Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review
Abstract
1. Introduction
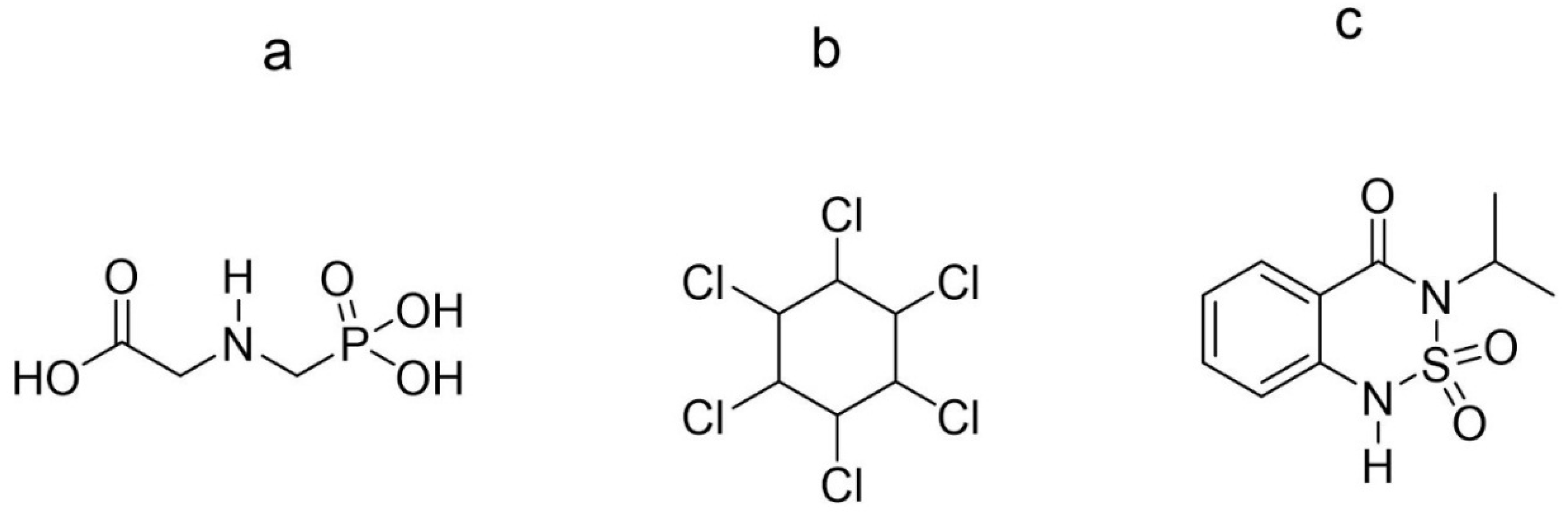
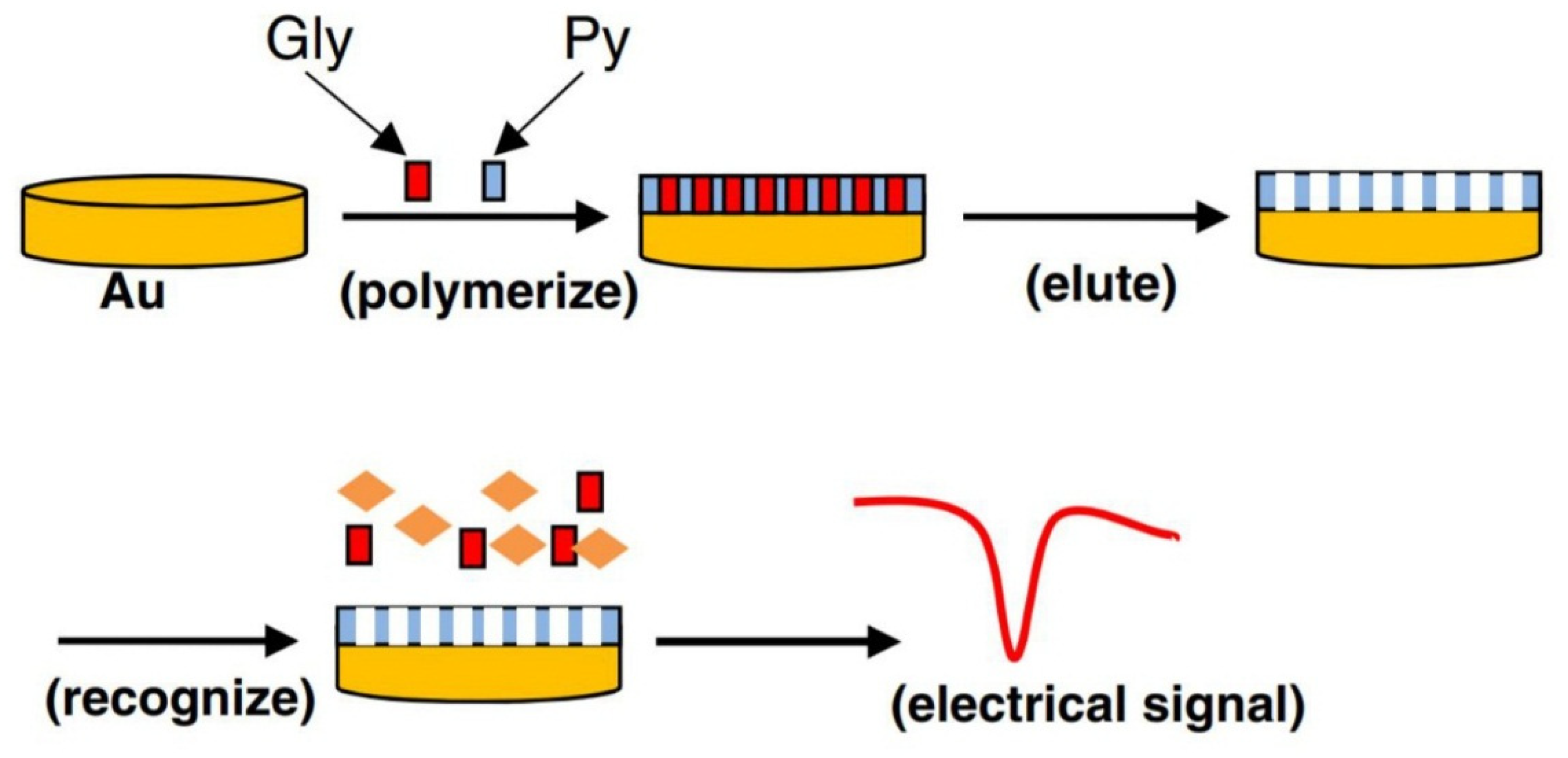
2. Electrochemical Detection of Glyphosate
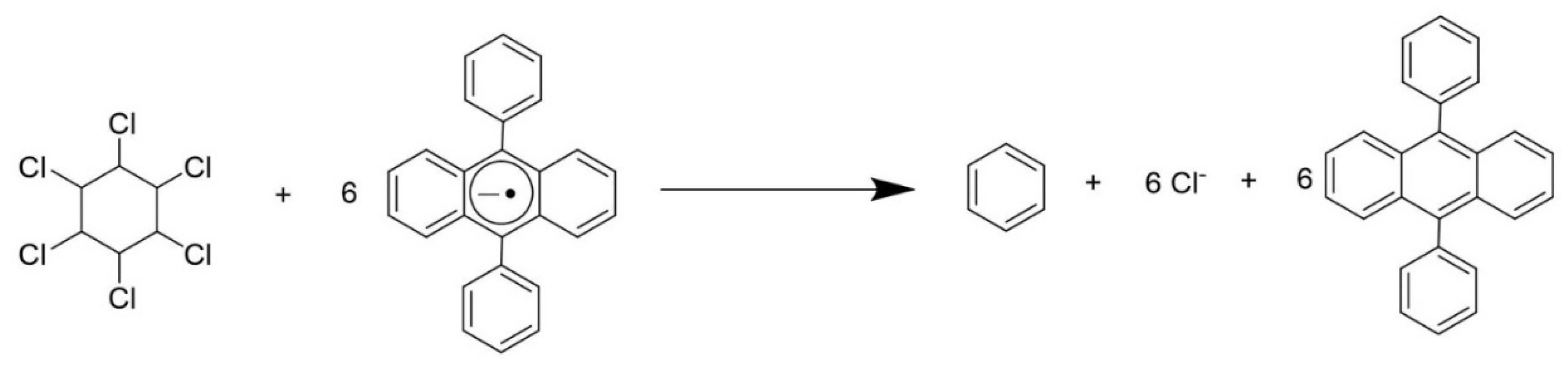
3. Electrochemical Detection of Lindane
4. Electrochemical Detection of Bentazone
5. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du Plessis, A. Freshwater Challenges of South Africa and its Upper Vaal River; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kostovetskiĭ, I.I.; Tolstopiatova, G.V.; Chegrinets, G. la Contamination of surface waters by agricultural pesticides. Gig. Sanit. 1973, 38, 99–100. [Google Scholar] [PubMed]
- Ermakova, E.Y.; Korotkov, Y.F.; Kuznetsov, M.G.; Nikolaev, N.A. Cleaning contaminated water by gravity flotation. Chem. Pet. Eng. 2010, 46, 40–44. [Google Scholar] [CrossRef]
- Qian, H.; Pretzer, L.A.; Velazquez, J.C.; Zhao, Z.; Wong, M.S. Gold nanoparticles for cleaning contaminated water. J. Chem. Technol. Biotechnol. 2013, 88, 735–741. [Google Scholar] [CrossRef]
- Dietrich, A.M.; Thomas, A.; Zhao, Y.; Smiley, E.; Shanaiah, N.; Ahart, M.; Charbonnet, K.A.; Deyonker, N.J.; Alexander, W.A.; Gallagher, D.L. Partitioning, aqueous solubility, and dipole moment data for cis- and trans-(4-methylcyclohexyl)methanol, principal contaminants of the West Virginia chemical spill. Environ. Sci. Technol. Lett. 2015, 2, 123–127. [Google Scholar] [CrossRef]
- Jiang, X.; Li, D.; Xu, X.; Ying, Y.; Li, Y.; Ye, Z.; Wang, J. Immunosensors for detection of pesticide residues. Biosens. Bioelectron. 2008, 23, 1577–1587. [Google Scholar] [CrossRef]
- Kumar, N.; Pathera, A.K.; Kumar, M. The effects of pesticides on human health. Ann. Agri-Bio Res. 2012, 17, 125–127. [Google Scholar]
- Hiltbold, A.E. Persistence of Pesticides in Soil, 2nd ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1974. [Google Scholar]
- Edwards, C.A. Factors that affect the persistence of pesticides in plants and soils. Pure Appl. Chem. 1975, 42, 39–56. [Google Scholar] [CrossRef]
- Cogger, C.G.; Stark, J.D.; Bristow, P.R.; Getzin, L.W.; Montgomery, M. Transport and Persistence of Pesticides in Alluvial Soils: II. Carbofuran. J. Environ. Qual. 1998, 27, 551. [Google Scholar] [CrossRef]
- Hara, J.; Kawabe, Y. Long-term Persistence of Cyclodiene Pesticide in Soil. In Proceedings of the AIP Conference Proceedings; AIP: Sendai, Japan, 2007; pp. 32–35. [Google Scholar]
- Treatment Facility Cleaning Up Contaminated Ground Water. Groundw. Monit. Remediat. 1987, 7, 10. [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S. Recent Advances in Environmental Analysis. Anal. Chem. 2011, 83, 4579–4613. [Google Scholar] [CrossRef]
- Wang, J. Real-time electrochemical monitoring: Toward green analytical chemistry. Acc. Chem. Res. 2002, 35, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Q. Remote electrochemical biosensor for field monitoring of phenolic compounds. Anal. Chim. Acta 1995, 312, 39–44. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780471678793. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2001; ISBN 9780471043720. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Alatraktchi, F.A.; Johansen, H.K.; Molin, S.; Svendsen, W.E. Electrochemical sensing of biomarker for diagnostics of bacteria-specific infections. Nanomedicine 2016, 11, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Albisser, A.M.; Leibel, B.S.; Ewart, T.G.; Davidovac, Z.; Botz, C.K.; Zingg, W.; Schipper, H.; Gander, R. Clinical Control of Diabetes by the Artificial Pancreas. Diabetes 1974, 23, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Yamasaki, Y.; Kawamori, R.; Hakui, N.; Abe, H. Wearable artificial endocrine pancreas with Needle-type glucose sensor. Lancet 1982, 320, 1129–1131. [Google Scholar] [CrossRef]
