Monitoring Winter Stress Vulnerability of High-Latitude Understory Vegetation Using Intraspecific Trait Variability and Remote Sensing Approaches
Abstract
1. Introduction
- (1)
- What is the range of intraspecific variability of common traits of dwarf shrubs and mosses in subarctic spring?
- (2)
- Which traits are reliable indicators of plant stress?
- (3)
- How do the indices derived from ordinary and modified RGB cameras correlate with common plant traits?
2. Materials and Methods
2.1. Site Description
2.2. Data Collection
2.3. Data Processing
2.3.1. Greenness Indices
| Name | Equation | Device | Market Price | Calibration | Comments | Source |
|---|---|---|---|---|---|---|
| Greenseeker NDVI | Automatically calculated NDVI output; range 0–1 1 | GreenSeeker handheld crop sensor (Trimble Inc., Sunnyvale, CA, USA) | ca. 700 $ | Not needed | Active sensor | [41,42] |
| Mapir NDVI | Survey2 Camera – NDVI (Mapir Inc., San Diego, CA, USA) | ca. 400 $ | Mapir target | Passive sensor | [43] | |
| BNDVI | Modified Canon Eos 450D (no IR filter) (MaxMax, LDP LLC, Carlstadt, CA, USA) | Body + conversion = 250 $ | White balance (WB) on gray area 2 | Widely used NDVI surrogate | [44] | |
| GNDVI | Same as for BNDVI | Same as for BNDVI | White balance (WB) on gray area 2 | More linear correlation with chlorophyll than NDVI | [28,29] | |
| GRVI | α7 (ILCE-7) (Sony Corp., Tokyo, Japan) | ca. 700 $, but could be any RGB camera | 3-step gray card 2, when EV = −0.7 | RGB index | [45] | |
| Channel G% | Same as for GRVI | Same as for GRVI | 3-step gray card and white balance on white area 2 when EV = −0.7 | RGB index | [46,47] |
2.3.2. Analysis of the Data
3. Results
3.1. Descriptive Statistics
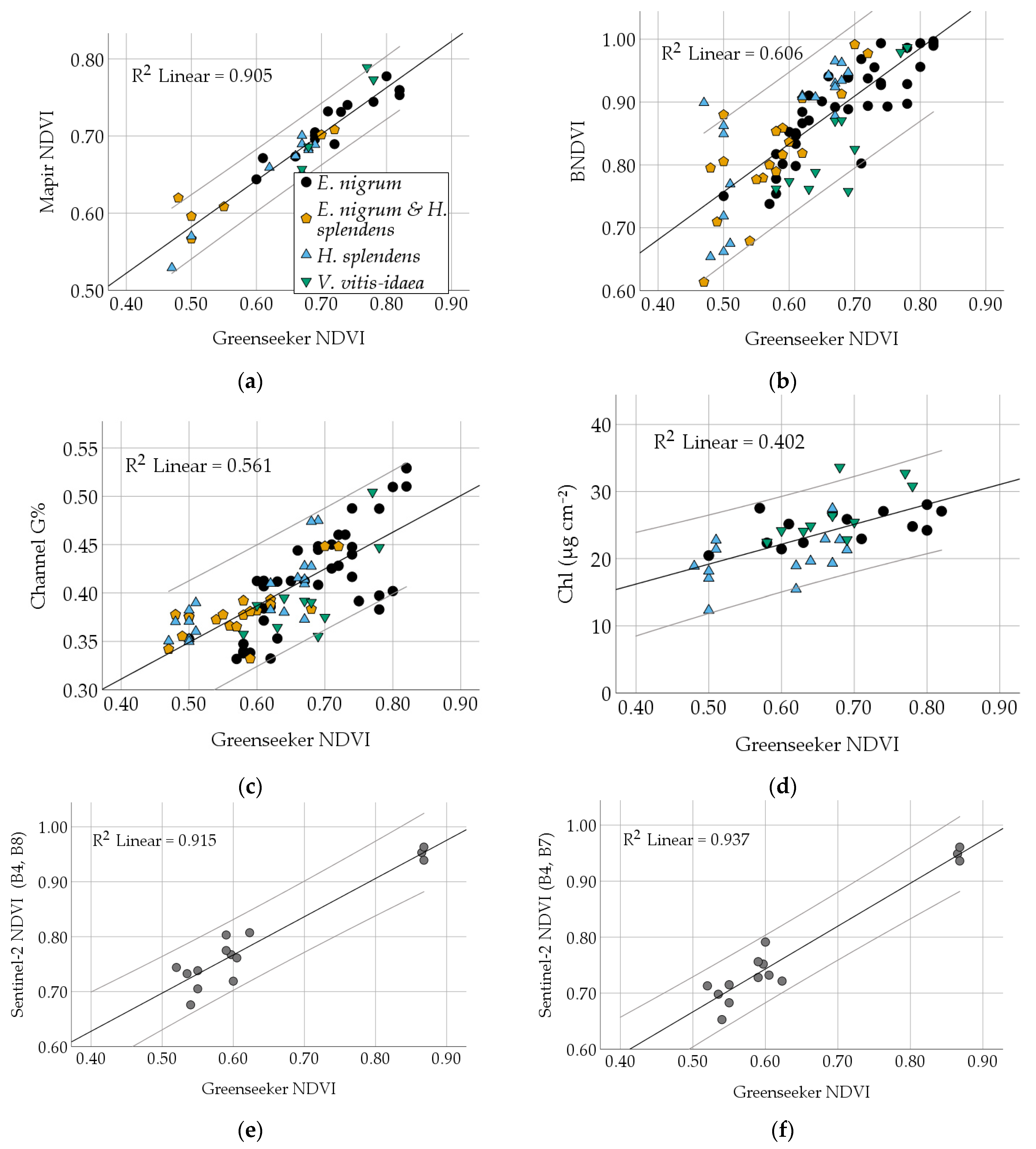
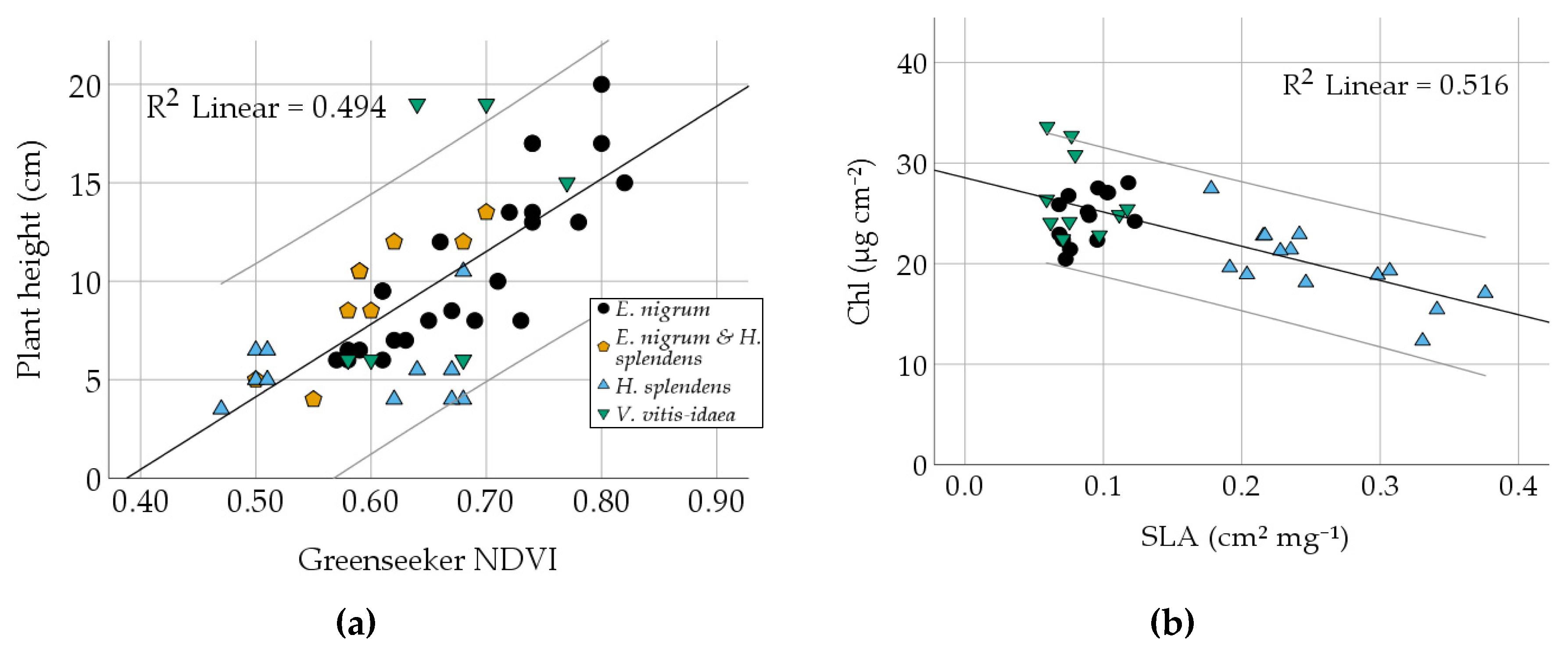
3.2. Comparison of Different Vegetation Indices and Plant Traits
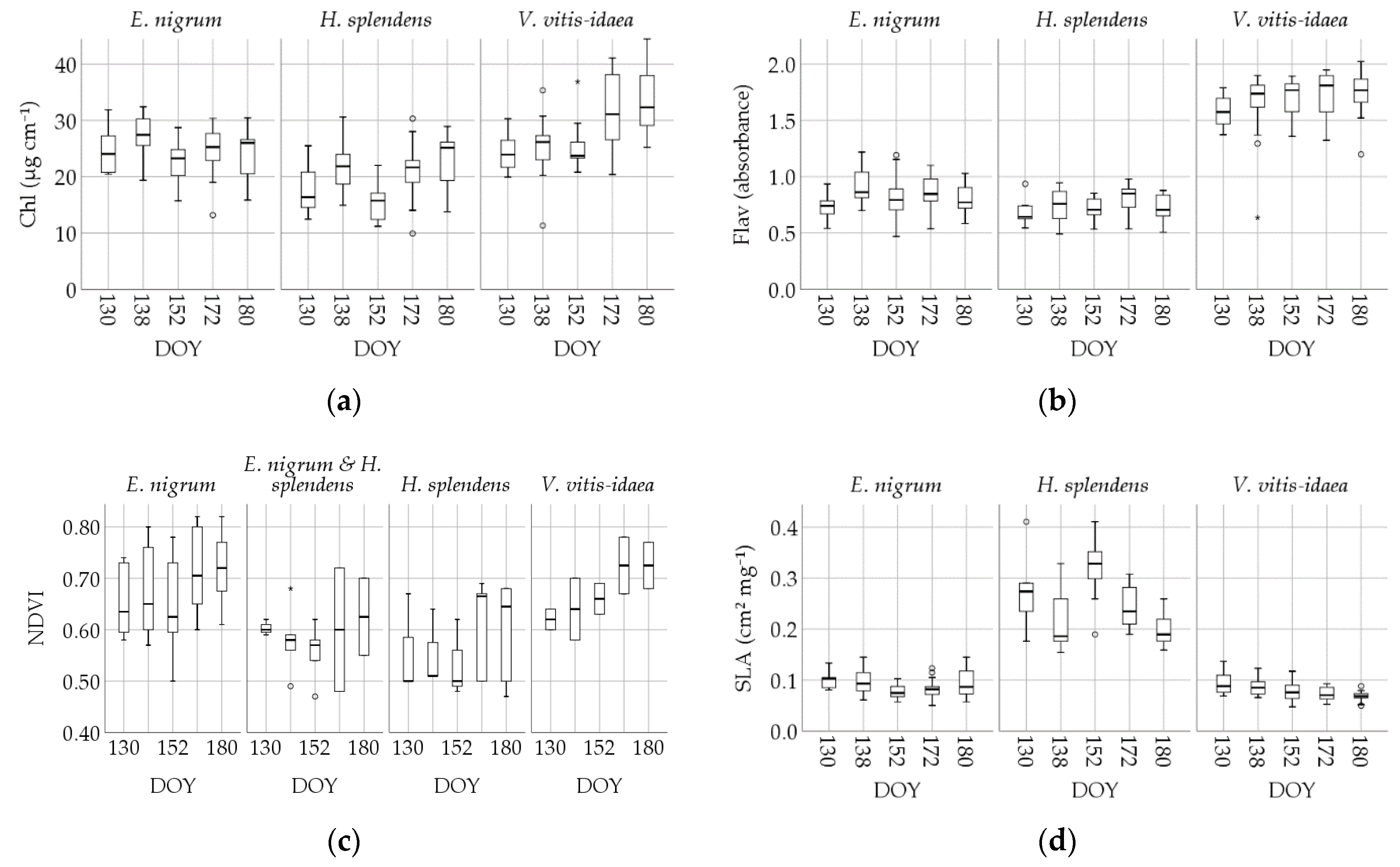
3.3. Intraspecific Trait Variablility
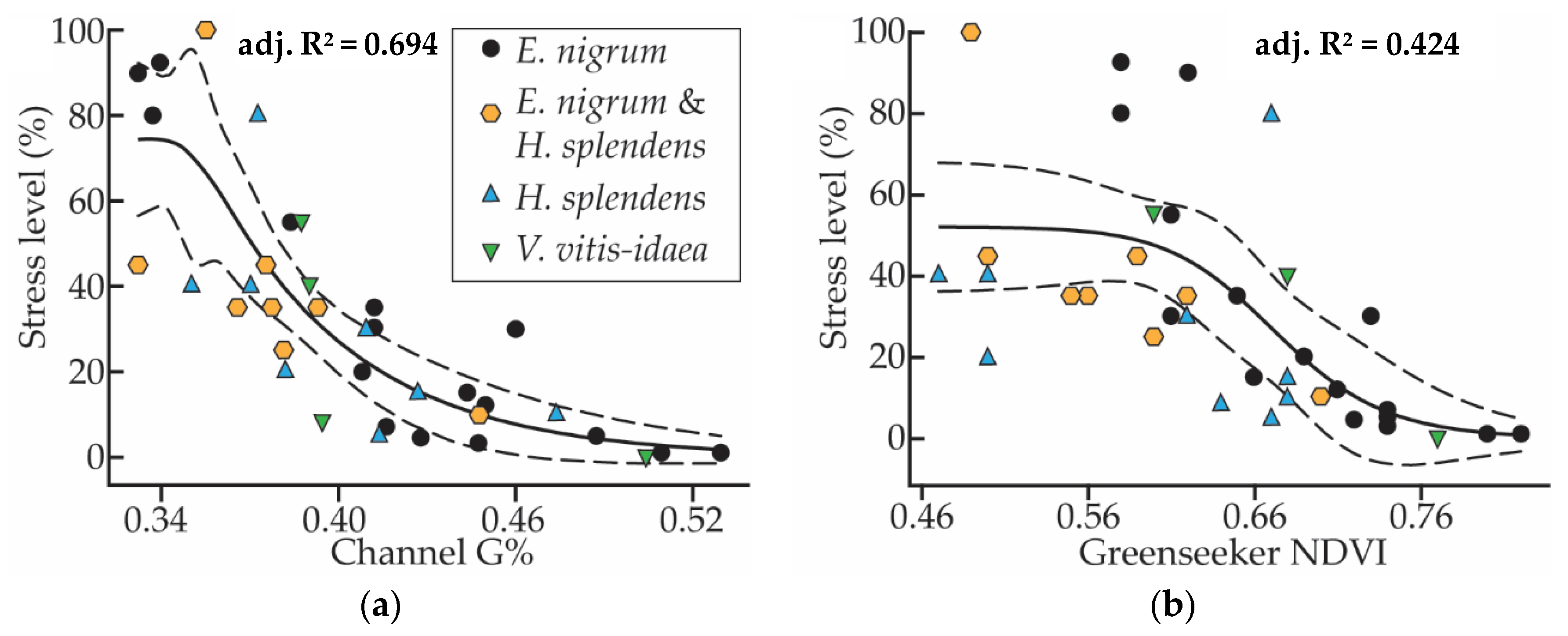
3.4. Suitable Traits for Stress Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report; Pachauri, R.K., Mayer, L., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2015. [Google Scholar]
