A Self-Calibrating IoT Portable Electrochemical Immunosensor for Serum Human Epididymis Protein 4 as a Tumor Biomarker for Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
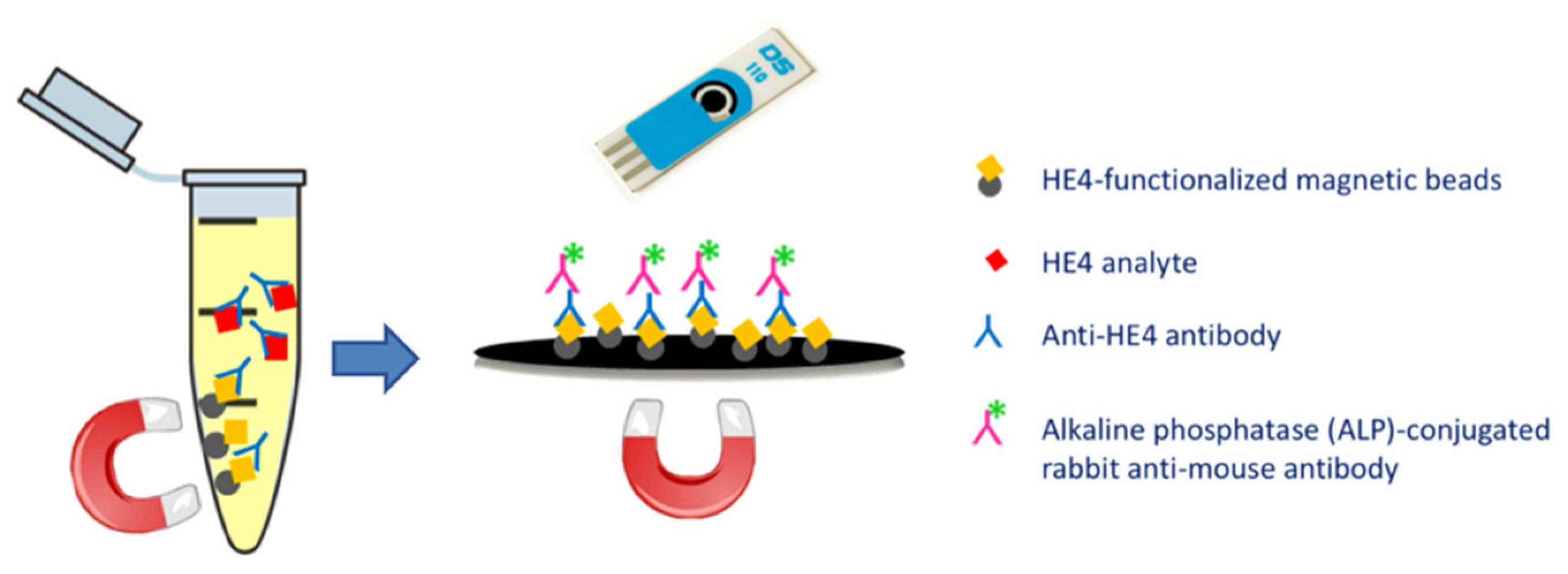
2.2. Magnetic Immunoassay Set-Up
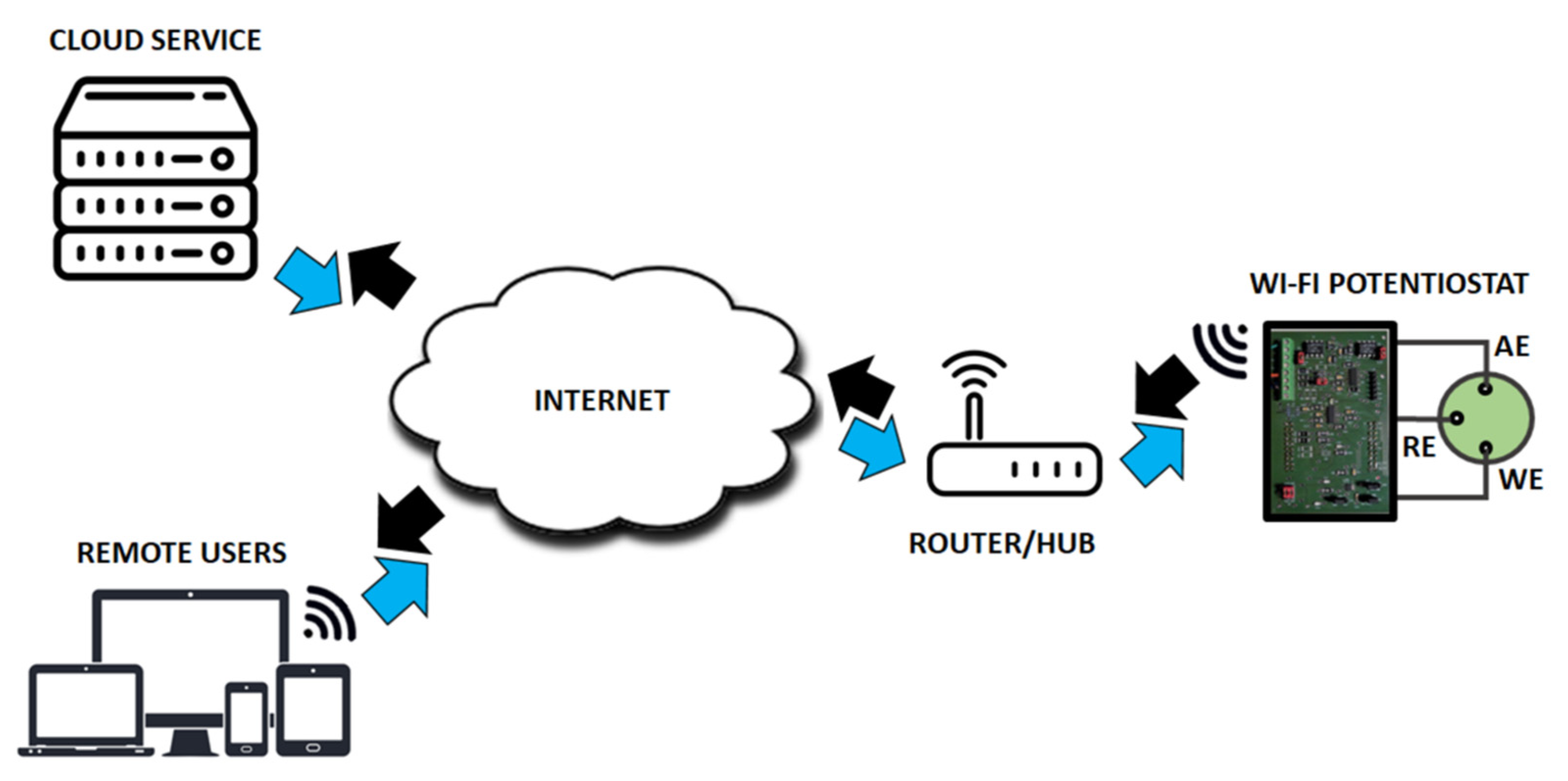
2.3. IoT Architecture
2.4. On-Board Data Management
2.5. Immunosensor Validation
3. Results and Discussion
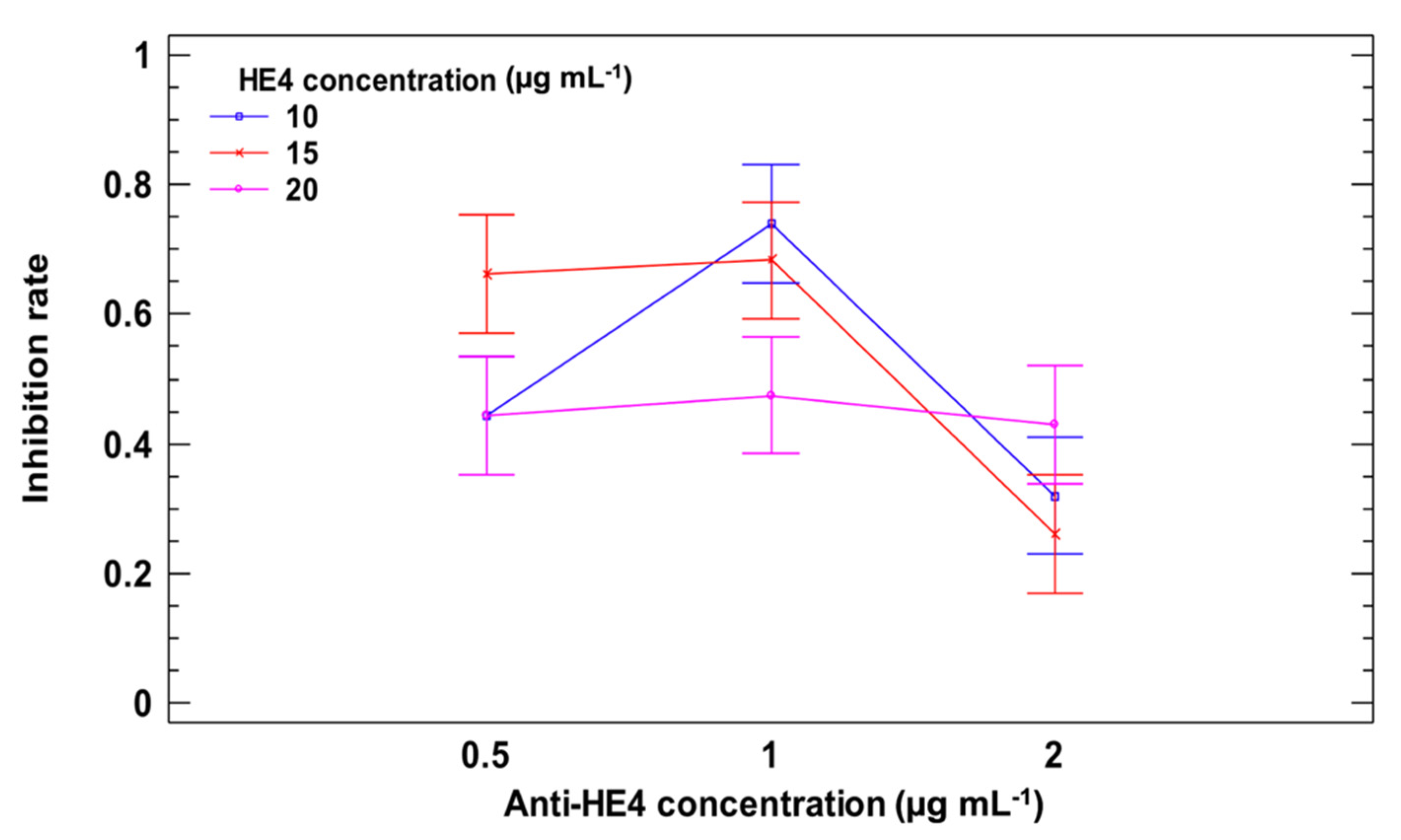
3.1. MBs Functionalization and Immunocompetition Study
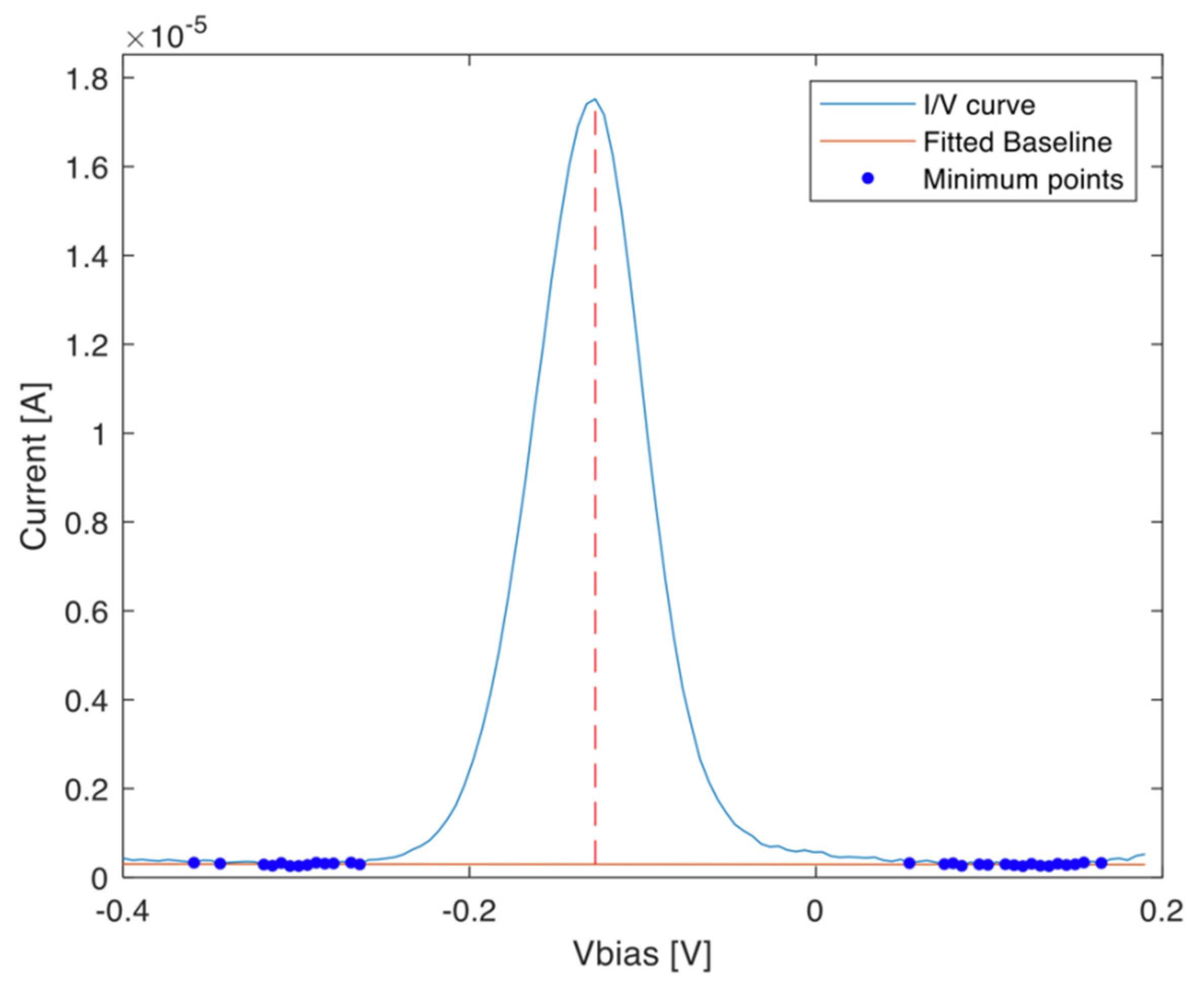
3.2. Data Acquisition and Processing
3.3. Electrochemical Immunoassay Analytical Performance
3.4. Selectivity Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Granato, T.; Porpora, M.G.; Longo, F.; Angeloni, A.; Manganaro, L.; Anastasi, E. HE4 in the differential diagnosis of ovarian masses. Clin. Chim. Acta 2015, 446, 147–155. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
- Bianchi, F.; Giannetto, M.; Careri, M. Advances in molecular analysis of biomarkers for autoimmune and carcinogenic diseases. Anal. Bioanal. Chem. 2014, 406, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Raghav, R.; O’Kennedy, R.; Srivastava, S. Advances in ovarian cancer diagnosis: A journey from immunoassays to immunosensors. Enzyme Microb. Technol. 2016, 89, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017, 87, 918–930. [Google Scholar] [CrossRef]
- Giannetto, M.; Bianchi, M.V.; Mattarozzi, M.; Careri, M. Competitive amperometric immunosensor for determination of p53 protein in urine with carbon nanotubes/gold nanoparticles screenprinted electrodes: A potential rapid and noninvasive screening tool for early diagnosis of urinary tract carcinoma. Anal. Chim. Acta 2017, 991, 133–141. [Google Scholar] [CrossRef]
