Pilot Site Deployment of an IoT Solution for Older Adults’ Early Behavior Change Detection †
Abstract
1. Introduction
2. Literature Review and Related Work
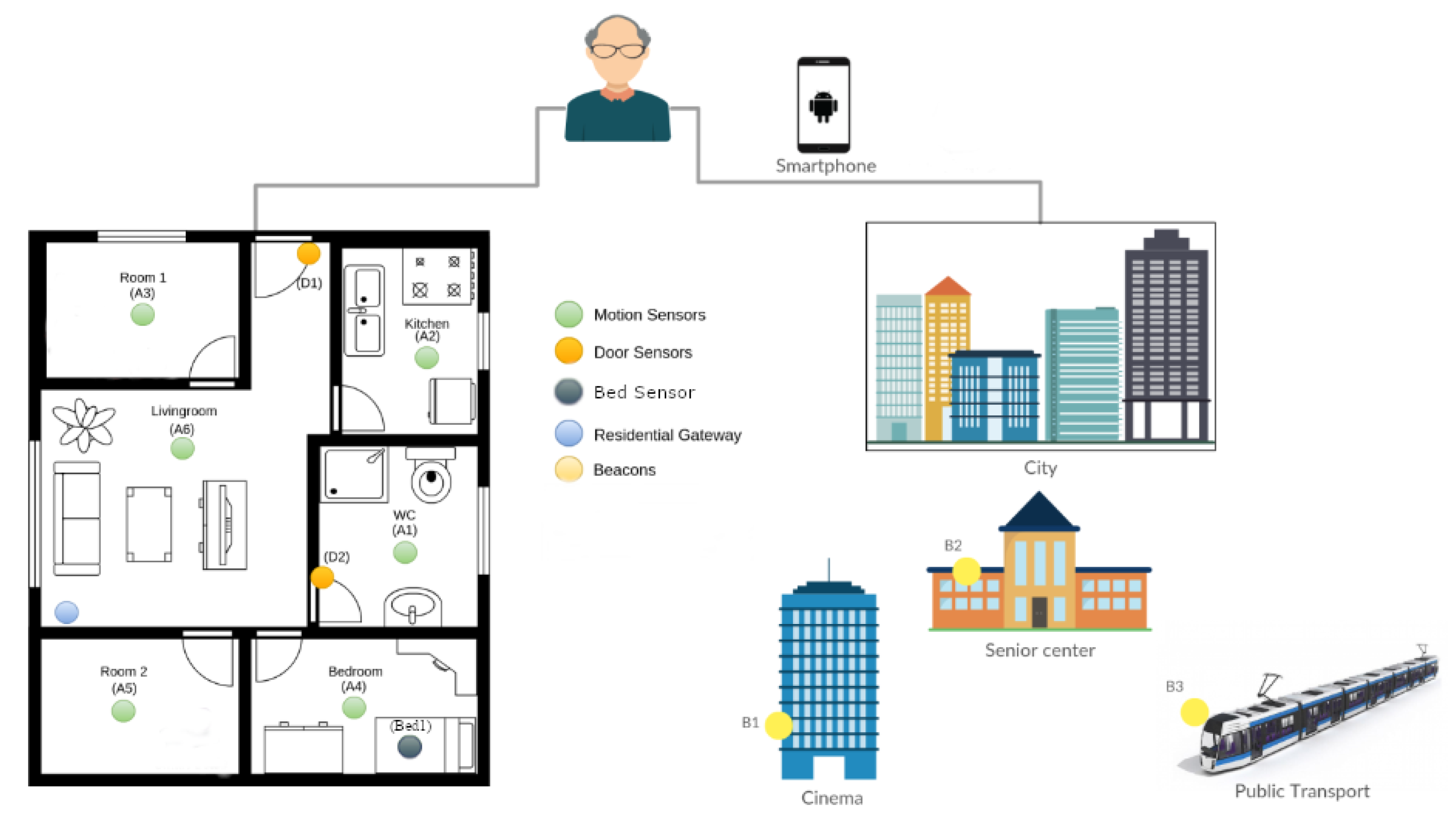
