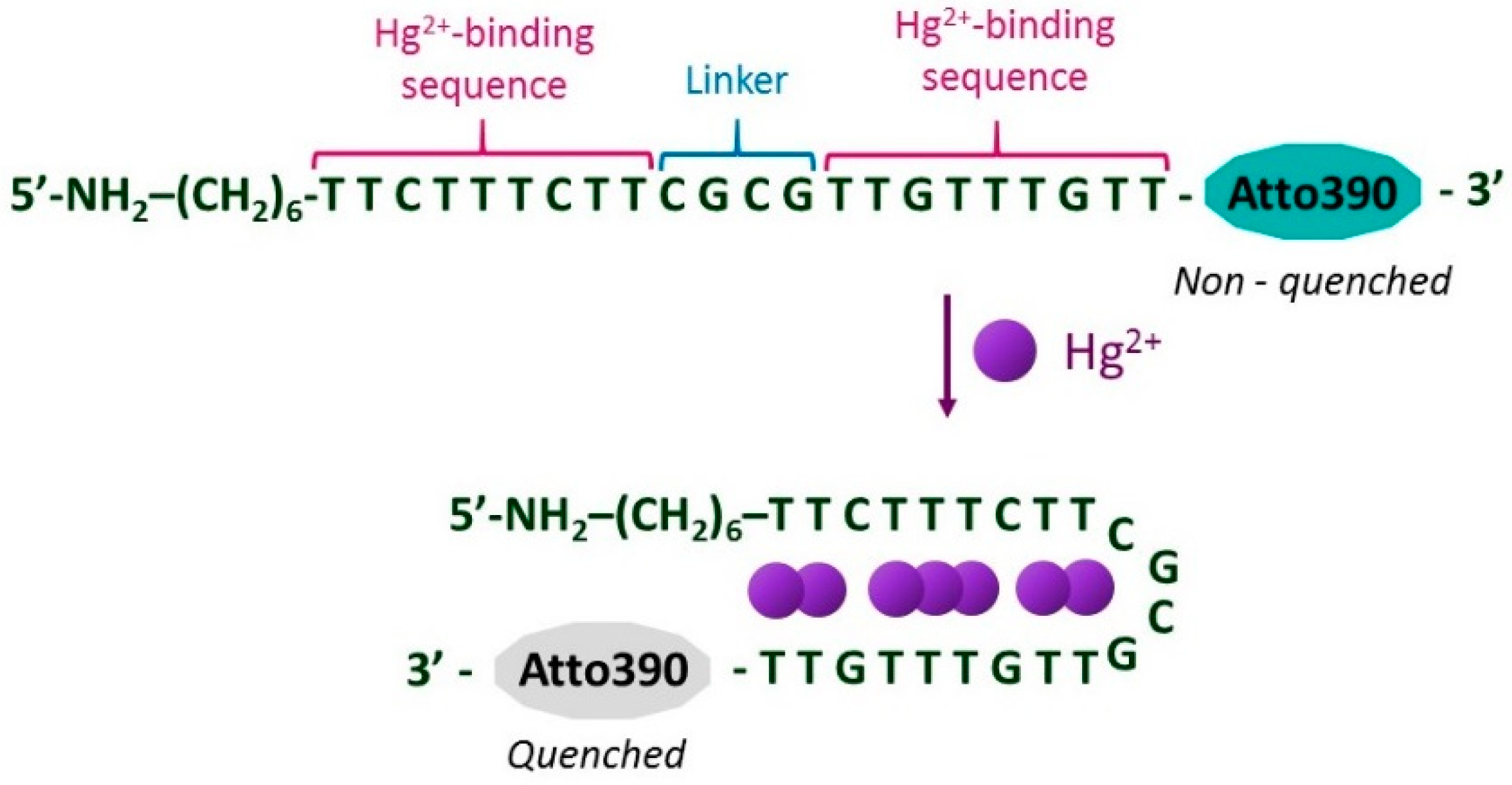
2.5. Sensing Mechanism
2.6. Characterization of the Sensors
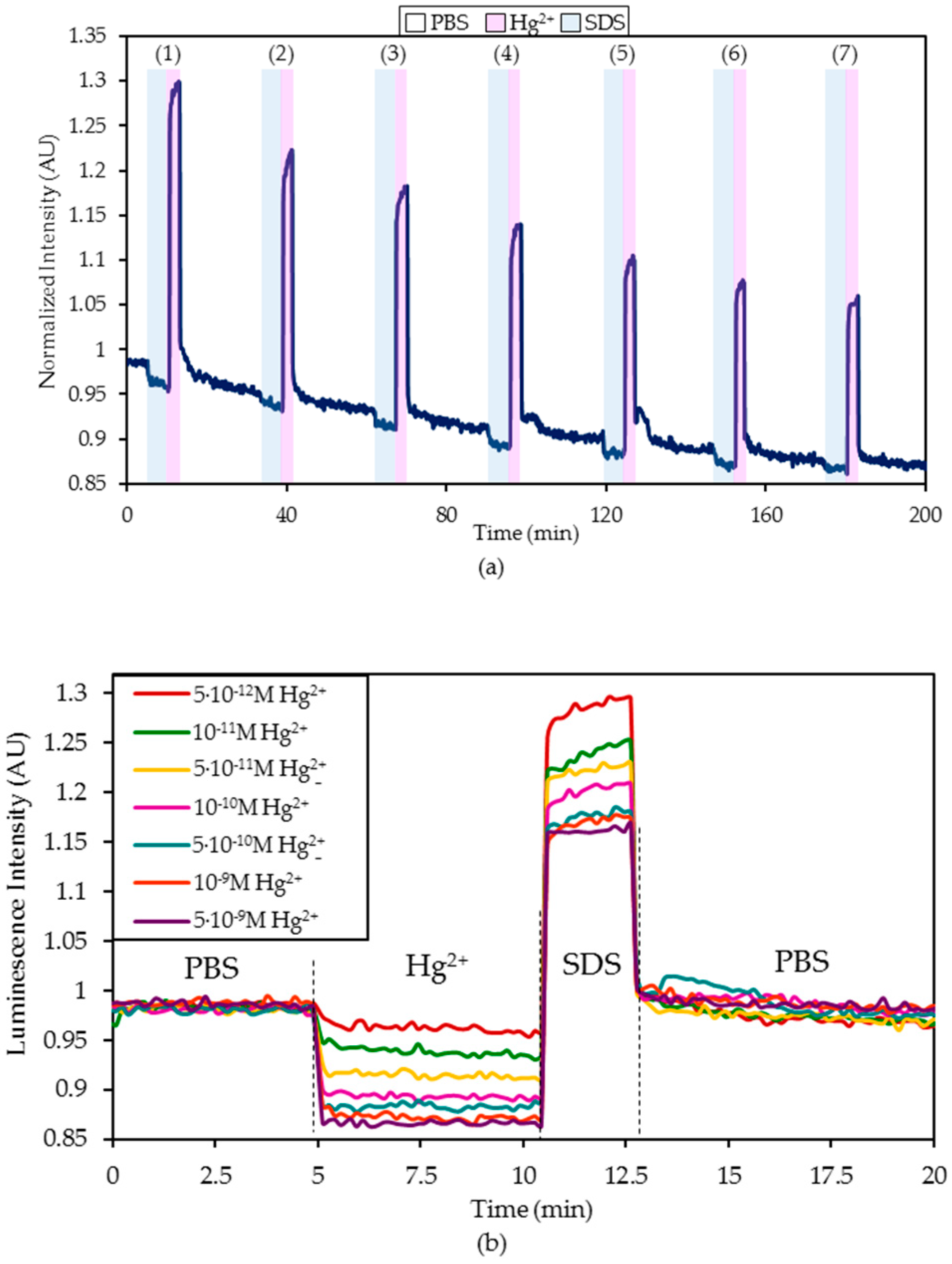
- First, the sensor was exposed to the continuous illumination of the LED for at least 90 minutes to estimate the mathematical characterization of the photobleaching suffered by the fluorophore labelled to the ON sequence [41]. During this test, the sensor was in a PBS (pH 7.4) solution.
- Hereafter, it was exposed for five minutes to a PBS (pH 7.4) solution with a certain Hg2+ concentration and then regenerated by dipping it in a 0.5% (w/w) SDS solution.
- Afterward, the sensor was introduced again into a PBS (pH 7.4) solution for 20 minutes until the fluorescence became stabilized.