- Ju, J.; Masek, J.G. The vegetation greenness trend in Canada and US Alaska from 1984–2012 Landsat data. Remote Sens. Environ. 2016, 176, 1–16. [Google Scholar] [CrossRef]
- Myneni, R.B.; Keeling, C.D.; Tucker, C.J.; Asrar, G.; Nemani, R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 1997, 386, 698–702. [Google Scholar] [CrossRef]
- Verbyla, D. The greening and browning of Alaska based on 1982–2003 satellite data. Glob. Ecol. Biogeogr. 2008, 17, 547–555. [Google Scholar] [CrossRef]
- Jia, G.J.; Epstein, H.E.; Walker, D.A. Greening of arctic Alaska, 1981–2001. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Epstein, H.E.; Bhatt, U.S.; Raynolds, M.K.; Walker, D.A.; Forbes, B.C.; Phoenix, G.K.; Bjerke, J.W.; Tømmervik, H.; Karlsen, S.-R.; Myneni, R.; et al. Tundra greenness. Bull. Am. Meteorol. Soc. 2019, 100, S163–S168. [Google Scholar]
- Myers-Smith, I.H.; Kerby, J.T.; Phoenix, G.K.; Bjerke, J.W.; Epstein, H.E.; Assmann, J.J.; John, C.; Andreu-Hayles, L.; Angers-Blondin, S.; Beck, P.S.A.; et al. Complexity revealed in the greening of the Arctic. Nat. Clim. Chang. 2020, 10, 106–117. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Bjerke, J.W. Arctic browning: Extreme events and trends reversing arctic greening. Glob. Chang. Biol. 2016, 22, 2960–2962. [Google Scholar] [CrossRef]
- Bokhorst, S.F.; Bjerke, J.W.; Bowles, F.W.; MELILLO, J.; Callaghan, T.V.; Phoenix, G.K. Impacts of extreme winter warming in the sub-Arctic: Growing season responses of dwarf shrub heathland. Glob. Chang. Biol. 2008, 14, 2603–2612. [Google Scholar] [CrossRef]
- Sorensen, P.O.; Finzi, A.C.; Giasson, M.A.; Reinmann, A.B.; Sanders-DeMott, R.; Templer, P.H. Winter soil freeze-thaw cycles lead to reductions in soil microbial biomass and activity not compensated for by soil warming. Soil. Biol. Biochem. 2018, 116, 39–47. [Google Scholar] [CrossRef]
- Bokhorst, S.F.; Bjerke, J.W.; Street, L.E.; Callaghan, T.V.; Phoenix, G.K. Impacts of multiple extreme winter warming events on sub-Arctic heathland: Phenology, reproduction, growth, and CO2 flux responses. Glob. Chang. Biol. 2011, 17, 2817–2830. [Google Scholar] [CrossRef]