- De La Franier, B.; Thompson, M. Early stage detection and screening of ovarian cancer: A research opportunity and significant challenge for biosensor technology. Biosens. Bioelectron. 2019, 135, 71–81. [Google Scholar] [CrossRef]
- Su, Z.; Graybill, W.S.; Zhu, Y. Detection and monitoring of ovarian cancer. Clin. Chim. Acta 2013, 415, 341–345. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Sadighbayan, K.; Tohid-kia, M.R.; Khosroushahi, A.Y.; Hasanzadeh, M. Development of electrochemical biosensors for tumor marker determination towards cancer diagnosis: Recent progress. Trends Anal. Chem. 2019, 118, 73–88. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Zheng, H.; Zheng, X.; Dai, H.; Hong, Z.; Lin, Y. Application of NiFe2O4 nanotubes as catalytically promoted sensing platform for ratiometric electrochemiluminescence analysis of ovarian cancer marker. Sensor. Actuators B Chem. 2019, 288, 80–87. [Google Scholar] [CrossRef]
- Fan, L.; Yan, Y.; Guo, B.; Zhao, M.; Li, J.; Bian, X.; Wu, H.; Cheng, W.; Ding, S. Trimetallic hybrid nanodendrites and magnetic nanocomposites-based electrochemical immunosensor for ultrasensitive detection of serum human epididymis protein 4. Sensor. Actuators B Chem. 2019, 296, 126697. [Google Scholar] [CrossRef]