3. Montpellier Pilot Setup
4. Recruitment and Engagement
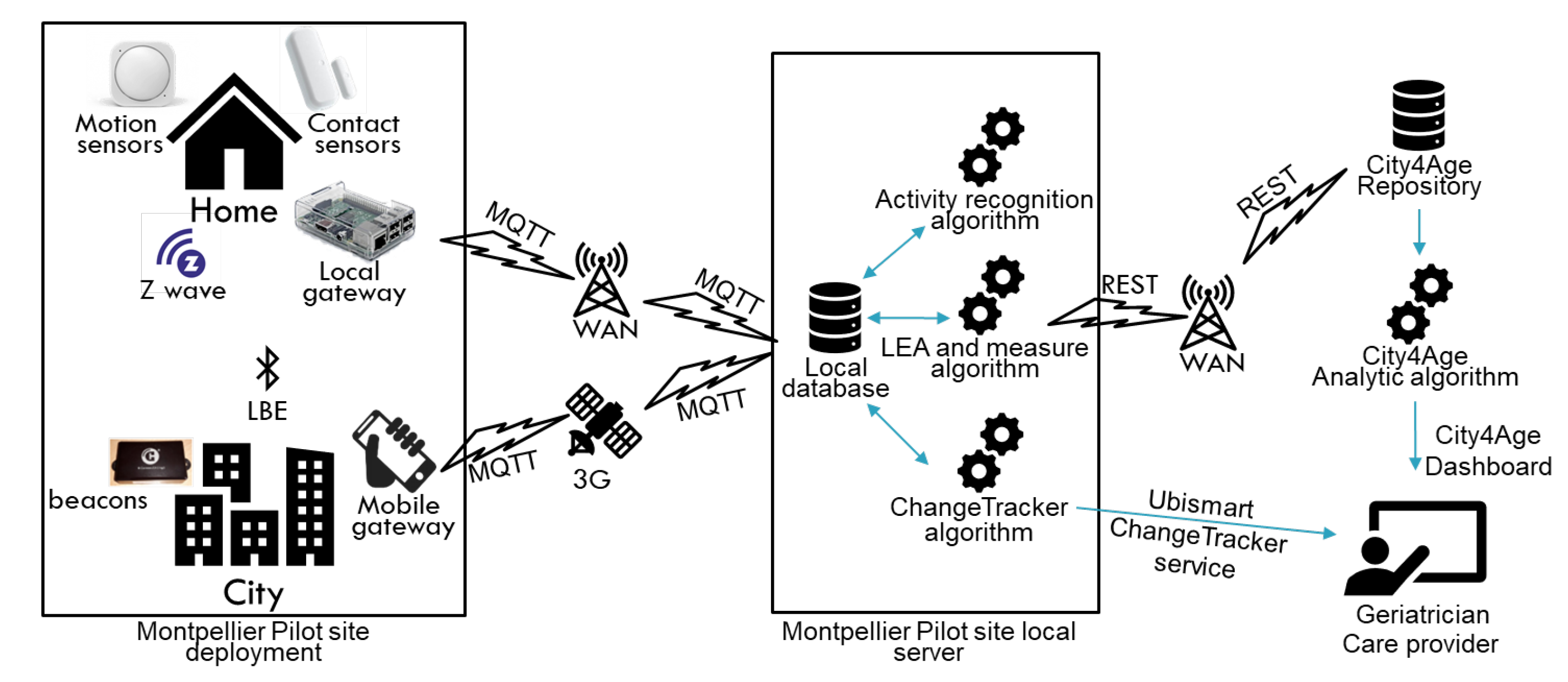
5. Technologies, Data Collection, and Data Analysis
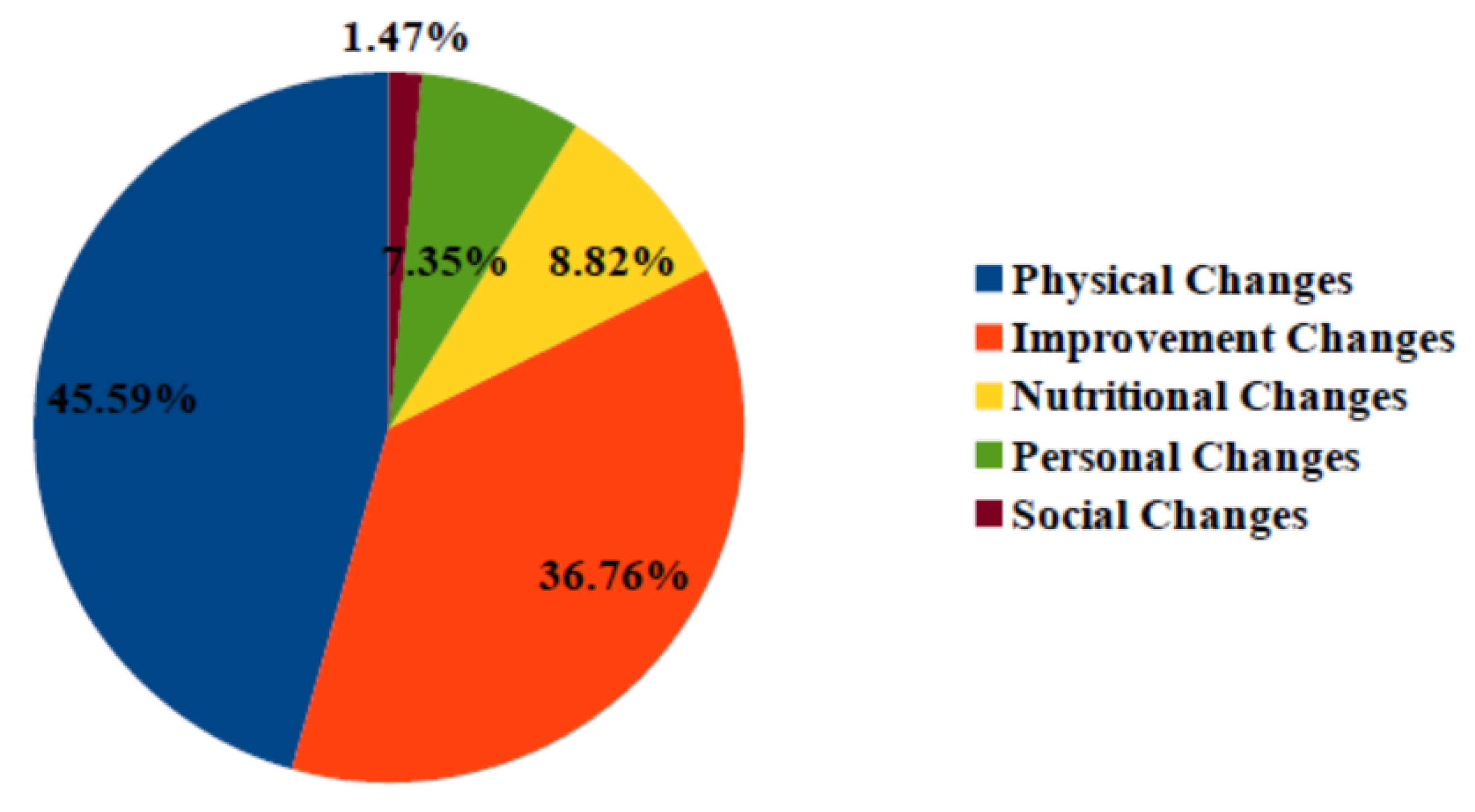