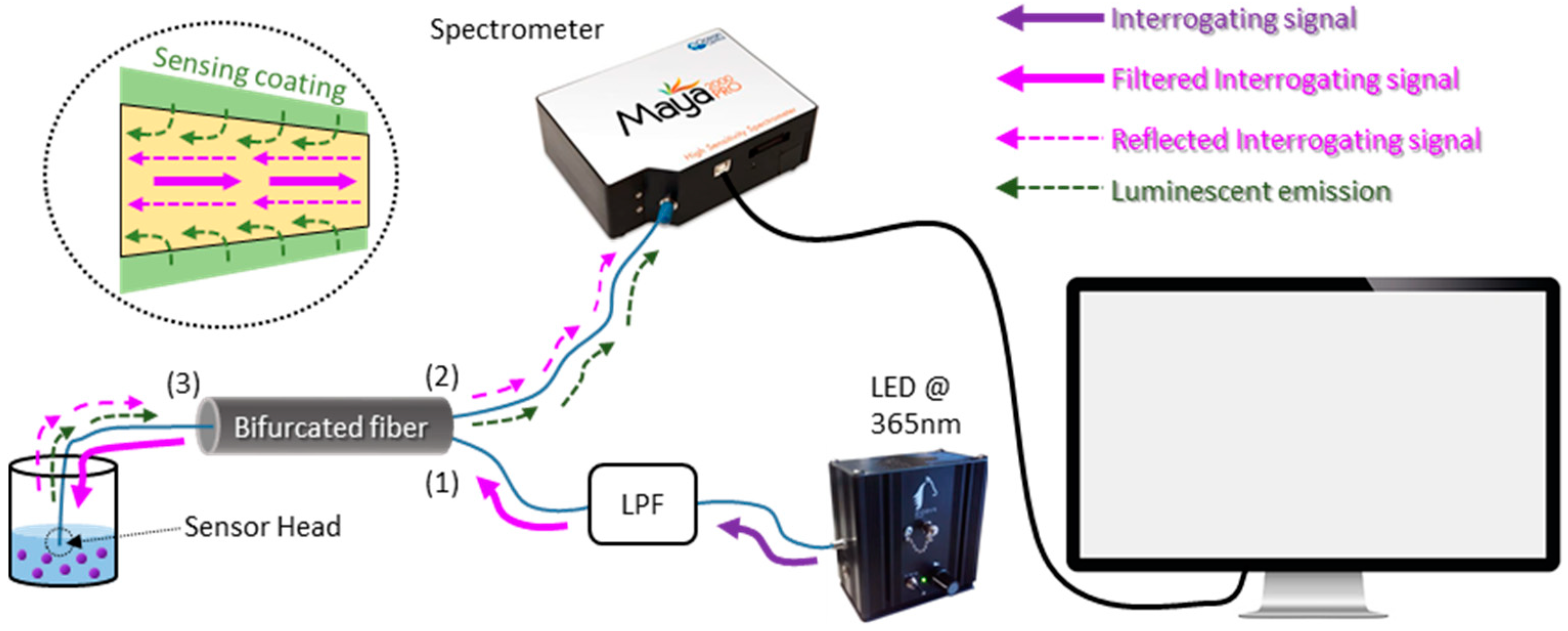
3. Experimental Set-Up
4. Results and Discussion
4.1. Detection of Hg2+ Ions in Phosphate Buffered Solution (PBS, pH 7.4) Solutions
4.2. Detection of Hg2+ Ions in Ultrapure Water
4.3. Detection of Hg2+ Ions in Tap Water
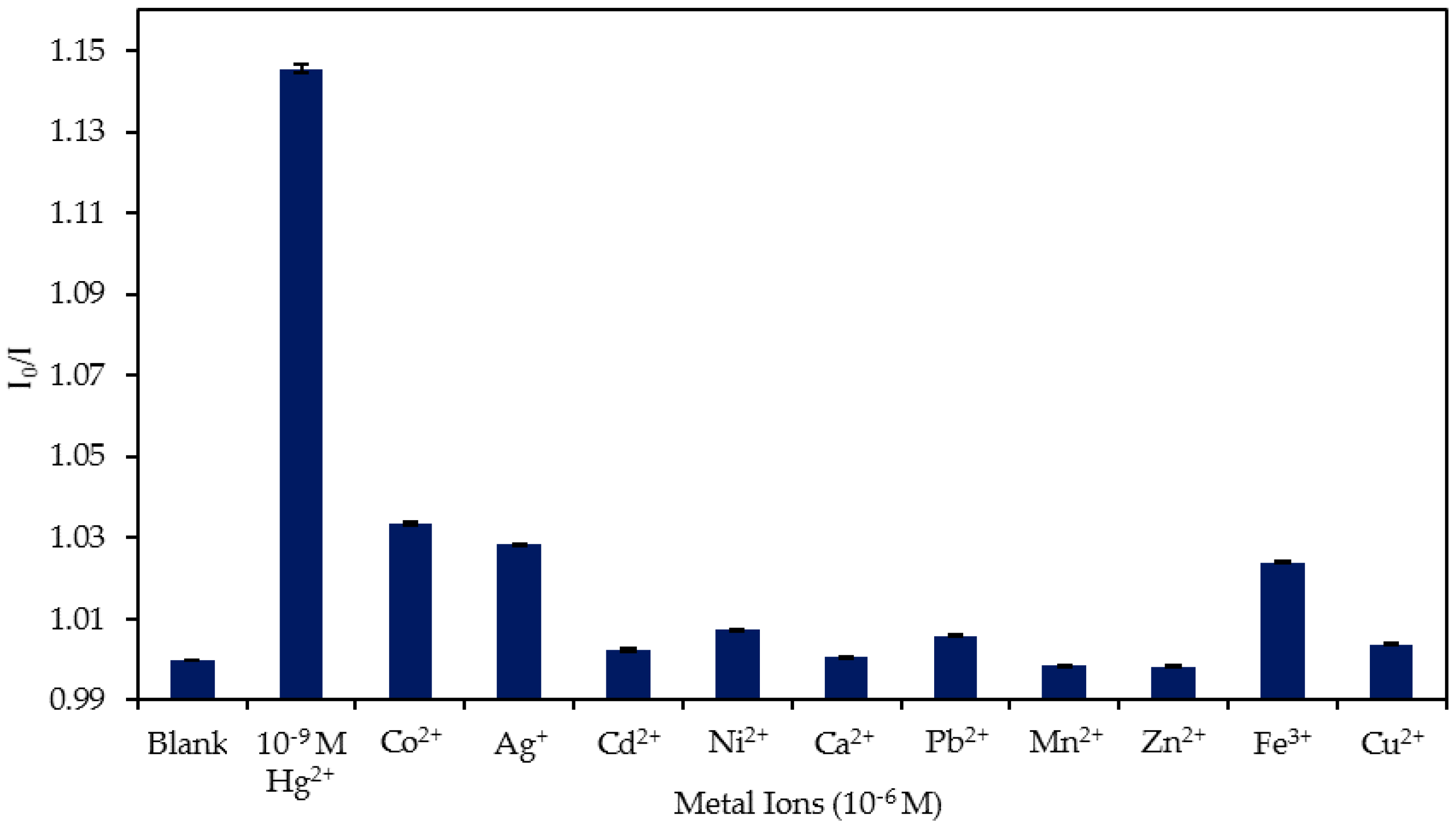
4.4. Study of the Cross-Sensitivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Drinking-Water. World Health Organization Fact Sheets. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/drinking-water (accessed on 22 October 2019).
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Blanchoud, H.; Moreau-Guigon, E.; Farrugia, F.; Chevreuil, M.; Mouchel, J.M. Contribution by urban and agricultural pesticide uses to water contamination at the scale of the Marne watershed. Sci. Total Environ. 2007, 375, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Dirilgen, N. Mercury and lead: Assessing the toxic effects on growth and metal accumulation by Lemna minor. Ecotoxicol. Environ. Saf. 2011, 74, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2014; Volume 229, pp. 1–18. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- C. of the E. U. European Parliament. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/15/EC as Regards Priority Substances in the Field of Water Policy Text with EEA relevance. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:348:0084:0097:EN:PDF (accessed on 20 February 2020).