- Yan, Q.; Cao, L.; Dong, H.; Tan, Z.; Liu, Q.; Zhang, W.; Zhao, P.; Li, Y.; Liu, Y.; Dong, Y. Sensitive amperometric immunosensor with improved electrocatalytic Au@Pd urchin-shaped nanostructures for human epididymis specific protein 4 antigen detection. Anal. Chim. Acta 2019, 1069, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Wang, W.; Yu, L.; Wu, K.; Boylan, K.L.M.; Isaksson Vogel, R.; Skubitz, A.P.N.; Wang, J.-P. Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosens. Bioelectron. 2019, 126, 301–307. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Oztekin, Y.; Ramanaviciene, A. Electrochemical formation of polypyrrole-based layerfor immunosensor design. Sensor. Actuators B 2014, 197, 237–243. [Google Scholar] [CrossRef]
- Tan, Y.; Li, M.; Ye, X.; Wang, Z.; Wang, Y.; Li, C. Ionic liquid auxiliary exfoliation of WS2 nanosheets and the enhanced effect of hollow gold nanospheres on their photoelectrochemical sensing towards human epididymis protein 4. Sensor. Actuators B 2018, 262, 982–990. [Google Scholar] [CrossRef]
- Giannetto, M.; Bianchi, V.; Gentili, S.; Fortunati, S.; De Munari, I.; Careri, M. An integrated IoT-Wi-Fi board for remote data acquisition and sharing from innovative immunosensors. Case of study: Diagnosis of celiac disease. Sensor. Actuators B Chem. 2018, 273, 1395–1403. [Google Scholar] [CrossRef]
- Loncaric, C.; Tang, Y.; Ho, C.; Parameswaran, M.A.; Yu, H.-Z. A USB-based electrochemical biosensor prototype for point-of-care diagnosis. Sensor. Actuators B Chem. 2012, 161, 908–913. [Google Scholar] [CrossRef]
- Adams, S.D.; Doeven, E.H.; Quayle, K.; Kouzani, A.Z. MiniStat: Development and evaluation of a mini-potentiostat for electrochemical measurements. IEEE Access 2019, 7, 31903–31912. [Google Scholar] [CrossRef]