- Landolfi, M.; Landi Degl’Innocenti, E. Net circular polarization in magnetic spectral lines produced by velocity gradients: Some analytical results. Sol. Phys. 1996, 164, 191–202. [Google Scholar] [CrossRef]
- Kim, H.J.; Hummel, J.W.; Birrell, S.J. Evaluation of Nitrate and Potassium Ion-Selective Membranes for Soil Macronutrient Sensing. Trans. ASABE 2006, 49, 597–606. [Google Scholar] [CrossRef]
- Sassolas, A.; Prieto-Simón, B.; Marty, J.-L. Biosensors for Pesticide Detection: New Trends. Am. J. Anal. Chem. 2012, 03, 210–232. [Google Scholar] [CrossRef]
- Mostafa, G.A.E. Electrochemical Biosensors for the Detection of Pesticides. Open Electrochem. J. 2010, 2, 22–42. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Chen, D.; Xu, Y.; Zhang, L. A micro-machined thin film electro-acoustic biosensor for detection of pesticide residuals. J. Zhejiang Univ. Sci. C 2014, 15, 383–389. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Alexander, S. Design and fabrication of molecularly imprinted polymer-based potentiometric sensor from the surface modified multiwalled carbon nanotube for the determination of lindane (γ-hexachlorocyclohexane), an organochlorine pesticide. Biosens. Bioelectron. 2015, 64, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Florea, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Tran-Thi, N.T.; Jaffrezic-Renault, N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Chaurasia, A.K.; Sawant, S.N.; Apte, S.K. Polyaniline-based highly sensitive microbial biosensor for selective detection of lindane. Anal. Chem. 2012, 84, 6672–6678. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.L.L.; Benimeli, C.; Madrid, R.E.; Giacomelli, C.E. A simple Streptomyces spore-based impedimetric biosensor to detect lindane pesticide. Sens. Actuators B Chem. 2015, 207, 447–454. [Google Scholar] [CrossRef]
- Rahemi, V.; Garrido, J.M.P.J.; Borges, F.; Brett, C.M.A.; Garrido, E.M.P.J. Electrochemical determination of the herbicide bentazone using a carbon nanotube β-cyclodextrin modified electrode. Electroanalysis 2013, 25, 2360–2366. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Sun, S.; Wei, C.; Xu, Z.J. A novel non-enzymatic lindane sensor based on CuO-MnO2 hierarchical nano-microstructures for enhanced sensitivity. Chem. Commun. 2015, 51, 4376–4379. [Google Scholar] [CrossRef]
- UTZ certified. List of Banned Pesticides and Pesticides Watchlist; Standard and Certification Department: Amasterdam, The Netherland, 2015. [Google Scholar]
- Registration Eligebility Decision(RED) Lemonene. United States Environmental Protection Agency, Office of prevention, pestices and toxic substances. EPA 738-R-94-034, September 1994. [Google Scholar]
- Van Bruggen, A.; He, M.M.; Shin, K.; Mai, V. Science of the Total Environment Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 617, 255–268. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Heal. 2016, 1–13. [Google Scholar] [CrossRef]