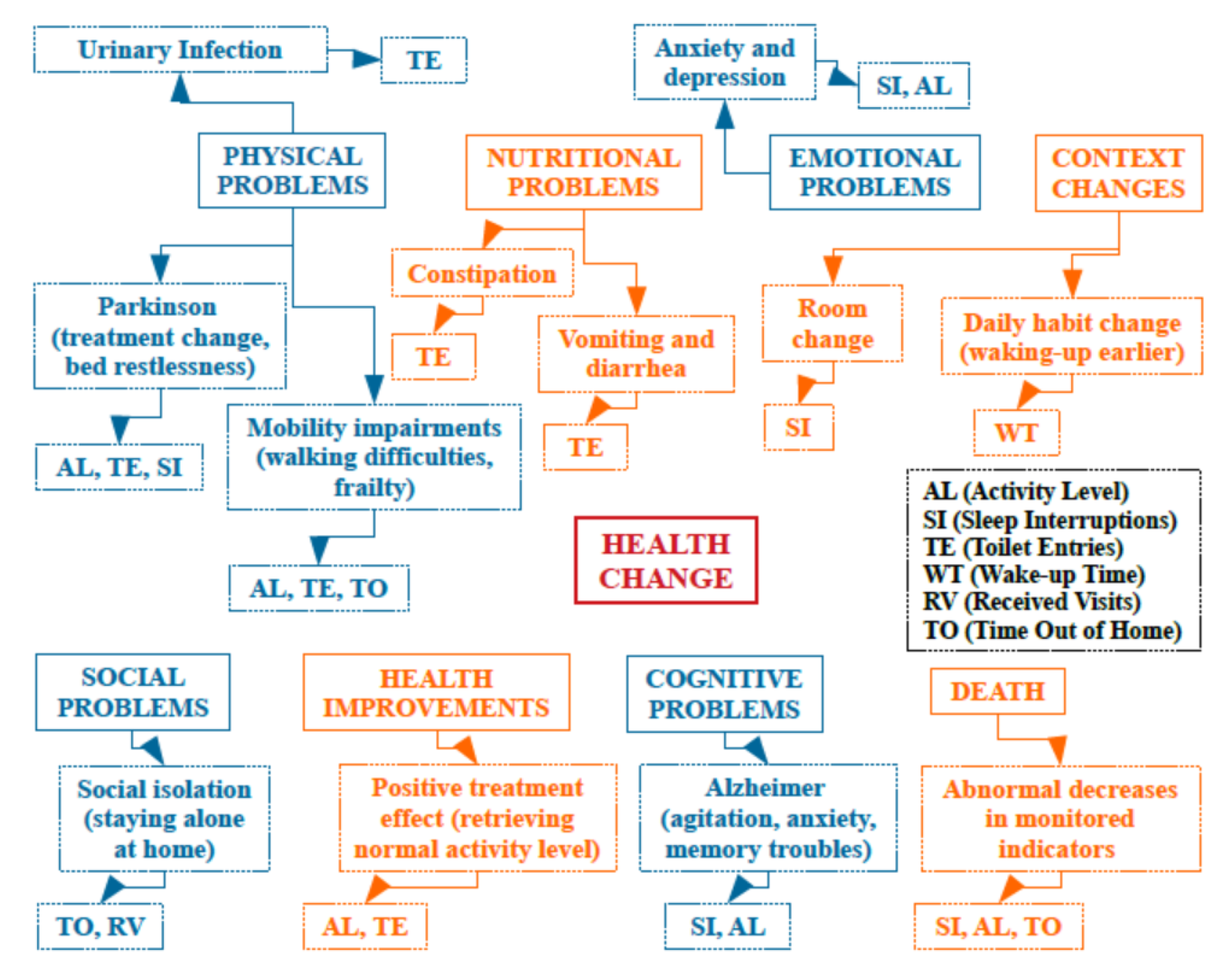
6. Behavior Change Detection
7. Intervention Process
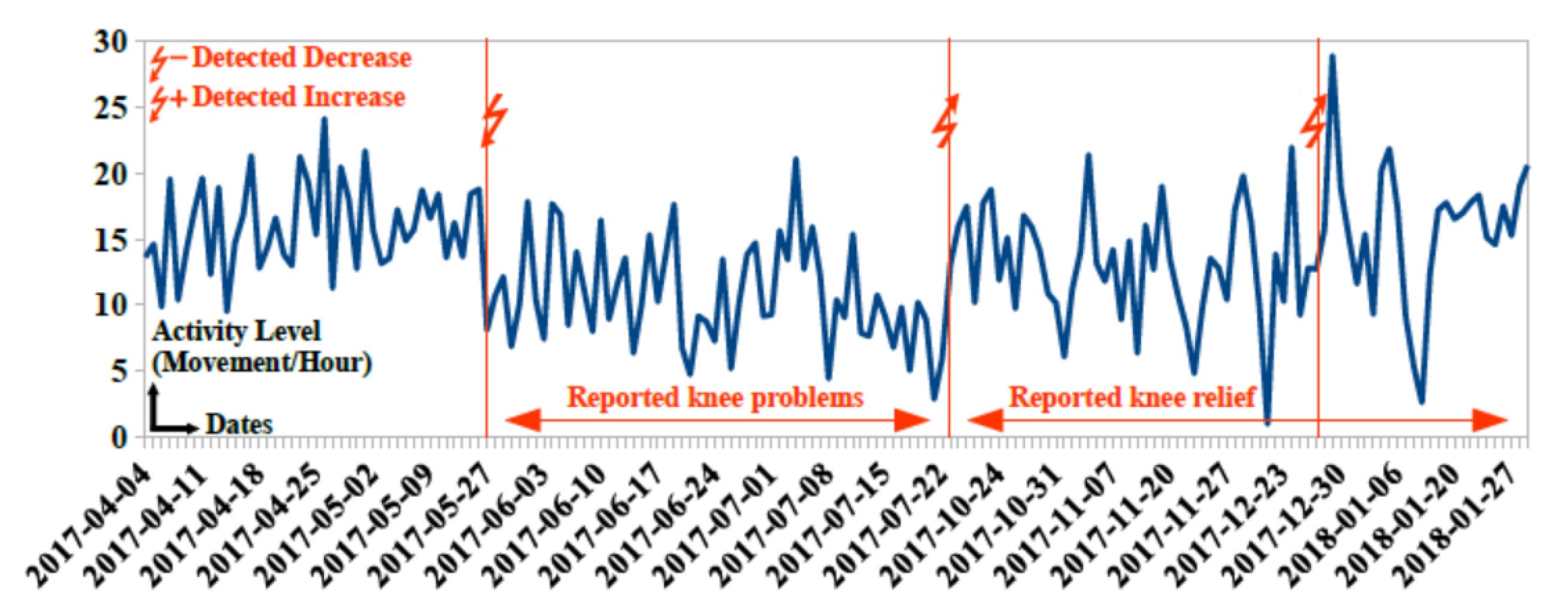
- After detecting a decrease in outdoor and indoor activities for participant 96, nursing home stuff decided to initiate home assistance
- Detecting a decrease in outdoor activities for participant 92 allowed the geriatrician to decide on the hospitalization of this participant.