- Pruna, R.; Palacio, F.; Baraket, A.; Zine, N.; Streklas, A.; Bausells, J.; Errachid, A.; López, M. A low-cost and miniaturized potentiostat for sensing of biomolecular species such as TNF-α by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2018, 100, 533–540. [Google Scholar] [CrossRef]
- Hanitra, I.N.; Criscuolo, F.; Pankratova, N.; Carrara, S.; De Micheli, G. Multi-Channel Front-End for Electrochemical Sensing of Metabolites, Drugs, and Electrolytes. IEEE Sens. J. 2019, 20, 3636–3645. [Google Scholar] [CrossRef]
- De Campos da Costa, J.P.; Bastos, W.B.; da Costa, P.I.; Zaghete, M.A.; Longo, E.; Carmo, J.P. Portable laboratory platform with electrochemical biosensors for immunodiagnostic of hepatitis C virus. IEEE Sens. J. 2019, 19, 10701–10709. [Google Scholar] [CrossRef]
- Ma, L.; Ju, F.; Tao, C.; Shen, X. Portable, Low Cost Smartphone-Based Potentiostat System for the Salivary α-Amylase Detection in Stress Paradigm. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1334–1337. [Google Scholar]
- Steinberg, M.D.; Kassal, P.; Kereković, I.; Murković Steinberg, I. A wireless potentiostat for mobile chemical sensing and biosensing. Talanta 2015, 143, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, P.; Slaughter, G. Wireless Bipotentiostat Circuit for Glucose and H2O2 Interrogation. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1567–1570. [Google Scholar]
- Chen, G.; Xie, J.; Zhang, Z.; Meng, W.; Zhang, C.; Kang, K.; Wu, Y.; Guo, Z. A portable digital-control electrochemical system with automatic ohmic drop compensation for fast scan voltammetry and its application to ultrasensitive detection of chromium(III). Sensor. Actuators B Chem. 2019, 301, 127135. [Google Scholar] [CrossRef]