- Noori, J.S.; Dimaki, M.; Mortensen, J.; Svendsen, W.E. Detection of glyphosate in drinking water: A fast and direct detection method without sample pretreatment. Sensors 2018, 18, 2961. [Google Scholar] [CrossRef]
- Zhang, C.; She, Y.; Li, T.; Zhao, F.; Jin, M.; Guo, Y.; Zheng, L.; Wang, S.; Jin, F.; Shao, H.; et al. A highly selective electrochemical sensor based on molecularly imprinted polypyrrole-modified gold electrode for the determination of glyphosate in cucumber and tap water. Anal. Bioanal. Chem. 2017, 409, 7133–7144. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V.; Desseille, K.L. An amperometric method for the detection of amitrole, glyphosate and its aminomethyl-phosphonic acid metabolite in environmental waters using passive samplers. Anal. Chim. Acta 2010, 675, 125–131. [Google Scholar] [CrossRef]
- Songa, E.A.; Arotiba, O.A.; Owino, J.H.O.; Jahed, N.; Baker, P.G.L.; Iwuoha, E.I. Electrochemical detection of glyphosate herbicide using horseradish peroxidase immobilized on sulfonated polymer matrix. Bioelectrochemistry 2009, 75, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mazouz, Z.; Touchente, Z.A.; Laradi, H.; Fourati, N.; Yaakoubi, N.; Touzani, R.; Chehimi, M.M.; Kalfat, R.; Othmane, A.; Zerrouki, C. Design of Novel Electrochemical Sensors for the Selective Detection of Glyphosate. Proceedings 2017, 1, 483. [Google Scholar] [CrossRef]
- Mazouz, Z.; Rahali, S.; Fourati, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly selective polypyrrole MIP-based gravimetric and electrochemical sensors for picomolar detection of glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef]
- Songa, E.A.; Waryo, T.; Jahed, N.; Baker, P.G.L.; Kgarebe, B.V.; Iwuoha, E.I. Electrochemical nanobiosensor for glyphosate herbicide and Its metabolite. Electroanalysis 2009, 21, 671–674. [Google Scholar] [CrossRef]
- Songa, E.A.; Somerset, V.S.; Waryo, T.; Baker, P.G.L.; Iwuoha, E.I. Amperometric nanobiosensor for quantitative determination of glyphosate and glufosinate residues in corn samples. Pure Appl. Chem. 2009, 81, 123–139. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An electrochemical sensor on the hierarchically porous Cu-BTC MOF platform for glyphosate determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Poorahong, S.; Thammakhet, C.; Thavarungkul, P.; Kanatharana, P. One-step preparation of porous copper nanowires electrode for highly sensitive and stable amperometric detection of glyphosate. Chem. Pap. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Coutinho, C.F.B.; Coutinho, L.F.M.; Mazo, L.H.; Lanças, F.M. Copper microelectrode as liquid chromatography detector for herbicide glyphosate. Electroanalysis 2007, 19, 1223–1226. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct electrochemical determination of glyphosate at copper phthalocyanine/multiwalled carbon nanotube film electrodes. Electroanalysis 2010, 22, 1586–1591. [Google Scholar] [CrossRef]
- Oliveira, P.C.; Maximiano, E.M.; Oliveira, P.A.; Camargo, J.S.; Fiorucci, A.R.; Arruda, G.J. Direct electrochemical detection of glyphosate at carbon paste electrode and its determination in samples of milk, orange juice, and agricultural formulation. J. Environ. Sci. Heal.—Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 817–823. [Google Scholar] [CrossRef] [PubMed]