- Detecting decrease in toilet visits for participant 94 and an increase in activity level for participant 95 allowed the geriatrician to change the medical treatment for these two participants
8. Validation
8.1. Technology Validation
8.2. Detection Process Validation
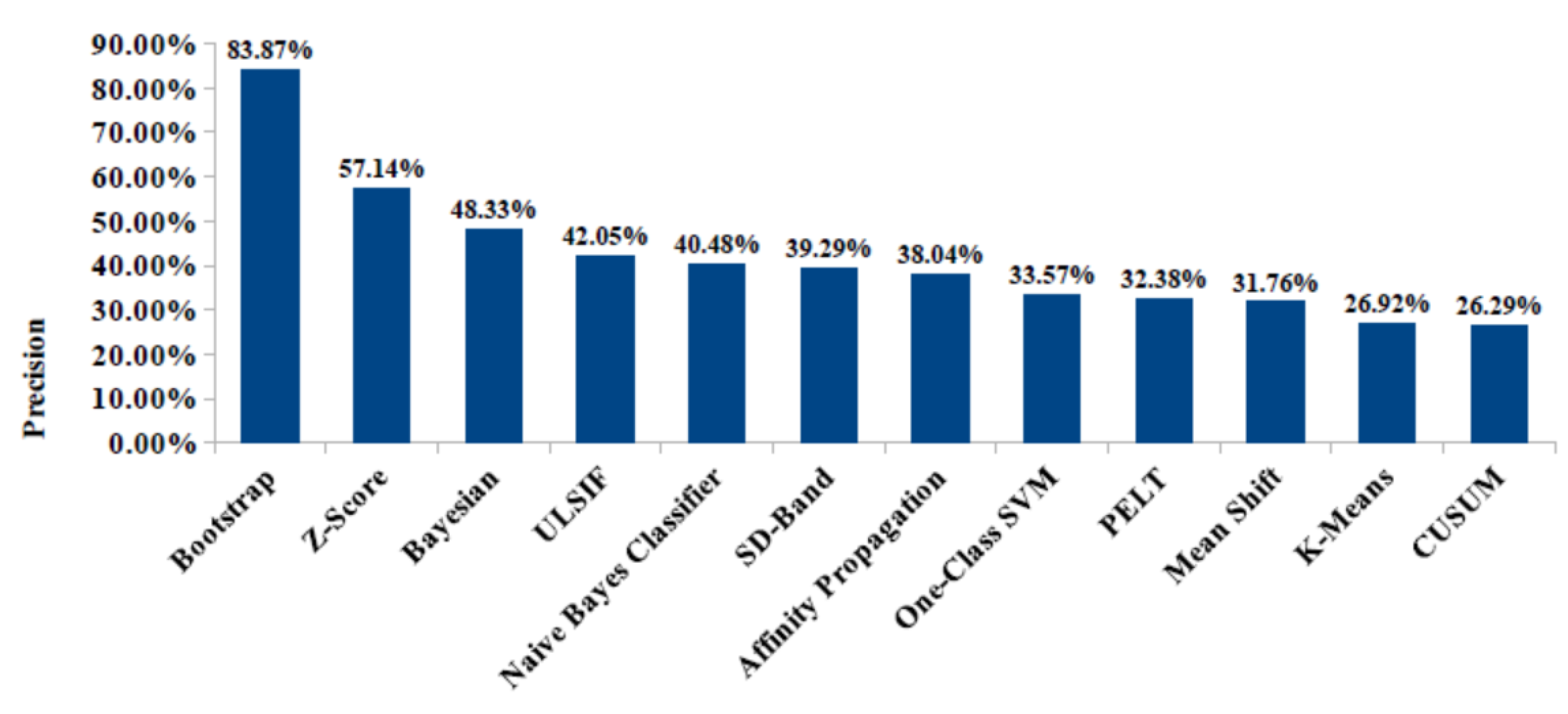
8.3. Results and Performance
8.4. Health Change Detection Ontology
8.5. Stakeholders’ Feedback
- Participant: I’m happy to participate in this research. Sensors do not bother me at all. They are now part of my house. I do not think about them. The results with the way we quantify my indoor movements and my activities are interesting.
- Geriatrician: We are working with patients with Parkinson’s disease. In this special disease, there are many problems concerning sleep and voiding function, and we have a solution to propose to them. However, in short consultations, we don’t have time to speak about all things and we know very few things about patients’ activities of daily living. We think that an unobtrusive technological solution will be interesting to help us to improve our assessment.
- Caregiver: My mother is participating in the project. The system doesn’t affect privacy. This is very important, and our feedback is positive. We could detect changes that correlate with my mother’s health status. This was beneficial for our discussions.