- Liu, C.; Chen, X.; Zong, B.; Mao, S. Recent advances in sensitive and rapid mercury determination with graphene-based sensors. J. Mater. Chem. A 2019, 7, 6616–6630. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef]
- Pokhrel, L.R. Novel carbon nanotube (CNT)-based ultrasensitive sensors for trace mercury (II) detection in water: A review. Sci. Total Environ. 2017, 574, 1379–1388. [Google Scholar] [CrossRef]
- National Research Council. Chapter 6: Chemical Sensors. In Expanding the Vision of Sensor Materials; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Nat. 2013, 5, 34–43. [Google Scholar] [CrossRef]
- Miyake, Y. MercuryII-mediated formation of thymine-HgII-thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.C.G.K.; Kumar, A.; Sivasakthi, K.; Thakur, C.S. Handheld, low-cost electronic device for rapid, real-time fluorescence-based detection of Hg2+, using aptamer-templated ZnO quantum dots. Sens. Actuators B Chem. 2019, 290, 73–78. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Ding, W.; Guo, S.; Wu, N. Fluorescent aptamer-functionalized graphene oxide biosensor for label-free detection of mercury(II). Biosens. Bioelectron. 2013, 41, 889–893. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Corres, J.M.; Arregui, F.J. Fluorescent sensors for the detection of heavy metal ions in aqueous media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef]
- Guo, L.; Yin, N.; Chen, G. Photoinduced electron transfer mediated by π-stacked thymine-Hg 2+-thymine base pairs. J. Phys. Chem. C 2011, 115, 4837–4842. [Google Scholar] [CrossRef]