- Bianchi, V.; Boni, A.; Fortunati, S.; Giannetto, M.; Careri, M.; De Munari, I. A Wi-Fi cloud-based portable potentiostat for electrochemical biosensors. IEEE Trans. Instrum. Meas. 2019, in press. [Google Scholar] [CrossRef]
- Mayer, M.; Baeumner, A.J. ABC spotlight on analytics 4.0. Anal. Bioanal. Chem. 2018, 410, 5095–5097. [Google Scholar] [CrossRef]
- Bassoli, M.; Bianchi, V.; De Munari, I.; Ciampolini, P. An IoT approach for an AAL Wi-Fi-based monitoring system. IEEE Trans. Instrum. Meas. 2017, 66, 3200–3209. [Google Scholar] [CrossRef]
- Bassoli, M.; Bianchi, V.; De Munari, I. A plug and play IoT Wi-Fi smart home system for human monitoring. Electronics 2018, 7, 200. [Google Scholar] [CrossRef]
- Huang, Y.; Li, G. Descriptive Models for Internet of Things. In Proceedings of the International Conference on Intelligent Control and Information Processing, Dalian, China, 12–15 August 2010; pp. 483–486. [Google Scholar]
- Manfredi, A.; Giannetto, M.; Mattarozzi, M.; Costantini, M.; Mucchino, C.; Careri, M. Competitive immunosensor based on gliadin immobilization on disposable carbon-nanogold screen-printed electrodes for rapid determination of celiotoxic prolamins. Anal. Bioanal. Chem. 2016, 408, 7289–7298. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain nonlinear problems in least squares. Quart. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquaradt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Taisbak, C.M. Cube roots of integers: A conjecture about Heron’s method in Metrika III. 20. Hist. Math. 2014, 41, 103–106. [Google Scholar] [CrossRef]