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waxman, A. WHO global strategy on diet, physical activity and health. Food Nutr. Bull. 2004, 25, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ridley, S. The recognition and early management of critical illness. Ann. R. Coll. Surgeons Eng. 2005, 87, 315. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. In-depth behavior understanding and use: The behavior informatics approach. Inf. Sci. 2010, 180, 3067–3085. [Google Scholar] [CrossRef]
- Tardieu, É.; Mahmoudi, R.; Novella, J.L.; Oubaya, N.; Blanchard, F.; Jolly, D.; Drame, M. External validation of the short emergency geriatric assessment (SEGA) instrument on the SAFES cohort. Geriatrie et Psychologie Neuropsychiatrie du Vieillissement 2016, 14, 49–55. [Google Scholar] [CrossRef]
- Cockrell, J.R.; Folstein, M.F. Mini-mental state examination. Princ. Pract. Eriatric Psychiatry 2002, 140–141. [Google Scholar] [CrossRef]
- Parmelee, P.A.; Katz, I.R. Geriatric depression scale. J. Am. Geriatr. Soc. 1990, 38, 1379. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Commenges, D.; Gagnon, M.; Letenneur, L.; Sauvel, C.; Dartigues, J.F. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J. Am. Geriatr. Soc. 1992, 40, 1129–1134. [Google Scholar] [CrossRef]
- Lafont, S.; Barberger-Gateau, P.; Sourgen, C.; Dartigues, J. Relation entre performances cognitives globales et dépendance évaluée par la grille AGGIR. Revue D’épidémiologie et de Santé Publique 1999, 47, 7–17. [Google Scholar]
- Lökk, J. Lack of information and access to advanced treatment for Parkinson’s disease patients. J. Multi. Healthc. 2011, 4, 433. [Google Scholar] [CrossRef]
- Wilson, D.; Consolvo, S.; Fishkin, K.; Philipose, M. In-Home Assessment of the Activities of Daily Living of the Elderly. Extended Abstracts of CHI 2005: Workshops-HCI Challenges in Health Assessment. 2005. Available online: https://homes.cs.washington.edu/~matthai/pubs/chi05_ws.pdf (accessed on 27 March 2020).
- Acampora, G.; Cook, D.J.; Rashidi, P.; Vasilakos, A.V. A survey on ambient intelligence in healthcare. Proc. IEEE 2013, 101, 2470–2494. [Google Scholar] [CrossRef]
- Coronato, A. Uranus: A middleware architecture for dependable AAL and vital signs monitoring applications. Sensors 2012, 12, 3145–3161. [Google Scholar] [CrossRef] [PubMed]
- Tunca, C.; Alemdar, H.; Ertan, H.; Incel, O.D.; Ersoy, C. Multimodal wireless sensor network-based ambient assisted living in real homes with multiple residents. Sensors 2014, 14, 9692–9719. [Google Scholar] [CrossRef] [PubMed]
- Alsina-Pagès, R.M.; Navarro, J.; Alías, F.; Hervás, M. homesound: Real-time audio event detection based on high performance computing for behaviour and surveillance remote monitoring. Sensors 2017, 17, 854. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Najafi, B. Sensor-Based Daily Physical Activity: Towards Prediction of the Level of Concern about Falling in Peripheral Neuropathy. Sensors 2020, 20, 505. [Google Scholar] [CrossRef]
- Addante, F.; Gaetani, F.; Patrono, L.; Sancarlo, D.; Sergi, I.; Vergari, G. An Innovative AAL System Based on IoT Technologies for Patients with Sarcopenia. Sensors 2019, 19, 4951. [Google Scholar] [CrossRef]
- Paragliola, G.; Coronato, A. Gait anomaly detection of subjects with Parkinson’s disease using a deep time series-based approach. IEEE Access 2018, 6, 73280–73292. [Google Scholar] [CrossRef]
- Coronato, A.; De Pietro, G.; Paragliola, G. A situation-aware system for the detection of motion disorders of patients with autism spectrum disorders. Expert Syst. Appl. 2014, 41, 7868–7877. [Google Scholar] [CrossRef]
- Monekosso, D.N.; Remagnino, P. Behavior analysis for assisted living. IEEE Trans. Autom. Sci. Eng. 2010, 7, 879–886. [Google Scholar] [CrossRef]
- Mora, N.; Grossi, F.; Russo, D.; Barsocchi, P.; Hu, R.; Brunschwiler, T.; Michel, B.; Cocchi, F.; Montanari, E.; Nunziata, S.; et al. IoT-Based Home Monitoring: Supporting Practitioners’ Assessment by Behavioral Analysis. Sensors 2019, 19, 3238. [Google Scholar] [CrossRef]
- Grgurić, A.; Mošmondor, M.; Huljenić, D. The SmartHabits: An intelligent privacy-aware home care assistance system. Sensors 2019, 19, 907. [Google Scholar] [CrossRef]
- Aloulou, H.; Mokhtari, M.; Tiberghien, T.; Biswas, J.; Yap, P. An adaptable and flexible framework for assistive living of cognitively impaired people. IEEE J. Biomed. Health Inf. 2013, 18, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, H.; Mokhtari, M.; Tiberghien, T.; Biswas, J.; Phua, C.; Lin, J.H.K.; Yap, P. Deployment of assistive living technology in a nursing home environment: Methods and lessons learned. BMC Med. Inf. Decis. Making 2013, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.L.; Dey, A.K. Sensor-based observations of daily living for aging in place. Pers. Ubiquitous Comput. 2015, 19, 27–43. [Google Scholar] [CrossRef]