- Freeman, R.; Finder, T.; Willner, I. Multiplexed analysis of Hg2+ and Ag+ ions by nucleic acid functionalized CdSe/ZnS quantum dots and their use for logic gate operations. Angew. Chemie-Int. Ed. 2009, 48, 7818–7821. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Yan, J.; Zhu, C.; Zhang, C.; Chen, A. Dulplex analysis of mercury and silver ions using a label-free fluorescent aptasensor. Int. J. Environ. Anal. Chem. 2018, 98, 349–359. [Google Scholar] [CrossRef]
- Liu, C.-W.; Huang, C.-C.; Chang, H.-T. Highly selective DNA-based sensor for lead (II) and mercury (II) ions. Anal. Chem. 2009, 81, 2383–2387. [Google Scholar] [CrossRef]
- López-Higuera, J.M.; Cobo, L.R.; Incera, A.Q.; Cobo, A. Fiber optic sensors in structural health monitoring. J. Light. Technol. 2011, 29, 587–608. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.G. Potentiometric performance of flexible pH sensor based on polyaniline nanofiber arrays. Nano Converg. 2019, 6, 9. [Google Scholar] [CrossRef]
- Zhang, Z. High-Sensitivity Gas-Pressure Sensor Based on Fiber-Tip PVC Diaphragm Fabry-Pérot Interferometer. J. Light. Technol. 2017, 35, 4067–4071. [Google Scholar] [CrossRef]
- Yang, J.; Che, X.; Shen, R.; Wang, C.; Li, X.; Chen, W. High-sensitivity photonic crystal fiber long-period grating methane sensor with cryptophane-A-6Me absorbed on a PAA-CNTs/PAH nanofilm. Opt. Express 2017, 25, 20258–20267. [Google Scholar] [CrossRef]
- Zubiate, P. Fiber-based early diagnosis of venous thromboembolic disease by label-free D-dimer detection. Biosens. Bioelectron. X 2019, 2, 100026. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Layer-by-layer nano-assembly: A powerful tool for optical fiber sensing applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- De Acha, N.; Elosúa, C.; Matías, I.R.; Arregui, F.J. Enhancement of luminescence-based optical fiber oxygen sensors by tuning the distance between fluorophore layers. Sens. Actuators B Chem. 2017, 248, 836–847. [Google Scholar] [CrossRef]