- del Aguirre, M.C.; Urreta, S.E.; Gomez, C.G. A Cu 2+ -Cu/glassy carbon system for glyphosate determination. Sens. Actuators B Chem. 2019, 675–683. [Google Scholar] [CrossRef]
- Cahuantzi-Muñoz, S.L.; González-Fuentes, M.A.; Ortiz-Frade, L.A.; Torres, E.; Ţălu, Ş.; Trejo, G.; Méndez-Albores, A. Electrochemical Biosensor for Sensitive Quantification of Glyphosate in Maize Kernels. Electroanalysis 2019, 31, 927–935. [Google Scholar] [CrossRef]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. Doubly imprinted polymer nanofilm-modified electrochemical sensor for ultra-trace simultaneous analysis of glyphosate and glufosinate. Biosens. Bioelectron. 2014, 59, 81–88. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Akbari, A.; Norouzi, L. Development of a novel hollow fiber- pencil graphite modified electrochemical sensor for the ultra-trace analysis of glyphosate. Sens. Actuators B Chem. 2018, 272, 415–424. [Google Scholar] [CrossRef]
- Pintado, S.; Montoya, M.R.; Rodríguez-Amaro, R.; Mayén, M.; Mellado, J.M.R. Electrochemical determination of glyphosate in waters using electrogenerated copper ions. Int. J. Electrochem. Sci. 2012, 7, 2523–2530. [Google Scholar]
- Brønstad, J.O.; Friestad, H.O. Method for determination of glyphosate residues in natural waters based on polarography of the n-nitroso derivative. Analyst 1976, 101, 820–824. [Google Scholar] [CrossRef]
- Teófilo, R.F.; Reis, E.L.; Reis, C.; da-Silva, G.A.; Paiva, J.F.; Kubota, L.T. Glyphosate Determination in Soil, Water and Vegetables Using DPV Optimized by Response Surface Methodology. Port. Electrochim. Acta 2007, 26, 325–337. [Google Scholar] [CrossRef]
- Teófilo, R.F.; Reis, E.L.; Reis, C.; Silva, G.A.; Kubota, L.T. Glyphosate Determination. Optimization 2004, 15, 865–871. [Google Scholar]
- Méndez, M.A.; Súarez, M.F.; Cortés, M.T.; Sarria, V.M. Electrochemical properties and electro-aggregation of silver carbonate sol on polycrystalline platinum electrode and its electrocatalytic activity towards glyphosate oxidation. Electrochem. Commun. 2007, 9, 2585–2590. [Google Scholar] [CrossRef]
- Khenifi, A.; Derriche, Z.; Forano, C.; Prevot, V.; Mousty, C.; Scavetta, E.; Ballarin, B.; Guadagnini, L.; Tonelli, D. Glyphosate and glufosinate detection at electrogenerated NiAl-LDH thin films. Anal. Chim. Acta 2009, 654, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bettazzi, F.; Natale, A.R.; Torres, E.; Palchetti, I. Glyphosate determination by coupling an immuno-magnetic assay with electrochemical sensors. Sensors 2018, 18, 2965. [Google Scholar] [CrossRef] [PubMed]
- Sok, V.; Fragoso, A. Amperometric biosensor for glyphosate based on the inhibition of tyrosinase conjugated to carbon nano-onions in a chitosan matrix on a screen-printed electrode. Microchim. Acta 2019, 186, 569. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Jin, J.Y.; Takeuchi, T.; Miwa, T.; Suenami, K.; Takekoshi, Y.; Kanno, S. Integrated pulsed amperometric detection of glufosinate, bialaphos and glyphosate at gold electrodes in anion-exchange chromatography. J. Chromatogr. A 2001, 919, 313–320. [Google Scholar] [CrossRef]
- Oliveira, G.C.; Moccelini, S.K.; Castilho, M.; Terezo, A.J.; Possavatz, J.; Magalhães, M.R.L.; Dores, E.F.G.C. Biosensor based on atemoya peroxidase immobilised on modified nanoclay for glyphosate biomonitoring. Talanta 2012, 98, 130–136. [Google Scholar] [CrossRef]