- Bokhorst, S.F.; Phoenix, G.K.; Berg, M.P.; Callaghan, T.V.; Kirby-Lambert, C.; Bjerke, J.W. Climatic and biotic extreme events moderate long-term responses of above- and belowground sub-Arctic heathland communities to climate change. Glob. Chang. Biol. 2015, 21, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, J.W.; Treharne, R.; Vikhamar-Schuler, D.; Karlsen, S.R.; Ravolainen, V.; Bokhorst, S.F.; Phoenix, G.K.; Bochenek, Z.; Tømmervik, H. Understanding the drivers of extensive plant damage in boreal and Arctic ecosystems: Insights from field surveys in the aftermath of damage. Sci. Total Environ. 2017, 599, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, J.W.; Bokhorst, S.F.; Callaghan, T.V.; Phoenix, G.K.; Dorrepaal, E. Persistent reduction of segment growth and photosynthesis in a widespread and important sub-Arctic moss species after cessation of three years of experimental winter warming. Funct. Ecol. 2017, 31, 127–134. [Google Scholar] [CrossRef]
- Bjerke, J.W.; Rune Karlsen, S.; Arild Høgda, K.; Malnes, E.; Jepsen, J.U.; Lovibond, S.; Vikhamar-Schuler, D.; Tømmervik, H. Record-low primary productivity and high plant damage in the Nordic Arctic Region in 2012 caused by multiple weather events and pest outbreaks. Environ. Res. Lett. 2014, 9, 84006. [Google Scholar] [CrossRef]
- Tømmervik, H.; Bjerke, J.W.; Park, T.; Hanssen, F.; Myneni, R.B. Legacies of historical exploitation of natural resources are more important than summer warming for recent biomass increases in a boreal–Arctic transition region. Ecosystems 2019, 113, 11770. [Google Scholar] [CrossRef]
- Xu, L.; Myneni, R.B.; Chapin III, F.S.; Callaghan, T.V.; Pinzon, J.E.; Tucker, C.J.; Zhu, Z.; Bi, J.; Ciais, P.; Tømmervik, H.; et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang. 2013. [Google Scholar] [CrossRef]
- Kitić, G.; Tagarakis, A.; Cselyuszka, N.; Panić, M.; Birgermajer, S.; Sakulski, D.; Matović, J. A new low-cost portable multispectral optical device for precise plant status assessment. Comput. Electron. Agric. 2019, 162, 300–308. [Google Scholar] [CrossRef]
- Bokhorst, S.F.; Tømmervik, H.; Callaghan, T.V.; Phoenix, G.K.; Bjerke, J.W. Vegetation recovery following extreme winter warming events in the sub-Arctic estimated using NDVI from remote sensing and handheld passive proximal sensors. Environ. Exp. Bot. 2012, 81, 18–25. [Google Scholar] [CrossRef]