- Jorge, P.A.S.; Caldas, P.; Rosa, C.C.; Oliva, A.G.; Santos, J.L. Optical fiber probes for fluorescence based oxygen sensing. Sens. Actuators B Chem. 2004, 103, 290–299. [Google Scholar] [CrossRef]
- Li, Z.; Muhandiramlage, T.P.; Keogh, J.P.; Hall, H.K.; Aspinwall, C.A. Aptamer-functionalized porous phospholipid nanoshells for direct measurement of Hg2+ in urine. Anal. Bioanal. Chem. 2015, 407, 953–960. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Wang, H. Optofluidics-based DNA structure-competitive aptasensor for rapid on-site detection of lead(II) in an aquatic environment. Anal. Chim. Acta 2014, 849, 43–49. [Google Scholar] [CrossRef]
- Anderson, G.P.; Golden, J.P.; Ligler, F.S. A fiber optic biosensor: Combination tapered fibers designed for improved signal acquisition. Biosens. Bioelectron. 1993, 8, 249–256. [Google Scholar] [CrossRef]
- Golden, J.P.; Anderson, G.P.; Rabbany, S.Y.; Ligler, F.S. An Evanescent Wave Biosensor—Part II: Fluorescent Signal Acquisition from Tapered Fiber Optic Probes. IEEE Trans. Biomed. Eng. 1994, 41, 585–591. [Google Scholar] [CrossRef]
- Long, F. Reusable evanescent wave DNA biosensor for rapid, highly sensitive, and selective detection of mercury ions. Biosens. Bioelectron. 2011, 26, 4018–4023. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Taira, K.-I.; Sode, K.; Ikebukuro, K. Improvement of aptamer affinity by dimerization. Sensors 2008, 8, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew. Chemie Int. Ed. 2004, 43, 4300–4302. [Google Scholar] [CrossRef]