- Kumaravel, A.; Vincent, S.; Chandrasekaran, M. Development of an electroanalytical sensor for γ-hexachlorocyclohexane based on a cellulose acetate modified glassy carbon electrode. Anal. Methods 2013, 5, 931–938. [Google Scholar] [CrossRef]
- Bhattacharjee, D. Toxicity of organochlorine pesticide, Lindane to fish: A review. J. Chem. Pharm. Res. 2013, 5, 90–96. [Google Scholar]
- Beck, B. Lindane toxicity in beef-cattle. Can. Vet. J.-Revue Vet. Can. 1989, 30, 833. [Google Scholar]
- Peverly, A.A.; Karty, J.A.; Peters, D.G. Electrochemical reduction of (1R,2r,3S,4R,5r,6S)-hexachlorocyclohexane (Lindane) at silver cathodes in organic and aqueous-organic media. J. Electroanal. Chem. 2013, 692, 66–71. [Google Scholar] [CrossRef]
- Wang, P.; Ge, L.; Li, M.; Li, W.; Li, L.; Wang, Y.; Yu, J. Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer-Coated TiO2 Nanotubes for Lindane Specific Recognition and Detection. J. Inorg. Organomet. Polym. Mater. 2013, 23, 703–711. [Google Scholar] [CrossRef]
- Peters, D.G.; McGuire, C.M.; Pasciak, E.M.; Peverly, A.A.; Strawsine, L.M.; Wagoner, E.R.; Tyler Barnes, J. Electrochemical dehalogenation of organic pollutants. J. Mex. Chem. Soc. 2014, 58, 287–302. [Google Scholar] [CrossRef]
- Birkin, P.R.; Evans, A.; Milhano, C.; Montenegro, M.I.; Pletcher, D. The mediated reduction of lindane in DMF. Electroanalysis 2004, 16, 583–587. [Google Scholar] [CrossRef]
- Jordan, P.; Gamoke, B.C.; Foley, M.P.; Raghavachari, K.; Peters, D.G. Electrochemical reduction of (1R,2r,3S,4R,5r,6S)-hexachlorocyclohexane (Lindane) at carbon cathodes in dimethylformamide. J. Electroanal. Chem. 2011, 660, 120–126. [Google Scholar]
- Matsunaga, A.; Yasuhara, A. Dechlorination of polychlorinated organic compounds by electrochemical reduction with naphthalene radical anion as mediator. Chemosphere 2005, 59, 1487–1496. [Google Scholar] [CrossRef]
- Martins, P.C.; Medeiros, M.J.; Montenegro, M.I. Electrochemical behaviour of hexachlorocyclohexane. Port. Electrochim. Acta 1999, 17, 319–323. [Google Scholar] [CrossRef]
- Belaud, F.A.; Farwell, S.O.; Robocker, A.E.; Geer, R.D. Electrochemical Reduction and Anaerobic Degradation of Lindane. J. Agric. Food Chem. 1976, 24, 753–756. [Google Scholar]
- Prathap, M.U.A.; Sun, S.; Xu, Z.J. An electrochemical sensor highly selective for lindane determination: A comparative study using three different α-MnO2 nanostructures. RSC Adv. 2016, 6, 22973–22979. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. A Sensor for the Determination of Lindane Using PANI/Zn, Fe(III) Oxides and Nylon 6,6/MWCNT/Zn, Fe(III) Oxides Nanofibers Modified Glassy Carbon Electrode. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Prathap, M.U.; Srivastava, R. Electrochemical reduction of lindane (γ-HCH) at NiCo2O4 modified electrode. Electrochim. Acta 2013, 108, 145–152. [Google Scholar] [CrossRef]
- Paramo-Garcia, U.; Gutierrez-Grandos, S.; Garcia-Jimenez, M.G.; Ibanez, J.G. Catalytic behavior of cobalt(I) salen during the electrochemical reduction of lindane and hexachlorobenzene. J. New Mater. Electrochem. Syst. 2010, 13, 356–360. [Google Scholar]
- Wang, B.; Ding, G.; Zhu, J.; Zhang, W.; Guo, M.; Geng, Q.; Guo, D.; Cao, Y. Development of novel ionic liquids based on bentazone. Tetrahedron 2015, 71, 7860–7864. [Google Scholar] [CrossRef]