- Street, L.E.; Shaver G., R.; Williams, M.; Van Wijk, M.T. What is the relationship between changes in canopy leaf area and changes in photosynthetic CO 2 flux in arctic ecosystems? J. Ecol. 2007, 95, 139–150. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, H.R.; Schell, A.J.; Deering, W.D. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the 3rd Earth Resource Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1973. [Google Scholar]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Boelman, N.T.; Stieglitz, M.; Rueth, H.M.; Sommerkorn, M.; Griffin, K.L.; Shaver, G.R.; Gamon, J.A. Response of NDVI, biomass, and ecosystem gas exchange to long-term warming and fertilization in wet sedge tundra. Oecologia 2003, 135, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, J.U.; Hagen, S.B.; Høgda, K.A.; Ims, R.A.; Karlsen, S.R.; Tømmervik, H.; Yoccoz, N.G. Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sens. Environ. 2009, 113, 1939–1947. [Google Scholar] [CrossRef]
- Tømmervik, H.; Karlsen, S.-R.; Nilsen, L.; Johansen, B.; Storvold, R.; Zmarz, A.; Becker, P.S.; Johansen, K.-S.; Hogda, K.-A.; Goetz, S.; et al. Use of Unmanned Aircraft Systems (UAS) in a Multi-Scale Vegetation Index Study of Arctic Plant Communities in Adventdalen on Svalbard. Available online: https://www.nina.no/Portals/NINA/Bilder%20og%20dokumenter/Prosjekter/ArcticBiomass/Tommervik_et_2014_UAS_RPAS.pdf (accessed on 26 January 2020).
- Leblanc, S.G.; Chen, W.; Maloley, M.; Humphreys, E.; Elliott, C. NDVI Digital Camera for Monitoring Arctic Vegetation. In Proceedings of the 2014 IEEE International Geoscience & Remote Sensing Symposium and 35th Canadian Symposium on Remote Sensing (IGARSS 2014/35th CSRS), Quebec, Canada, 13–18 July 2014. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Anderson, H.; Nilsen, L.; Tømmervik, H.; Karlsen, S.; Nagai, S.; Cooper, E.J. Using Ordinary Digital Cameras in Place of Near-Infrared Sensors to Derive Vegetation Indices for Phenology Studies of High Arctic Vegetation. Remote Sens. 2016, 8, 847. [Google Scholar] [CrossRef]