- Ono, A. Development of novel oligonucleotide-based sensors which are highly Hg (II) selective and are insensitive to other heavy metal ions. Nucleic Acids Symp. Ser. (Oxf). 2004, 48, 29–30. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, F.S.; Zhu, B.; Zhu, L. Fluorescence Determination of Merucury (II) Using a Thymine Aptamer. Anal. Lett. 2015, 48, 2208–2216. [Google Scholar] [CrossRef]
- Mattos, A.B.; Freitas, T.A.; Silva, V.L.; Dutra, R.F. A dual quartz crystal microbalance for human cardiac troponin T in real time detection. Sens. Actuators B Chem. 2012, 161, 439–446. [Google Scholar] [CrossRef]
- Elosua, C.; de Acha, N.; Hernaez, M.; Matias, I.R.; Arregui, F.J. Layer-by-Layer assembly of a water-insoluble platinum complex for optical fiber oxygen sensors. Sens. Actuators B Chem. 2015, 207, 683–689. [Google Scholar] [CrossRef]
- Li, J. A ‘turn-off’ fluorescent biosensor for the detection of mercury (II) based on graphite carbon nitride. Talanta 2017, 162, 46–51. [Google Scholar] [CrossRef]
- Sung, T.-W.; Lo, Y.-L. Dual sensing of temperature and oxygen using PtTFPP-doped CdSe/SiO2 core-shell nanoparticles. Sens. Actuators B Chem. 2012, 173, 406–413. [Google Scholar] [CrossRef]
- Wenzel, M.J.; Mensah-Brown, A.; Josse, F.; Yaz, E.E. Online drift compensation for chemical sensors using estimation theory. IEEE Sens. J. 2011, 11, 225–232. [Google Scholar] [CrossRef]
- Bhuyan, A.K. On the mechanism of SDS-induced protein denaturation. Biopolymers 2010, 93, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Liu, C.-W.; Chang, H.-T. Fluorescence detection of mercury (II) and lead (II) ions using aptamer/reporter conjugates. Talanta 2011, 84, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantification of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Huang, P.-J.J.; Kempaiah, R.; Liu, J. Synergistic pH effect for reversible shuttling aptamer-based biosensors between graphene oxide and target molecules. J. Mater. Chem. 2011, 21, 8991–8993. [Google Scholar] [CrossRef]