- Mir, N.A.; Haque, M.M.; Khan, A.; Muneer, M.; Vijayalakshmi, S. Photocatalytic degradation of herbicide Bentazone in aqueous suspension of TiO2: Mineralization, identification of intermediates and reaction pathways. Environ. Technol. (United Kingd.) 2014, 35, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.T. Farm Chemicals Handbook; Meister Publishing Company: Willoughby, OH, USA, 1989; Volume 98, ISBN 0430-0750. [Google Scholar]
- Brooks, G.T. The agrochemicals handbook. Agric. Ecosyst. Environ. 1989, 26, 147–148. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; De Carlo, R.M.; Rivoira, L.; Del Bubba, M.; Pavani, M.; Riatti, M.; Onida, B. Adsorption of bentazone herbicide onto mesoporous silica: Application to environmental water purification. Environ. Sci. Pollut. Res. 2016, 23, 5399–5409. [Google Scholar] [CrossRef]
- Hedegaard, M.J.; Deliniere, H.; Prasse, C.; Dechesne, A.; Smets, B.F.; Albrechtsen, H.J. Evidence of co-metabolic bentazone transformation by methanotrophic enrichment from a groundwater-fed rapid sand filter. Water Res. 2018, 129, 105–114. [Google Scholar] [CrossRef]
- Environmental Protection Agency. 2012 Edition of the Drinking Water Standards and Health Advisories; EPA: Washington, DC, USA, 2012. [Google Scholar]
- Manuela Garrido, E.; Costa Lima, J.L.; M Delerue-Matos, C.; Maria Oliveira Brett, A. Electrochemical oxidation of bentazon at a glassy carbon electrode Application to the determination of a commercial herbicide. Talanta 1998, 46, 1131–1135. [Google Scholar] [CrossRef]
- Akinbulu, I.A.; Nyokong, T. Characterization of polymeric film of a new manganese phthalocyanine complex octa-substituted with 2-diethylaminoethanethiol, and its use for the electrochemical detection of bentazon. Electrochim. Acta 2009, 55, 37–45. [Google Scholar] [CrossRef]
- Norouzi, P.; Larijani, B.; Faridbod, F.; Ganjali, M.R. A novel method for ultra trace measurement of bentazon based on nanocomposite electrode and continuous coulometric FFT cyclic voltammetry. Int. J. Environ. Res. 2015, 9, 101–108. [Google Scholar]
- Jevtić, S.; Stefanović, A.; Stanković, D.M.; Pergal, M.V.; Ivanović, A.T.; Jokić, A.; Petković, B.B. Boron-doped diamond electrode—A prestigious unmodified carbon electrode for simple and fast determination of bentazone in river water samples. Diam. Relat. Mater. 2018, 81, 133–137. [Google Scholar] [CrossRef]
- Geto, A.; Noori, J.S.; Mortensen, J.; Svendsen, W.E.; Dimaki, M. Electrochemical determination of bentazone using simple screen-printed carbon electrodes. Environ. Int. 2019, 129, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Yañez, C.; Araya, M.; Bollo, S. Complexation of herbicide bentazon with native and modified β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 237–241. [Google Scholar] [CrossRef]
- Simões, F.R.; Mattoso, L.H.C.; Vaz, C.M.P. Conducting Polymers as Sensor Materials for the Electrochemical Detection of Pesticides. Sens. Lett. 2006, 4, 319–324. [Google Scholar] [CrossRef]
- Cerejeira, R.P.A.G.; Delerue-Matos, C.; Vaz, M.C.V.F. Development of an FIA system with amperometric detection for determination of bentazone in estuarine waters. Anal. Bioanal. Chem. 2002, 373, 295–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohamed, H.M. Screen-printed disposable electrodes: Pharmaceutical applications and recent developments. TrAC—Trends Anal. Chem. 2016, 82, 1–11. [Google Scholar] [CrossRef]