- Nilsson, M.-C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front Ecol. Environ. 2005, 3. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer: Sunderland, MA, USA, 2010; p. 662. [Google Scholar]
- Bjerke, J.W.; Bokhorst, S.F.; Zielke, M.; Callaghan, T.V.; Bowles, F.W.; Phoenix, G.K. Contrasting sensitivity to extreme winter warming events of dominant sub-Arctic heathland bryophyte and lichen species. J. Ecol. 2011, 99, 1481–1488. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sensing Reviews 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Pierce, L.L.; Running, S.W.; Walker, J. Regional-Scale Relationships of Leaf Area Index to Specific Leaf Area and Leaf Nitrogen Content. Ecol. Appl. 1994, 4, 313–321. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Li, S.; Lü, X.; Wang, X.; Han, X. Changes in specific leaf area of dominant plants in temperate grasslands along a 2500-km transect in northern China. Sci. Rep. 2017, 7, 10780. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.t.; Morgan, H.D.; Van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335. [Google Scholar] [CrossRef]
- Treharne, R.; Bjerke, J.W.; Tømmervik, H.; Stendardi, L.; Phoenix, G.K. Arctic browning: Impacts of extreme climatic events on heathland ecosystem CO2 fluxes. Glob. Chang. Biol. 2019, 25, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; López, J.; Lan, Y. Laboratory evaluation of the GreenSeeker™ hand-held optical sensor to variations in orientation and height above canopy. Int. J. Agric. Biol. Eng. 2012, 43–47. [Google Scholar] [CrossRef]
- Verhulst, N.; Govaerts, B.; Sayre, K.D.; Deckers, J.; François, I.M.; Dendooven, L. Using NDVI and soil quality analysis to assess influence of agronomic management on within-plot spatial variability and factors limiting production. Plant Soil. 2009, 317, 41–59. [Google Scholar] [CrossRef]
- Koucká, L.; Kopačková, V.; Fárová, K.; Gojda, M. UAV Mapping of an Archaeological Site Using RGB and NIR High-Resolution Data. Proceedings 2018, 2, 351. [Google Scholar] [CrossRef]
- MaxMax. Manufacturer Homepage; Product Specifications. Available online: https://maxmax.com/ndvi_camera_technical.htm (accessed on 6 September 2017).