- Hianik, T.; Ostatná, V.; Sonlajtnerova, M.; Grman, I. Influence of ionic strength, pH and aptamer configuration for binding affinity to thrombin. Bioelectrochemistry 2007, 70, 127–133. [Google Scholar] [CrossRef]
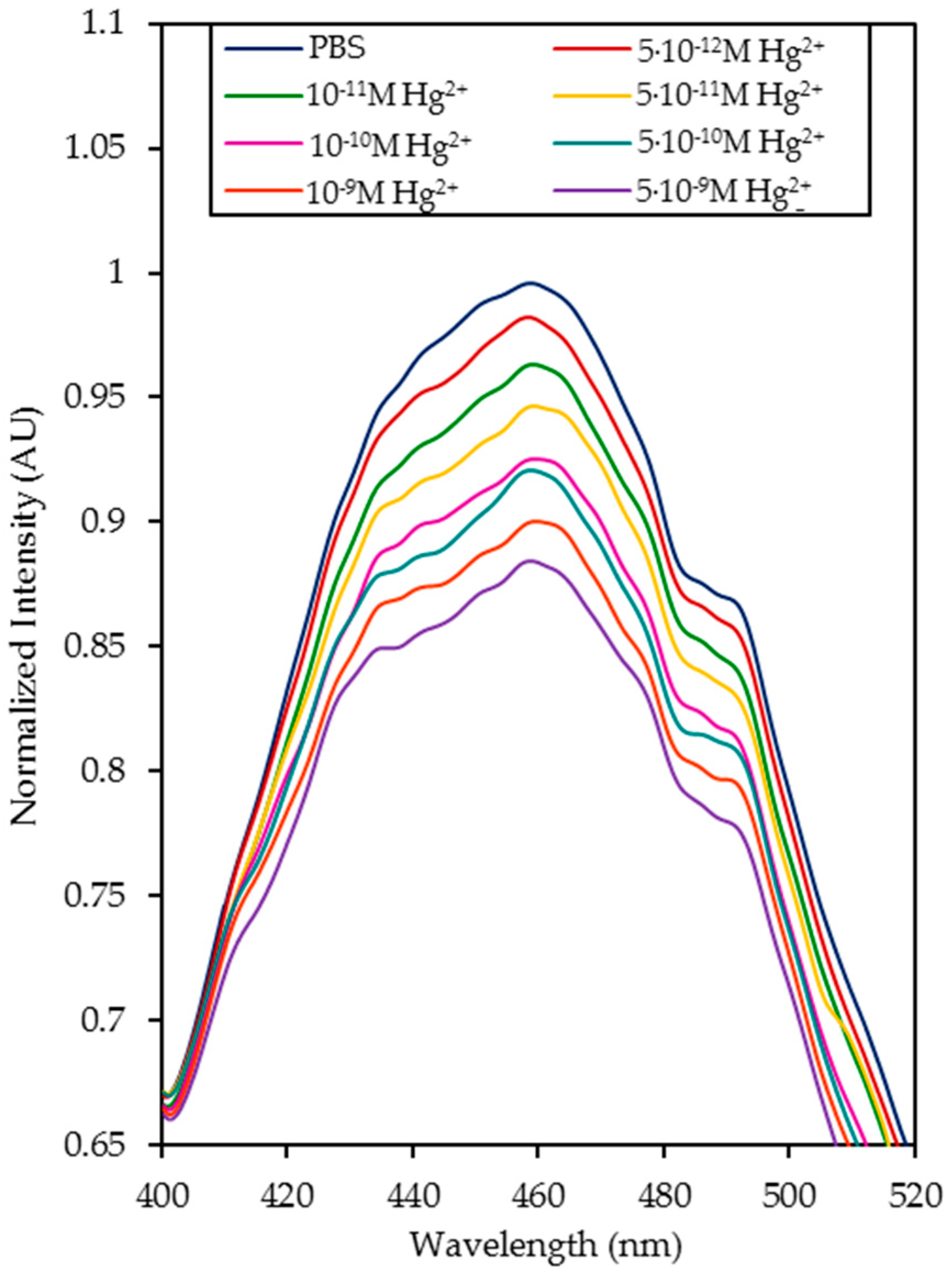
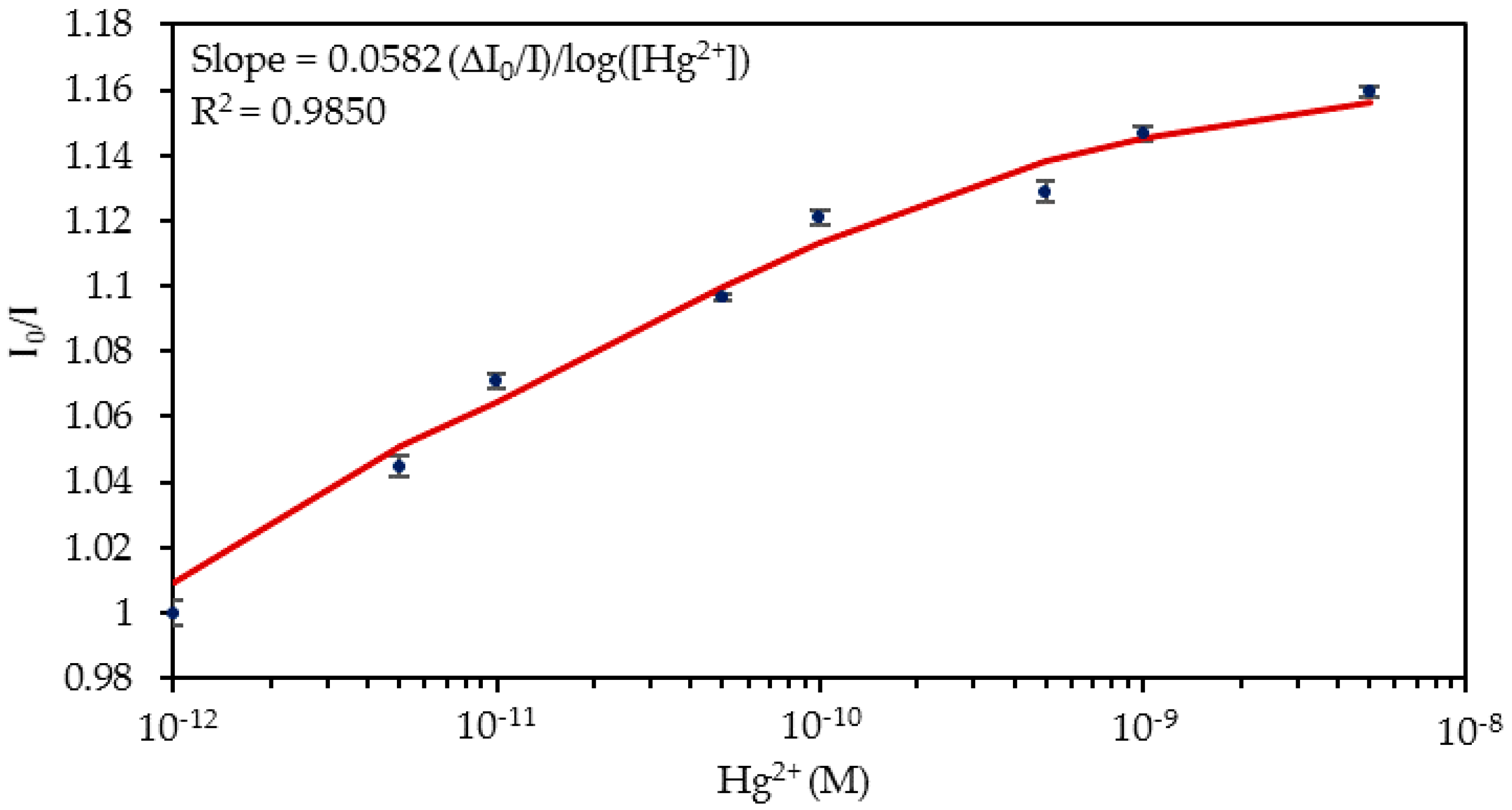
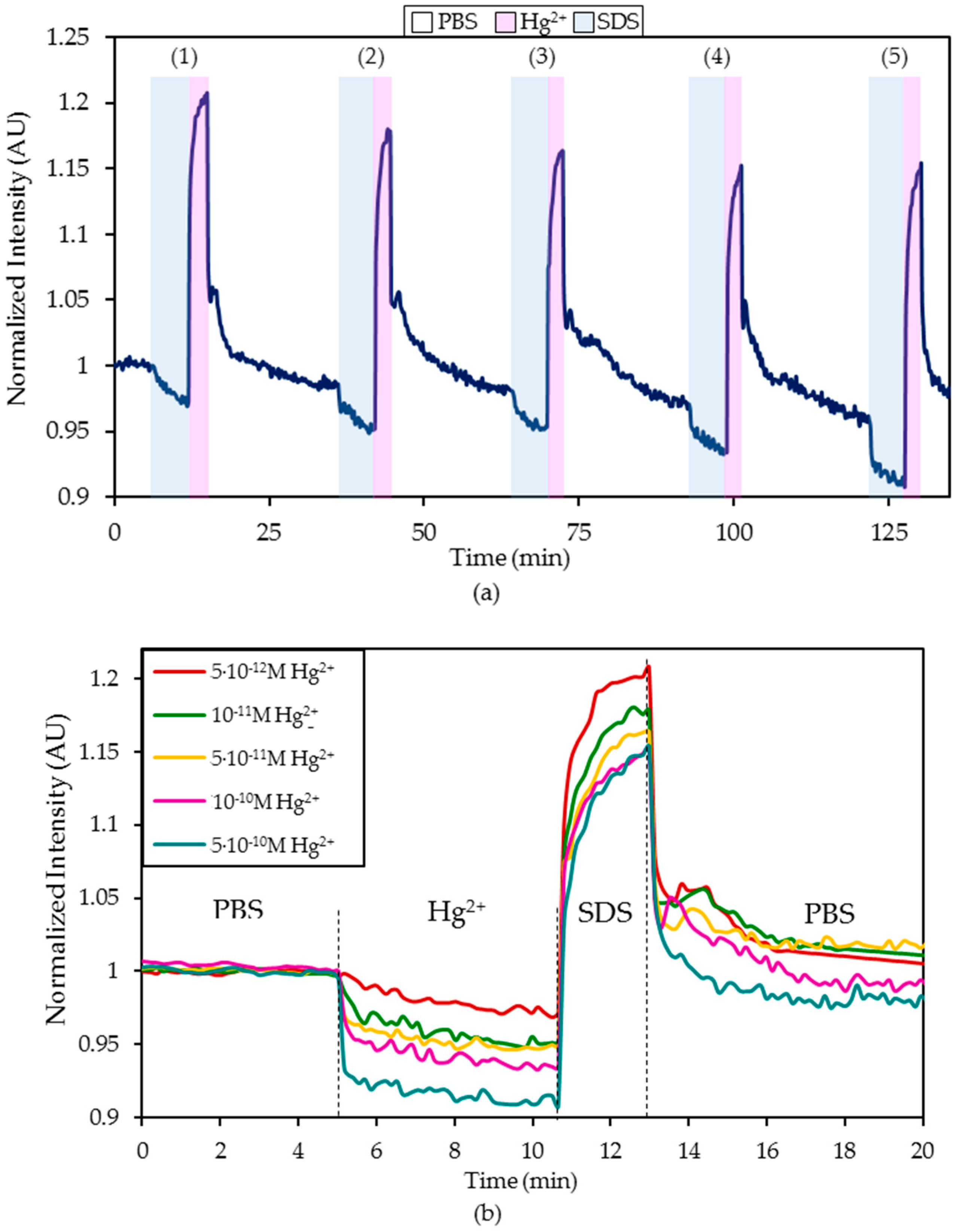
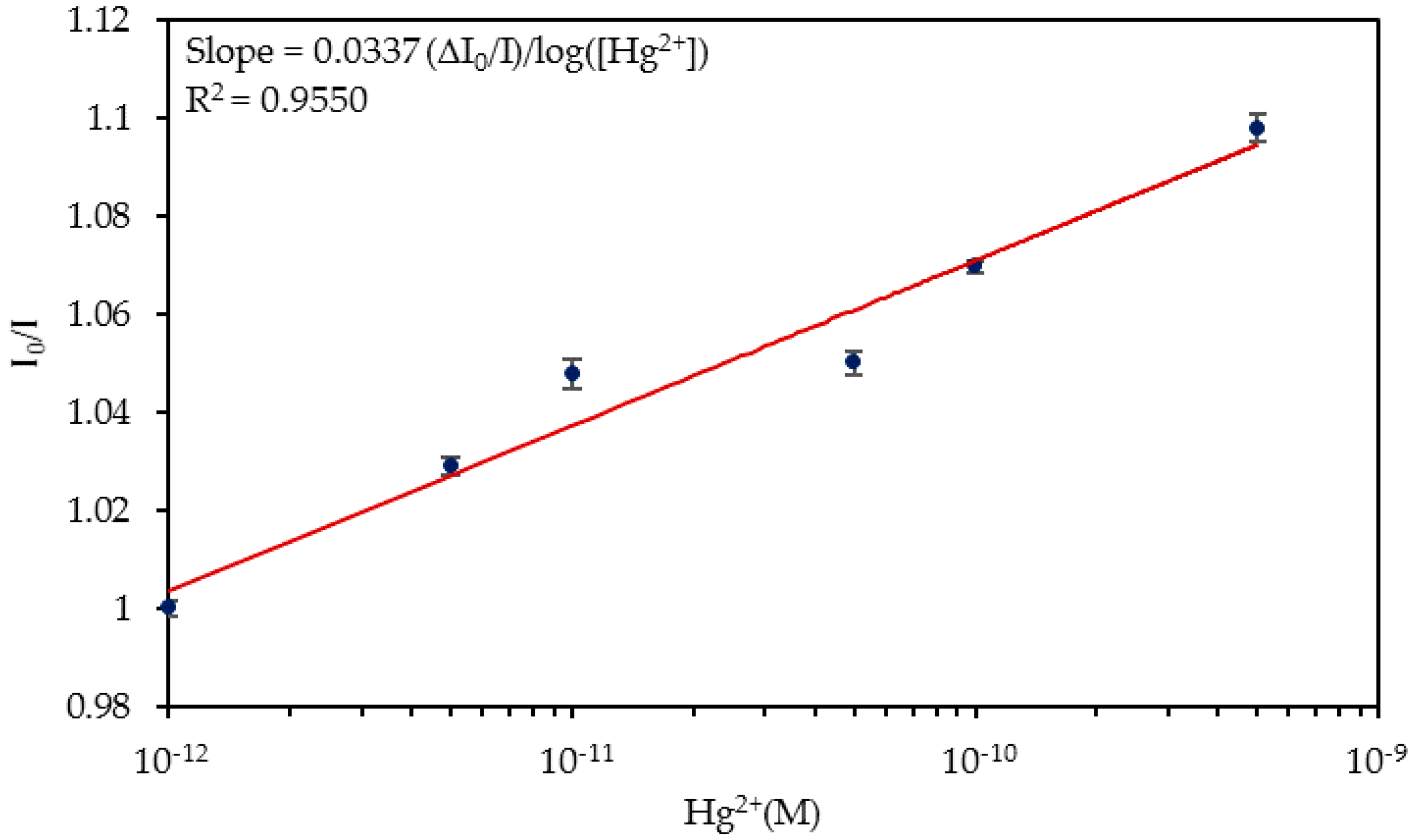
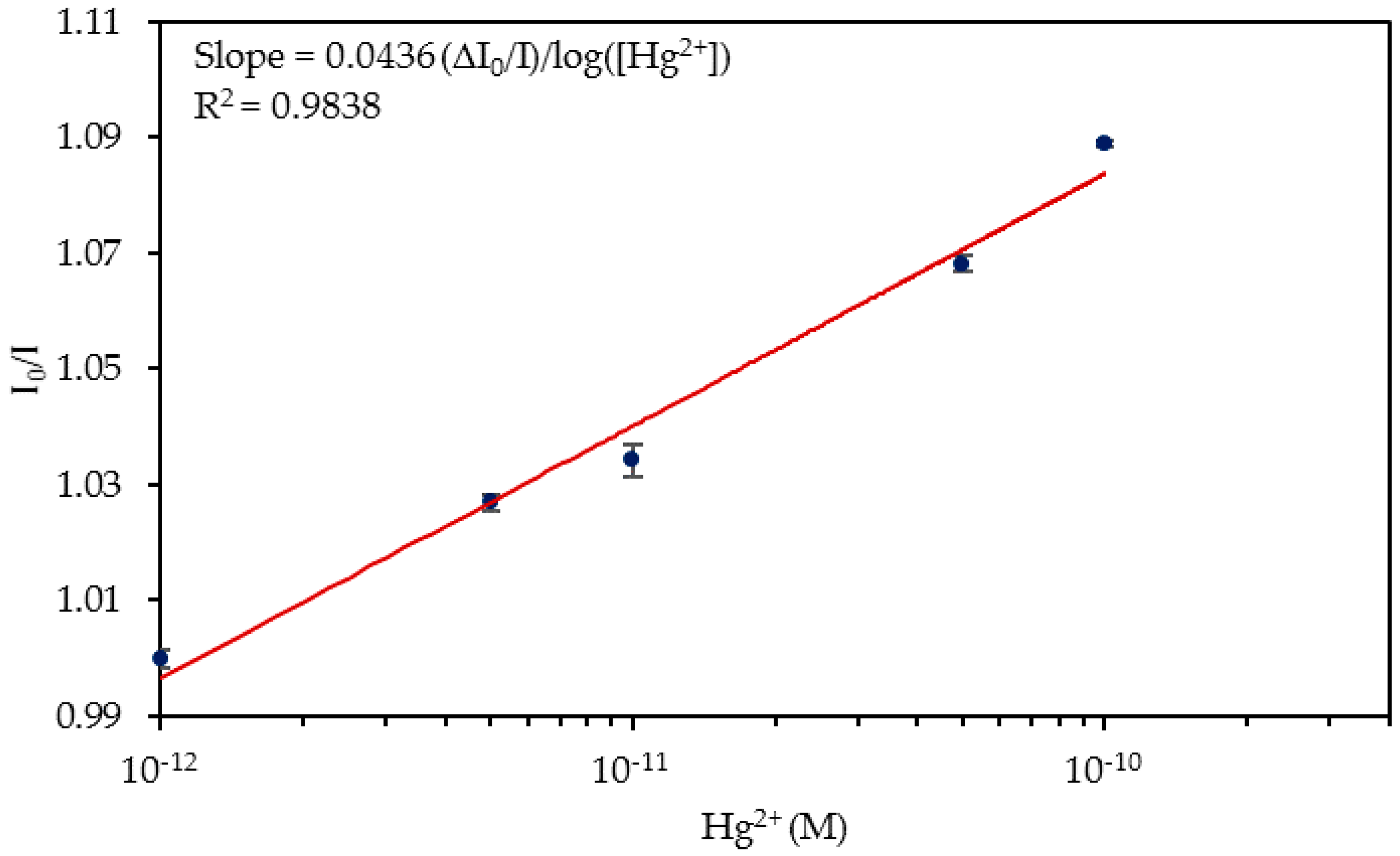
| Media | Sensitivity Δ(I0/I)/log([Hg2+]) | R2 |
|---|---|---|
| PBS solutions | 0.0582 | 0.9850 |
| Ultrapure water | 0.0337 | 0.9550 |
| Tap Water | 0.0436 | 0.9838 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Acha, N.; Elosúa, C.; Arregui, F.J. Development of an Aptamer Based Luminescent Optical Fiber Sensor for the Continuous Monitoring of Hg2+ in Aqueous Media. Sensors 2020, 20, 2372. https://doi.org/10.3390/s20082372
De Acha N, Elosúa C, Arregui FJ. Development of an Aptamer Based Luminescent Optical Fiber Sensor for the Continuous Monitoring of Hg2+ in Aqueous Media. Sensors. 2020; 20(8):2372. https://doi.org/10.3390/s20082372
Chicago/Turabian StyleDe Acha, Nerea, César Elosúa, and Francisco J. Arregui. 2020. "Development of an Aptamer Based Luminescent Optical Fiber Sensor for the Continuous Monitoring of Hg2+ in Aqueous Media" Sensors 20, no. 8: 2372. https://doi.org/10.3390/s20082372
APA StyleDe Acha, N., Elosúa, C., & Arregui, F. J. (2020). Development of an Aptamer Based Luminescent Optical Fiber Sensor for the Continuous Monitoring of Hg2+ in Aqueous Media. Sensors, 20(8), 2372. https://doi.org/10.3390/s20082372