| Electrode | Technique | Medium | pH | Potential | LOD | Linear Range | Matrix | Reference |
|---|---|---|---|---|---|---|---|---|
| Anti-glyphosate-IgG magnetic beads | Amperometry | 0.10 M Citrate/PBS | 5 | −0.1 V vs. Ag/AgCl | 0.03 nM | 0.29 nM–5.90 nM | Beer sample | [60] |
| HRP/PDMA-PSS/Au | Amperometry | PBS | −0.1 V vs. Ag/AgCl | 0.59 nM | 0.01–0.46 µM | Spiked corn sample | [44] | |
| HRP/PDMA-PSS/Au | Amperometry | 0.10 M PBS | 6.1 | −0.28 V vs. Ag/AgCl | 0.95 nM | 0.01–0.47 µM | [43] | |
| SPE/Chi/CNO/TYR | Amperometry | 20.0 mM PBS | 7 | −0.2 V vs. Ag/AgCl | 6.50 nM | 0.02–10.0 µM | Water and soil | [61] |
| HRP/PDMA-PSS/Au | Amperometry | PBS | 6.1 | −0.28 V vs. Ag/AgCl | 10.0 nM | 1.50 nM–0.082 µM | [40] | |
| Porous copper nanowires | Amperometry | 0.10 M PBS in 0.10 M KCl | 6.5 | 10.0 nM | 0.01–5.0 µM | Fresh Fruit, Vegetables | [46] | |
| Au | Amperometry | 0.10 M NaOH | 0.30 µM | 0.59–268 µM | urine, serum | [62] | ||
| NiAl-LDH/Pt | Amperometry | 0.10 M NaOH | 12.8 | 0.49 V vs. SCE | 1.0 µM | 0.01–0.90 mM | [59] | |
| Au | Amperometry | 0.10 M NaOH | 13 | 1.0 mV vs. SHE | 1.89 µM | 5.9 µM–1.06 mM | Extracted river water | [39] |
| Gold SPE | Amperometry | Tap water | 0.78 V | 2.0 µM | 18–300 µM | Ground water | [37] | |
| GCE/MWCNTs-HRP | CV | wide range buffer | 4 | −0.40 V vs. SCE | 1.32 pM | 0.10 nM–11.0 µM | Maize kernels | [51] |
| Cu/CPE, Cu/GCE | CV | 0.10 M PBS | 6.5 | 0–0.59 mM | [54] | |||
| Cu | Coulometry | 0.03 M PBS/Methanol | 6.8 | 0.05 V vs. | 0.59 µM | 0.59–200.0 µM | Tomato juice | [47] |
| MIP/GNPs-PGE | DPASV | ABS | 5.5 | −0.90 V vs. Ag/AgCl | 2.0 nM | 0.024–1.04 µM | Soil and human serum | [52] |
| HMDE | DPP | 1.0 HCl | −0.70 V vs. Ag/AgCl | 0.08 µM | 0.06–10.4 and 23.6–591.5 µM | Water, soil, vegetable | [56] | |
| Dropping Mercury Electrode | DPP | 0.10 M HCl | −0.80 V vs. SCE | 0.20 µM | 0.20–1.24 µM | Tap water | [55] | |
| Cu-BTC MOF/ITO | DPV | 0.10 M PBS | 5.5 | 0.10 V vs. SCE | 0.14 pM | 1.0 pM–10.0 µM | Green vegetable | [45] |
| HF-PGE/CuO/MWCNTs–IL | DPV | 0.10 PBS | 7 | 0.65 V vs. Ag/AgCl | 1.30 nM | 5.0 nM–1.10 µM | Soil and river water sample | [53] |
| MIPPy/Au | DPV | 0.10 M KCl | 0.20 V vs. SCE | 1.60 nM | 0.03–4.73 µM | Cucumber, Tap Water | [38] | |
| GCE/MWCNT/CuPc | DPV | 0.10 M PBS | 7.4 | −0.10 V vs. SCE | 12.20 nM | 0.83–9.90 µM | [48] | |
| Cu2+-Cu/GCE | DPV | ABS | 6 | −0.015 V vs. Ag/AgCl | 0.19 µM | 5.0–60.0 µM | Drinking water | [50] |
| Electro-aggregated silver carbonate modified-Pt | DPV and LSV | 0.1 M Na2CO3 | 40.0 µM | 0–3.80 mM | [58] | |||
| MIP-MOF | LSV | 10.0 mM [Fe(CN)6]3–/4– | 7.2 | −0.05 V vs. SCE | 4.73 nM | 5.91 nM–5.91 µM | Tap water sample | [28] |
| PPY-MIP/Au and PPy-MIP/ZnO | SWV | LiClO4 | 0.50 V vs. SCE | 0.10 pM | 0.10 pM–100 µM | [41] | ||
| PPY-MIP/Au | SWV | 0.01 M LiClO4 | 5 | 0.38 V vs. SCE | 1.0 pM | 0.10 pM–10.0 µM | [42] | |
| HMDE | SWV | 1.25 M HCl | −0.70 V vs. Ag/AgCl | 0.15 nM | 0.30 nM–0.59 µM | [57] | ||
| CPE | SWV | 0.20 M BR buffer | 5 | 0.95 V vs. Ag/AgCl | 2.0 nM | 0.04–2.80 µM | Milk, orange juice, agricultural formulation | [49] |
| Atemoya peroxidase immobilised on modified nanoclay | SWV | 0.10 M PBS | 7 | −0.10 V vs. Ag/AgCl | 0.18 µM | 0.59–26.90 µM | Spiked water | [63] |