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sensing Reviews 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.-L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kudo, G. Short-term effects of simulated environmental change on phenology, leaf traits, and shoot growth of alpine plants on a temperate mountain, northern Japan. Glob. Chang. Biol. 1997, 3, 108–115. [Google Scholar] [CrossRef]
- Pensa, M.; Karu, H.; Luud, A.; Kund, K. Within-species correlations in leaf traits of three boreal plant species along a latitudinal gradient. Plant Ecol. 2010, 208, 155–166. [Google Scholar] [CrossRef]
- Van Wijk, M.T.; Williams, M.; Shaver, G.R. Tight coupling between leaf area index and foliage N content in arctic plant communities. Oecologia 2005, 142, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, J.W.; Wierzbinski, G.; Tømmervik, H.; Phoenix, G.K.; Bokhorst, S. Stress-induced secondary leaves of a boreal deciduous shrub (Vaccinium myrtillus) overwinter then regain activity the following growing season. Nord. J. Bot. 2018, 36, e01894. [Google Scholar] [CrossRef]
- Tybirk, K.; Nilsson, M.-C.; Michelsen, A.; Kristensen, H.L.; Shevtsova, A.; Strandberg, M.T.; Johansson, M.; Nielsen, K.E.; Riis-Nielsen, T.; Strandberg, B.; et al. Nordic Empetrum dominated ecosystems: Function and susceptibility to environmental changes. Ambio 2000, 29, 90–97. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S.; Moya, I. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Lefebvre, T.; Millery-Vigues, A.; Gallet, C. Does leaf optical absorbance reflect the polyphenol content of alpine plants along an elevational gradient? Alp. Bot. 2016, 126, 177–185. [Google Scholar] [CrossRef]
- Nijland, W.; De Jong, R.; De Jong, S.M.; Wulder, M.A.; Bater, C.W.; Coops, N.C. Monitoring plant condition and phenology using infrared sensitive consumer grade digital cameras. Agric. For. Meteorol. 2014, 184, 98–106. [Google Scholar] [CrossRef]
- Leeuw, T.; Boss, E. The HydroColor app: Above water measurements of remote sensing reflectance and turbidity using a smartphone camera. Sensors 2018, 18, 256. [Google Scholar] [CrossRef]
- Westergaard-Nielsen, A.; Lund, M.; Hansen, B.U.; Tamstorf, M.P. Camera derived vegetation greenness index as proxy for gross primary production in a low Arctic wetland area. ISPRS Int. J. Geoinf. 2013, 86, 89–99. [Google Scholar] [CrossRef]
- Hunt, E.R.; Hively, W.D.; McCarty, G.W.; Daughtry, C.S.T.; Forrestal, P.J.; Kratochvil, R.J.; Carr, J.L.; Allen, N.F.; Fox-Rabinovitz, J.R.; Miller, C.D. NIR-Green-Blue High-Resolution Digital Images for Assessment of Winter Cover Crop Biomass. GIScience Remote Sens. 2011, 48, 86–98. [Google Scholar]
- Manna, S.; Raychaudhuri, B. Retrieval of leaf area index and stress conditions for Sundarban mangroves using Sentinel-2 data. Int. J. Remote Sens. 2019, 1–21. [Google Scholar] [CrossRef]
- Barbanti, L.; Adroher, J.; Damian, J.M.; Di Virgilio, N.; Falsone, G.; Zucchelli, M.; Martelli, R. Assessing wheat spatial variation based on proximal and remote spectral vegetation indices and soil properties. Ital. J. Agronomy 2018, 13, 21. [Google Scholar] [CrossRef]
| Mapir | Greenseeker | Sentinel-2 | Sentinel-2 | Sentinel-2 | |
|---|---|---|---|---|---|
| Survey 2 NDVI | Handheld Crop Sensor | Band 4 | Band 7 | Band 8 | |
| Red band | 660 nm (50) | 660 nm (25) | 665 nm (30) | ||
| NIR band | 850 nm (70) | 780 nm (25) | 783 nm (20) | 833 nm (106) | |
| Spatial resolution | 16 MP camera | an oval depending on the height of the sensor: at 60 cm, length is 25 cm | 10 m | 20 m | 10 m |
| Name | Mean | Std. Error | Std. Deviation | N | 5% PCTL | Median | 95% PCTL | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|