- Sprint, G.; Cook, D.J.; Schmitter-Edgecombe, M. Unsupervised detection and analysis of changes in everyday physical activity data. J. Biomed. Inf. 2016, 63, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.L.; Riley, T.; Mattek, N.; Pavel, M.; Kaye, J.A. Sleep habits in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2014, 28, 145. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.; Mattek, N.; Dodge, H.H.; Campbell, I.; Hayes, T.; Austin, D.; Hatt, W.; Wild, K.; Jimison, H.; Pavel, M. Unobtrusive measurement of daily computer use to detect mild cognitive impairment. Alzheimer’s Dement. 2014, 10, 10–17. [Google Scholar] [CrossRef]
- Allin, S.; Bharucha, A.; Zimmerman, J.; Wilson, D.; Robinson, M.; Stevens, S.; Wactlar, H.; Atkeson, C. Toward the automatic assessment of behavioral distrubances of dementia. In Proceedings of the Fifth International Conference on Ubiquitous Computing, 2nd International Workshop on Ubiquitous Computing for Pervasive Healthcare Applications, Seattle, WA, USA, 12–15 October 2003. [Google Scholar]
- Xiang, T.; Gong, S. Video behavior profiling for anomaly detection. IEEE Trans. Pattern Anal. Mach. Intell. 2008, 30, 893–908. [Google Scholar] [CrossRef]
- Avvenuti, M.; Baker, C.; Light, J.; Tulpan, D.; Vecchio, A. Non-intrusive patient monitoring of Alzheimer’s disease subjects using wireless sensor networks. In Proceedings of the 2009 World Congress on Privacy, Security, Trust and the Management of e-Business, Saint John, NB, Canada, 25–27 August 2009; pp. 161–165. [Google Scholar]
- Loy, C.C.; Xiang, T.; Gong, S. Surveillance video behaviour profiling and anomaly detection. Proc. SPIE 2009, 7486, 74860E. [Google Scholar]
- Nater, F.; Grabner, H.; Van Gool, L. Exploiting simple hierarchies for unsupervised human behavior analysis. In Proceedings of the 2010 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Francisco, CA, USA, 13–18 June 2010; pp. 2014–2021. [Google Scholar]
- Lally, P.; Van Jaarsveld, C.H.; Potts, H.W.; Wardle, J. How are habits formed: Modelling habit formation in the real world. Eur. J. Soc. Psychol. 2010, 40, 998–1009. [Google Scholar] [CrossRef]
- Aloulou, H.; Mokhtari, M.; Abdulrazak, B. Deployment of an IoT Solution for Early Behavior Change Detection. In Proceedings of the International Conference on Smart Living and Public Health, New York, NY, USA, 14–16 October 2019; pp. 27–35. [Google Scholar]
- Aloulou, H.; Mokhtari, M.; Tiberghien, T.; Endelin, R.; Biswas, J. Uncertainty handling in semantic reasoning for accurate context understanding. Knowl. Based Syst. 2015, 77, 16–28. [Google Scholar] [CrossRef]
- Kaddachi, F.; Aloulou, H.; Abdulrazak, B.; Fraisse, P.; Mokhtari, M. Long-term behavior change detection approach through objective technological observations toward better adaptation of services for elderly people. Health Technol. 2018, 8, 329–340. [Google Scholar] [CrossRef]
- Kaddachi, F.; Aloulou, H.; Abdulrazak, B.; Fraisse, P.; Mokhtari, M. Technological Approach for Early and Unobtrusive Detection of Possible Health Changes Toward More Effective Treatment. In Proceedings of the International Conference on Smart Homes and Health Telematics, Singapore, 10–12 July 2018; pp. 47–59. [Google Scholar]






| Id | Birth Date | S | Marital Status | Education | Care |
|---|---|---|---|---|---|
| 91 | 1934 | F | W | primary | Yes |
| 92 | 1949 | M | D | secondary | Yes |
| 93 | 1939 | F | W | tertiary | No |
| 94 | 1956 | F | D | tertiary | Yes |
| 95 | 1959 | M | S | tertiary | Yes |
| 96 | 1923 | M | W | secondary | Yes |
| 97 | 1923 | F | W | primary | Yes |
| 98 | 1925 | F | W | none | Yes |
| 99 | 1926 | F | W | none | No |
| 100 | 1928 | M | W | secondary | Yes |
| 101 | 1928 | F | W | none | Yes |
| 102 | 1932 | F | W | secondary | Yes |
| 103 | 1929 | F | W | secondary | Yes |
| 170 | 1928 | F | W | secondary | Yes |
| 171 | 1927 | F | W | secondary | Yes |
| 172 | 1922 | F | W | secondary | Yes |
| 173 | 1919 | F | W | secondary | Yes |
| 174 | 1933 | M | W | secondary | Yes |
| Patient | Regular Habits | Health Info |
|---|---|---|