- Eurachem. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics. Available online: http://www.eurachem.org (accessed on 3 March 2020).
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA: A review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, V.; Mattarozzi, M.; Giannetto, M.; Boni, A.; De Munari, I.; Careri, M. A Self-Calibrating IoT Portable Electrochemical Immunosensor for Serum Human Epididymis Protein 4 as a Tumor Biomarker for Ovarian Cancer. Sensors 2020, 20, 2016. https://doi.org/10.3390/s20072016
Bianchi V, Mattarozzi M, Giannetto M, Boni A, De Munari I, Careri M. A Self-Calibrating IoT Portable Electrochemical Immunosensor for Serum Human Epididymis Protein 4 as a Tumor Biomarker for Ovarian Cancer. Sensors. 2020; 20(7):2016. https://doi.org/10.3390/s20072016
Chicago/Turabian StyleBianchi, Valentina, Monica Mattarozzi, Marco Giannetto, Andrea Boni, Ilaria De Munari, and Maria Careri. 2020. "A Self-Calibrating IoT Portable Electrochemical Immunosensor for Serum Human Epididymis Protein 4 as a Tumor Biomarker for Ovarian Cancer" Sensors 20, no. 7: 2016. https://doi.org/10.3390/s20072016
APA StyleBianchi, V., Mattarozzi, M., Giannetto, M., Boni, A., De Munari, I., & Careri, M. (2020). A Self-Calibrating IoT Portable Electrochemical Immunosensor for Serum Human Epididymis Protein 4 as a Tumor Biomarker for Ovarian Cancer. Sensors, 20(7), 2016. https://doi.org/10.3390/s20072016