| Greenseeker NDVI | 0.64 | 0.0099 | 0.092 | 88 | 0.48 | 0.63 | 0.80 | 0.018 | −0.701 |
| Mapir NDVI | 0.69 | 0.0104 | 0.062 | 35 | 0.56 | 0.69 | 0.78 | −0.611 | 0.246 |
| BNDVI | 0.86 | 0.0096 | 0.090 | 88 | 0.68 | 0.87 | 0.99 | −0.569 | −0.233 |
| Channel G% | 0.40 | 0.0050 | 0.047 | 87 | 0.48 | 0.39 | 0.80 | 0.848 | 0.283 |
| GRVI | −0.098 | 0.0087 | 0.081 | 88 | −0.212 | −0.108 | 0.072 | 0.659 | 0.349 |
| GNDVI | 0.47 | 0.0085 | 0.079 | 88 | 0.34 | 0.47 | 0.60 | −0.395 | 0.393 |
| Plant height | 9.46 | 0.0638 | 4.508 | 50 | 4.00 | 8.25 | 19.00 | 0.748 | −0.430 |
| Stress level | 32.07 | 4.6593 | 27.956 | 36 | 0.85 | 30.00 | 93.63 | 1.009 | 0.274 |
| Soil depth | 13.73 | 0.7238 | 6.790 | 88 | 4.00 | 12.00 | 25.00 | 0.267 | −1.301 |
| SLA | 0.149 | 0.0151 | 0.093 | 38 | 0.059 | 0.103 | 0.343 | 0.993 | −0.238 |
| Chl | 23.46 | 0.7151 | 4.401 | 38 | 15.28 | 22.92 | 32.78 | −0.014 | 0.617 |
| Flav | 1.01 | 0.0672 | 0.414 | 38 | 0.61 | 0.81 | 1.86 | 1.065 | −0.420 |
| NBI | 25.31 | 0.0151 | 7.241 | 38 | 14.34 | 25.93 | 36.04 | −0.018 | −1.166 |
| BNDVI | Channel G% | GRVI | GNDVI | Plant Height | Stress Level | Soil Depth | SLA | Chl | Flav | NBI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDVI | Correlation | 0.779 | 0.749 | 0.689 | 0.440 | 0.703 | −0.600 | 0.454 | −0.451 | 0.634 | 0.260 | 0.190 |
| Sig. | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.116 | 0.254 | |
| RMSE | 0.0571 | 0.0314 | 0.0592 | 0.0716 | 3.2404 | 22.682 | 6.086 | 0.0841 | 3.4572 | |||
| Slope/ Intercept | 0.762/0.376 | 0.379/0.159 | 0.606/−0.485 | 0.377/0.232 | 36.892/−14.313 | −188.3/ 152.9 | 33.324/ −7.562 | −0.446/ 0.436 | 29.706/ 4.320 | |||
| N | 88 | 87 | 88 | 88 | 50 | 36 | 88 | 38 | 38 | 38 | 38 | |
| BNDVI | Correlation | 0.730 | 0.641 | 0.547 | 0.468 | −0.654 | 0.246 | −0.232 | 0.433 | −0.029 | 0.327 | |
| Sig. | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.021 | 0.161 | 0.007 | 0.863 | 0.045 | ||
| RMSE | 0.0324 | 0.0627 | 0.0668 | 4.026 | 21.469 | 4.027 | ||||||
| slope/intercept | 0.378/0.076 | 0.576/ −0.595 | 0.480/0.059 | 26.478/ −13.720 | −233.09/228.97 | 19.167/ 7.033 | ||||||
| N | 87 | 88 | 88 | 50 | 36 | 88 | 38 | 38 | 38 | 38 | ||
| Channel G% | Correlation | 0.909 | 0.454 | 0.515 | −0.768 | 0.395 | −0.141 | 0.376 | −0.051 | 0.354 | ||
| Sig. | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.397 | 0.020 | 0.759 | 0.029 | |||
| RMSE | 0.0340 | 0.0714 | 3.946 | 18.176 | ||||||||
| Slope/Intercept | 1.565/ −0.728 | 0.164/0.768 | 49.185/ −10.268 | −419.85/203.61 | ||||||||
| N | 87 | 87 | 49 | 36 | 87 | 38 | 38 | 38 | 38 | |||
| GRVI | Correlation | 0.338 | 0.554 | −0.745 | 0.393 | −0.124 | 0.387 | 0.019 | 0.301 | |||
| Sig. | 0.001 | 0.000 | 0.000 | 0.000 | 0.459 | 0.016 | 0.908 | 0.066 | ||||
| RMSE | 3.7909 | 18.909 | ||||||||||
| slope/intercept | 30.426/12.371 | −246.65/11.770 | ||||||||||
| N | 88 | 50 | 36 | 88 | 38 | 38 | 38 | 38 | ||||
| GNDVI | Correlation | 0.045 | −0.220 | 0.070 | −0.324 | 0.226 | −0.120 | 0.294 | ||||
| Sig. | 0.758 | 0.196 | 0.515 | 0.047 | 0.172 | 0.472 | 0.074 | |||||
| N | 50 | 36 | 88 | 38 | 38 | 38 | 38 | |||||
| Plant height | Correlation | −0.553 | 0.425 | −0.357 | 0.365 | 0.383 | −0.160 | |||||
| Sig. | 0.001 | 0.002 | 0.103 | 0.095 | 0.079 | 0.477 | ||||||
| RMSE | 22.129 | 6.3474 | ||||||||||
| slope/intercept | −3.085/58.926 | 0.654/ 7.765 | ||||||||||
| N | 34 | 50 | 22 | 22 | 22 | 22 | ||||||
| Stress level | Correlation | −0.336 | −0.110 | −0.232 | −0.090 | −0.021 | ||||||
| Sig. | 0.045 | 0.707 | 0.425 | 0.760 | 0.944 | |||||||
| N | 36 | 14 | 14 | 14 | 14 | |||||||
| Soil depth | Correlation | −0.025 | 0.103 | −0.441 | 0.685 | |||||||