| Electrode | Technique | Medium | Potential | LOD | Linear Range | Matrix | Reference |
|---|---|---|---|---|---|---|---|
| PANI-microbial biosensor | Amperometry | 0.40 V | 6.90 nM | 0.02–1.72 µM | [29] | ||
| α-MnO2-NW/GCE | Amperometry/DPV | 0.05 M TBAB solution in 60:40 methanol–water | −1.45 V vs. Ag/AgCl | 114 nM | 1.10–510 µM | Spiked tap water | [75] |
| Vitreous carbon | CV, SWV | 0.1 M of TBAB in ethanol | −2.0 V vs. Ag/AgCl | 50.0 nM | [73] | ||
| CA/GCE | CV, DPV | 0.05 M TBAB 60:40 methanol–water | −1.50 V vs. Ag/AgCl | 37.0 µM | 50.0–1000 µM | Lindane lotion | [64] |
| Silver | CV | ACN, DMF, EtOH, ACN–H2O, DMF–H2O, EtOH–H2O 0.050 M TBABF4 | −0.89 V–−1.65 V vs. SCE | [67] | |||
| CuO–MnO2 | DPV | 0.05 M TBAB solution in 60:40 methanol–water | −1.50 V vs. Ag/AgCl | 4.80 nM | 1.0−700 µM | Tap water | [32] |
| NiCo2O4/GCE | DPV | 0.05 M TBAB solution in 60:40 (v/v) methanol–water | −1.50 V vs. Ag/AgCl | 5.90 µM | 10.0–170 µM | Tap water | [77] |
| Streptomyces strain M7 biosensor | EIS | 0.03 µM | [30] | ||||
| MWCNT-MIP-Cu | Potentiometry | 0.10 nM | 1.0 nM–1.0 mM | water, fruits and vegetables | [27] | ||
| GCE/PANI-ZnO, GCE/PANI-Fe3O4, GCE/Nylon 6,6/MWCNT/ZnO, GCE/Nylon 6,6/MWCNT/Fe3O4 Concentration | SWV | 60:40 methanol/water containing 0.05M TBAB | −0.80 V vs. Ag/AgCl | 32.0 nM | 9.90 pM–5.0 µM | Tap waters | [76] |
| vitreous carbon | SWV | 0.10 M Bu4NBF4 in DMF ((DPA as mediator) | −1.73 V vs. Ag/AgCl | 40.0–1000 µM | [70] | ||
| GCE | 0.10 M TBABF4 in DMF | −1.40 V vs. Ag/AgCl | [71] | ||||
| Hg/Pt | 0.10 M TBAB in DMSO | −1.52 V vs. SCE | Sewage sludge, soil | [74] |
| Electrode | Technique | Medium | pH | Potential | LOD | Linear Range | Matrix | Reference |
|---|---|---|---|---|---|---|---|---|
| GCE | FIA/Amperometry | ABS | 4.5 | 1.10 V vs. Ag/AgCl | 1.0 µM | 2.50–50.0 µM | estuarine water | [93] |
| MWCNT-IL/RGO/SiC/CILE | Continuous Coulometric FFT CV | 0.05 M PBS | 4.5 | 0.70 V | 0.25 nM | 1.0–150 nM | [88] | |
| PANI-β-CD/fMWCNT | CV | PBS | 6 | 0.85 V vs. Ag/AgCl | 1.60 µM | 10.0–80.0 µM | River water | [31] |
| PANI-CPE | CV | 0.05 M PBS | 6.9 | [92] | ||||
| BDD | DPV | B-R | 4 | 1.07 V vs. Ag/AgCl | 0.50 µM | 2.0–100 µM | River water | [89] |
| GCE | DPV | 0.20 M ABS | 3.4 | 0.94 V vs. Ag/AgCl | 10.0 µM | 15.10–2.30 µM | Basagran | [86] |
| β-CD-GCE | DPV | 0.10 M BR | 6 | 0.93 V vs. Ag/AgCl | 2.0–14.0 mM | [91] | ||
| poly-n-AcMnODEAETPc-GCE | SWV | 0.10 M PBS | 5 | 0.80 V vs. Ag/AgCl | 0.25 µM | 50.0–750 µM | [87] | |
| SPE | SWV | 0.10 M PBS | 7 | 0.71 V | 34.0 nM | 0.19–50.0 µM | Ground and lake water | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noori, J.S.; Mortensen, J.; Geto, A. Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors 2020, 20, 2221. https://doi.org/10.3390/s20082221
Noori JS, Mortensen J, Geto A. Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors. 2020; 20(8):2221. https://doi.org/10.3390/s20082221
Chicago/Turabian StyleNoori, Jafar Safaa, John Mortensen, and Alemnew Geto. 2020. "Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review" Sensors 20, no. 8: 2221. https://doi.org/10.3390/s20082221
APA StyleNoori, J. S., Mortensen, J., & Geto, A. (2020). Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors, 20(8), 2221. https://doi.org/10.3390/s20082221