| 91 | Wakes up at 7 h. Goes to toilet. Takes breakfast. Goes out for 1 hour to take care of animals. Goes out between 12 h 30 and 14 h for lunch with his daughter. Reads newspapers. Frequently goes out during the day. Friend visits on Sundays midday. Goes out shopping Wednesdays. | Very active person. No special diseases. Recent mobility impairments. Recent social isolation. Recent nutritional problems. |
| 98 | Wakes up at 8 h. Home aid 4 times per day. Stays most often at home. Sometimes goes out with daughter or caregiver. | Alzheimer. Diabetes. Vision and audition problems. |
| 101 | Wakes up at 7 h 30–8 h. Home aid visits 3 times per day (morning, midday and evening). Niece and neighbor visits during the day. Sleeps earlier than before (at 20 h, and before at 22 h). | Alzheimer. Some falls and hospitalizations. |
| 102 | Wakes up at 6 h–7 h. Home aid visits each day in the morning. Lives alone. Daughter house is nearby. Monthly visits to and from daughter. | Heart problems. Urinary infection. |
| Technology | Model | Raw Data | Inferred Data | Number | Location |
|---|---|---|---|---|---|
| Movement sensor | Z-wave MultiSensor | Presence/absence of movements | Walking patterns, received visits, sleep interruptions, toilet entries | 4–5/part | One sensor/room |
| Contact sensor | Z-wave Door/Window Sensor | Openings/closings of objects | Come home, go out, prepare meal, take medicines, read books | 3–4/part | On specific objects |
| Bed sensor | Fiber optic bedsensor | Bed movements, heart beats, respiration rate, rhythm, depth | Sleep time, wake-up time, sleep duration, bed restlessness, cardiac events, respiration | 1/part | On the bed |
| Beacon sensor | BLE beacon Sensor | Unique identifier bound to specific locations in the city | Shops visits, restaurants visits, cinema visits, transport usage | 4–5/part | Attached in specific locations in the city |
| Category | Sub-Category | Examples | Relevance | Technology |
|---|---|---|---|---|
| Activity of Daily Living | House activities | Clean, tidy-up rooms, reading, watching TV, put laundry, wash dishes | Physical, cognitive impairments, autonomy loss | Door, movement |
| Upper hygiene | Shave, dress one’s hair | |||
| Inferior hygiene | Hygiene of intimate, inferior members, legs, feet, nails | |||
| Elimination | Urinary and fecal elimination | |||
| Mobility | Moving | Between the rooms, to areas of interest in the city | physical problems | beacons, movement, door |
| Position changes | Walk, get up, turn around, sit | |||
| Social Life | Go out | Use means of transport, shopping, free time activities | Social isolation | beacons |
| Nutrition | Eat | Protein, fruit, vegetable | digestive problems, depression | movement, door |
| Metric | Description | Examples |
|---|---|---|
| Time | Start and end times of executing monitored activities | eating time, sleep time, wake up time, watch TV time |
| Place | Where monitored activities are executed | shopping place, entertainment place, physical activities place cultural activities place |
| Number | quantity and amount of human activity execution | number of sleep interruptions, number of toilet entries, number of meals |
| Duration | length of executing monitored activities | sleep duration, watch TV duration, out of home duration |
| Category | Collected Measures | Periodicity |
|---|---|---|
| Indoor measures | NB_ROOM_CHANGES, NB_BEDROOM_VISITS, TIME_BEDROOM, NB_LIVINGROOM_VISITS, TIME_LIVINGROOM, NB_RESTROOM_VISITS, TIME_RESTROOM, NB_KITCHEN_VISITS, TIME_KITCHEN, NB_BATHROOMS_VISITS, TIME_BATHROOM, NB_MEALS, TIME_MEALS, TIME_HOME, TIME_OUTDOOR, NB_OUTDOOR, TIME_SLEEP | /day |
| Outdoor measures | NB_SHOPS_VISITS, TIME_SHOPS, NB_SUPERMARKET_VISITS, TIME_SUPERMARKET, NB_RESTAURANTS_VISITS, TIME_RESTAURANTS, NB_CINEMA_VISITS, TIME_CINEMA, NB_PHARMACY_VISITS | /week |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloulou, H.; Mokhtari, M.; Abdulrazak, B. Pilot Site Deployment of an IoT Solution for Older Adults’ Early Behavior Change Detection. Sensors 2020, 20, 1888. https://doi.org/10.3390/s20071888
Aloulou H, Mokhtari M, Abdulrazak B. Pilot Site Deployment of an IoT Solution for Older Adults’ Early Behavior Change Detection. Sensors. 2020; 20(7):1888. https://doi.org/10.3390/s20071888
Chicago/Turabian StyleAloulou, Hamdi, Mounir Mokhtari, and Bessam Abdulrazak. 2020. "Pilot Site Deployment of an IoT Solution for Older Adults’ Early Behavior Change Detection" Sensors 20, no. 7: 1888. https://doi.org/10.3390/s20071888
APA StyleAloulou, H., Mokhtari, M., & Abdulrazak, B. (2020). Pilot Site Deployment of an IoT Solution for Older Adults’ Early Behavior Change Detection. Sensors, 20(7), 1888. https://doi.org/10.3390/s20071888