| Sig. | 0.883 | 0.539 | 0.006 | 0.000 | ||||||||
| RMSE | 0.3768 | 5.3469 | ||||||||||
| slope/intercept | −0.027/ 1.385 | 0.721/ 15.182 | ||||||||||
| N | 38 | 38 | 38 | 38 | ||||||||
| SLA | Correlation | −0.718 | −0.512 | 0.086 | ||||||||
| Sig. | 0.000 | 0.001 | 0.607 | |||||||||
| RMSE | 3.1102 | 0.3605 | ||||||||||
| slope/intercept | −34.048/28.541 | −2.282/ 1.353 | ||||||||||
| N | 38 | 38 | 38 | |||||||||
| Chl | Correlation | 0.541 | 0.045 | |||||||||
| Sig. | 0.000 | 0.790 | ||||||||||
| RMSE | 0.3530 | |||||||||||
| slope/intercept | 0.051/−0.180 | |||||||||||
| N | 38 | 38 | ||||||||||
| Flav | Correlation | −0.787 | ||||||||||
| Sig. | 0.000 | |||||||||||
| RMSE | 4.5306 | |||||||||||
| slope/intercept | −13.759/39.239 | |||||||||||
| N | 38 | |||||||||||
| Name | Specie | Mean | N | Std. Deviation | Std. Error | Median | 5% PCTL | 25% PCTL | 75% PCTL | 95% PCTL |
|---|---|---|---|---|---|---|---|---|---|---|
| Greenseeker NDVI | E. nigrum | 0.65 | 59 | 0.094 | 0.012 | 0.62 | 0.49 | 0.58 | 0.72 | 0.82 |
| H. splendens | 0.59 | 19 | 0.086 | 0.020 | 0.62 | 0.47 | 0.50 | 0.67 | 0.68 (90%) | |
| V. vitis-idaea | 0.67 | 10 | 0.066 | 0.020 | 0.67 | 0.58 | 0.62 | 0.72 | 0.78 (90%) | |
| Channel G% | E. nigrum | 0.405 | 58 | 0.050 | 0.006 | 0.393 | 0.333 | 0.372 | 0.444 | 0.511 |
| H. splendens | 0.396 | 19 | 0.037 | 0.008 | 0.383 | 0.350 | 0.383 | 0.415 | 0.474 (90%) | |
| V. vitis-idaea | 0.397 | 10 | 0.046 | 0.015 | 0.389 | 0.355 | 0.363 | 0.408 | 0.497 (90%) | |
| Plant height | E. nigrum | 10.4 | 33 | 3.975 | 0.692 | 9.5 | 4,7 | 7.0 | 13.3 | 17.9 |
| H. splendens | 5.5 | 11 | 1.955 | 0.589 | 5.0 | 3.5 | 4.0 | 6.5 | 9.7 (90%) | |
| V. vitis-idaea | 11.8 | 6 | 6.55 | 2.676 | 10.5 | 6.0 | 6.0 | 19.0 | - | |
| SLA | E. nigrum | 0.089 | 69 | 0.022 | 0.0027 | 0.085 | 0.057 | 0.072 | 0.105 | 0.130 |
| H. splendens | 0.257 | 69 | 0.086 | 0.0104 | 0.246 | 0.164 | 0.189 | 0.291 | 0.411 | |
| V. vitis-idaea | 0.080 | 61 | 0.019 | 0.0024 | 0.079 | 0.052 | 0.066 | 0.092 | 0.117 | |
| Chl | E. nigrum | 24.72 | 70 | 4.267 | 0.510 | 25.26 | 16.25 | 21.44 | 27.57 | 30.67 |
| H. splendens | 19.88 | 69 | 5.122 | 0.617 | 20.01 | 11.90 | 15.59 | 23.88 | 28.54 | |
| V. vitis-idaea | 28.25 | 61 | 6.491 | 0.831 | 26.55 | 20.21 | 23.63 | 31.39 | 40.96 | |
| Flav | E. nigrum | 0.833 | 69 | 0.164 | 0.020 | 0.818 | 0.542 | 0.727 | 0.943 | 1.145 |
| H. splendens | 0.736 | 67 | 0.126 | 0.015 | 0.728 | 0.519 | 0.642 | 0.842 | 0.942 | |
| V. vitis-idaea | 1.671 | 61 | 0.232 | 0.030 | 1.706 | 1.297 | 1.558 | 1.843 | 1.944 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritz, E.; Bjerke, J.W.; Tømmervik, H. Monitoring Winter Stress Vulnerability of High-Latitude Understory Vegetation Using Intraspecific Trait Variability and Remote Sensing Approaches. Sensors 2020, 20, 2102. https://doi.org/10.3390/s20072102
Ritz E, Bjerke JW, Tømmervik H. Monitoring Winter Stress Vulnerability of High-Latitude Understory Vegetation Using Intraspecific Trait Variability and Remote Sensing Approaches. Sensors. 2020; 20(7):2102. https://doi.org/10.3390/s20072102
Chicago/Turabian StyleRitz, Elmar, Jarle W. Bjerke, and Hans Tømmervik. 2020. "Monitoring Winter Stress Vulnerability of High-Latitude Understory Vegetation Using Intraspecific Trait Variability and Remote Sensing Approaches" Sensors 20, no. 7: 2102. https://doi.org/10.3390/s20072102
APA StyleRitz, E., Bjerke, J. W., & Tømmervik, H. (2020). Monitoring Winter Stress Vulnerability of High-Latitude Understory Vegetation Using Intraspecific Trait Variability and Remote Sensing Approaches. Sensors, 20(7), 2102. https://doi.org/10.3390/s20072102





